Abstract
Obese adipose tissue (AT) is characterized by increased recruitment and infiltration of multiple immune cell populations, in particular T cells (CD4+ or CD8+ subsets) and macrophages, that interact with adipocytes through paracrine signaling (i.e., cross-talk). Adipocyte/ immune cell cross-talk results in increased inflammatory and chemoattractant mediator production that contributes to local (i.e., AT) and systemic metabolic dysfunction. Therefore, co-culture models of adipocytes and immune cell populations represent an important experimental approach to study how paracrine interactions between cell types promote obese AT inflammation and dysfunction, and to identify intervention strategies to attenuate this cellular cross-talk. In this commentary, we will discuss the development of physiologically relevant adipocyte (differentiated and mature 3T3-L1 pre-adipocyte cell line) and primary immune cell population (namely CD4+ T cells, CD8+ T cells and CD11b+ macrophages) co-culture models that recapitulate the critical features of the obese AT microenvironment via i) culturing cellular ratios that reproduce the cellular abundance of immune cells observed in obese AT, and ii) stimulation with a concentration of lipopolysaccharide (LPS) that mimics circulating endotoxin levels in obese humans and rodents. The co-culture models discussed are comprised of i) a cell contact-dependent model wherein the cells are in direct physical contact, ii) a cell contact-independent model, wherein cells are physically separated by trans-well semi-permeable membrane that prevents physical cell contact but permits soluble mediators to cross, and iii) a cell contact-independent model where conditioned media is generated from intact primary AT, or adipocyte/immune cell co-cultures to influence another cell type. Finally, we summarize the utility of these co-culture models by discussing recent findings demonstrating how n-3 polyunsaturated fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] derived from fish oil can attenuate inflammatory and chemotactic paracrine signaling between adipocytes and immune cell populations to improve AT function.
Keywords
Co-culture, Paracrine interactions, Cross-talk, Adipose tissue, Adipocyte, CD4+ and CD8+ T cells, Macrophages, n-3 polyunsaturated fatty acids
Introduction
This invited Commentary is on the methods paper entitled “Studying adipocyte and immune cell cross talk using a co-culture system” in Immunometabolism: Methods and Protocols [1]. Co-culturing individual immune cell populations (as primary cells or cell lines) with adipocytes represents a model system to study the paracrine interactions (or cross-talk) between cell types that can impact adipose tissue (AT) function. This is particularly relevant in obese AT, wherein paracrine interactions between cell types promotes the secretion of inflammatory mediators that contribute to increased local (i.e. within the AT) and systemic low-grade inflammation and metabolic dysfunction, including insulin resistance (IR) [2-5].
AT is comprised of adipocytes and multiple immune cell types within the stromal vascular cellular fraction, as reviewed elsewhere [6]. As lean AT converts into obese AT during prolonged periods of overnutrition, there is a change in both the number and activity of immune cell populations. In lean AT, regulatory T cells [Tregs; CD4+, forkhead box P3 (FOXP3+)] and M2-polarized macrophages (F4/80+, CD11b+, CD11c-) have been shown to contribute to the maintenance of insulin sensitivity, in part, via secretion of anti-inflammatory mediators [3,5,7]. Conversely, in obese AT, decreases in Treg and M2 cellular abundance combined with increased immune cell recruitment and infiltration changes the cellular composition of the AT stromal vascular fraction and contributes to inflammation and metabolic dysfunction of the tissue [8-17]. Specifically, the increased abundance of immune cells within obese AT includes macrophages exhibiting polarization to the inflammatory M1 phenotype (F4/80+, CD11b+, CD11c+) [8-13], CD4+ T cells [4,9,14,18], CD8+ T cells [4,9,14], natural killer (NK) cells [19-21], B cells [22,23] and dendritic cells [24,25]. Thus, understanding the influence of the paracrine interactions between immune cell populations and adipocytes in obese AT that underlie obesity-associated inflammation and metabolic dysfunction (both locally and systemically) will help elucidate appropriate intervention strategies to attenuate inflammatory mediator production and improve AT function.
Our research group [26-32] and others [33-36] have demonstrated the anti-inflammatory mechanisms through which long-chain (LC) omega-3 (n-3) polyunsaturated fatty acids (PUFA), eicosapentaenoic acid (20:5:n-3, EPA) and docosahexaenoic acid (22:6n-3, DHA), serve as an intervention strategy to improve obesity-associated AT inflammation and metabolic dysfunction. As such, high fat (HF) diet supplementation with fish oil derived LC n-3 PUFA can attenuate the severity of AT and systemic inflammation and associated metabolic dysfunction [26], which can be attributed, at least in part, to the paracrine interactions (or cross-talk) between immune cell subsets and adipocytes, as reviewed elsewhere [2]. Since research on adipocyte – immune cell cross-talk has centred on macrophages and T cell subsets, these immune cell populations are the focus of this commentary. We will discuss the development of the adipocyte-immune cell co-culture models and highlight our research findings demonstrating the ability of n-3 PUFA to mitigate paracrine signaling between co-cultured adipocytes and T cells (CD8+ and CD4+) or macrophages with an emphasis on the secretory profile (inflammatory and chemotactic mediators), however, it is worth noting that we have also shown a beneficial impact of n-3 PUFA on the NLRP3 inflammasome and/or macrophage M1/M2 polarization status in these models [29,31,32,37].
Adipocyte – Immune Cell Co-Culture Models
The adipocyte – immune cell (either macrophages, CD8+ or CD4+ T cells) co-culture model we developed [27-29,31,32,37-39], utilizes the murine 3T3-L1 preadipocyte cell line, which requires differentiation into lipid-laden mature adipocytes prior to co-culture with immune cells and provides a standardized component of the model between co-culture studies that is combined with varying immune cell populations [1]. To recapitulate a critical feature of the obese phenotype and provide an inflammatory stimulus, co-cultures are stimulated with a physiologically relevant dose of lipopolysaccharide (LPS, 10 ng/mL) derived from Escherichia coli serotype 055:B5, which reproduces the level of endotoxin units reported in obese humans and rodents (5-6 endotoxin units/mL) [40-42], that is not utilized in other adipocyte-immune cell co-culture models [43-47]. Moreover, we have conducted studies involving the pre-stimulation of adipocytes with LPS for 24 hr to recapitulate already inflamed AT prior to co-culture with either macrophages [27,28] or CD8+ T cells [31], as this would be the AT microenvironment that newly infiltrating immune cells would encounter in vivo [2,9]. Our initial co-culture work utilized the RAW264.7 macrophage cell line [38], which is commonly employed by other groups in adipocyte co-cultures treated with dietary bioactives [43-47]. More recently, we have utilized mouse primary splenic CD11b+ macrophages in co-culture with 3T3-L1 mature adipocytes [28], which increases the translational potential of this model compared to studies using primary splenocytes (comprised of multiple undefined cell types) [48], or immortalized cell lines (e.g. RAW264.7 macrophages) [43-47]. In this connection, our co-culture work with CD8+ and CD4+ T cells has exclusively utilized primary cells purified from the spleen of both lean and obese mice [29,31,32,37,39].
To further increase the translational relevance of the coculture model, we have utilized a physiologically relevant cellular ratio of the immune cell population co-cultured with adipocytes to recapitulate their abundance within obese AT, which is in contrast to co-culture models utilizing equal numbers of adipocytes and macrophages (1:1 cellular ratio) [43-47]. In our model, macrophages are co-cultured with adipocytes at 17% of cells in culture [28,38], which is reflective of the level of macrophage cellular infiltration in epididymal AT of db/db mice and recapitulates the in vivo cellular ratio of macrophages:adipocytes [49]. Similarly, T cells are co-cultured with adipocytes to recapitulate the murine obese AT cellular ratio of adipocytes to T cells [9], with co-cultures comprised of 10% CD8+ T cells [31,37,39] and 5% CD4+ T cells [29,32].
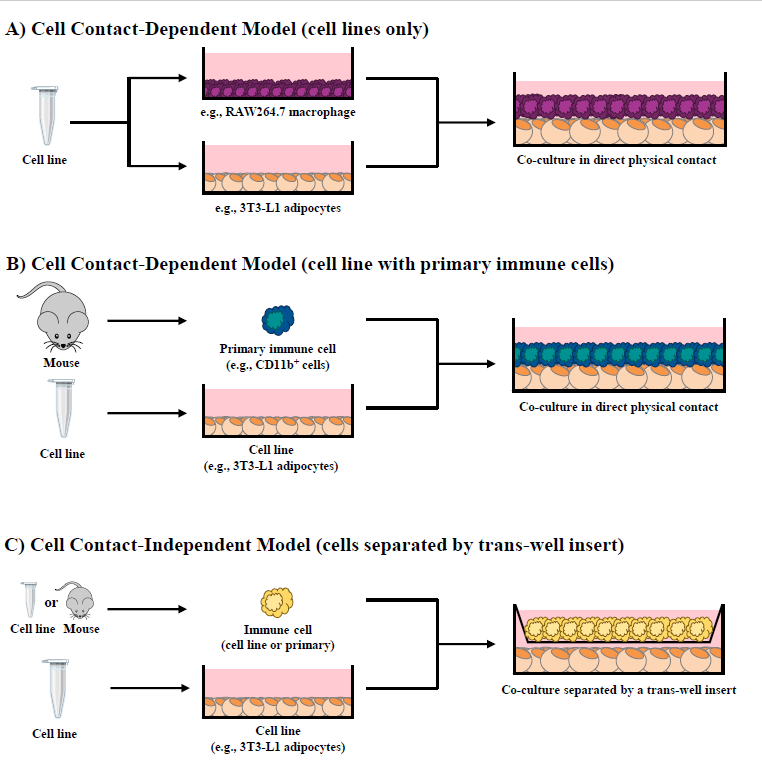
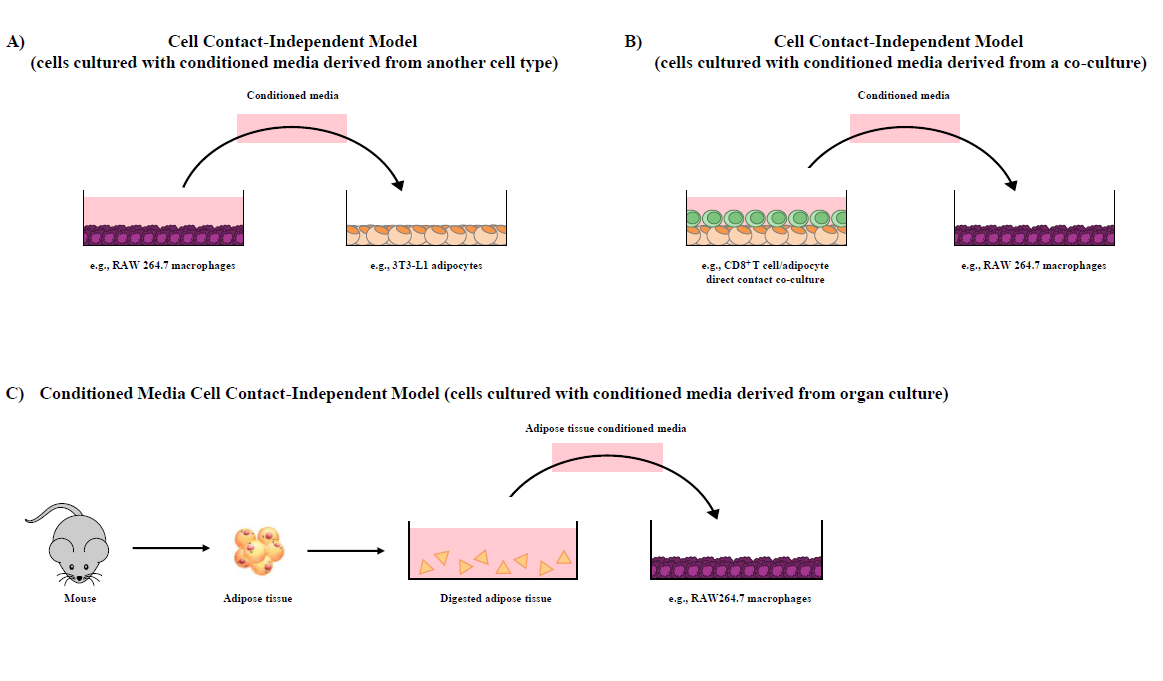
Our research group has shown that cellular co-cultures can be established for 12 hr [38], 24 hr [28,31,32,37,39] or 48 hr [29] and can be utilized to discern the difference in outcomes resultant from i) direct cell contact (i.e., contactdependent, Figure 1A and 1B), a combined outcome of both physical cellular interactions and paracrine signaling mechanisms, and ii) trans-well in direct cell contact (i.e., contact-independent, Figure 1C), wherein cells are separated by a semi-permeable 0.4 μM polyester membrane trans-well insert that precludes physical contact between cell types but permits the movement of soluble mediators across the trans-well insert and the discernment of paracrine signaling effects [1]. To highlight a variation of the adipocyte/immune cell co-culture models, our research group has also utilized conditioned media (contact-independent) generated from i) one cultured cell line to another (Figure 2A), ii) adipocyte/CD8+ T cell co-culture conditioned media added to RAW264.7 macrophage cultures (Figure 2B) [31,37,39], and iii) intact primary AT conditioned media added to adipocyte/CD4+ T cell co-cultures [32] or macrophages [27] (Figure 2C).
CD8+ T cell and Adipocyte Co-Culture
CD8+ T cells have been shown to accumulate in obese AT prior to the accumulation of macrophages [9,50,51] and co-localize to crown-like structures in the AT [9]. In vivo depletion of CD8+ T cells via neutralizing antibody injections reduces the expression of macrophage chemotactic signals in AT and cellular trafficking, thereby reducing the magnitude of inflammation and systemic IR [9]. Similarly, the severity of the obese phenotype in CD8a-/- mice is attenuated but can be reversed through adoptive transfer of CD8+ T cells resulting in inflammatory mediator production and IR [9], thus, demonstrating the essential role of this cell type in the development and maintenance of obesity-associated AT dysfunction.
To highlight the utility of our co-culture models to study the paracrine signaling between adipocytes and immune cells, our initial contact-dependent co-culture studies used 3T3-L1 mature adipocytes cultured with purified splenic CD8+ T cells isolated from lean mice fed either a fish oil [37] or flaxseed oil [39] supplemented diet as the source of either marine- or plant-derived n-3 PUFA, respectively. Despite the difference in marine versus plant sources of n-3 PUFA that enriched the CD8+ T cells, in contact dependent co-culture with adipocytes stimulated with LPS, the resultant anti-inflammatory and anti-chemotactic effect was consistent and characterized by reduced TNFα, IL-6, MCP-1, MCP-3 and MIP-1β secreted protein into the culture media compared to control [37,39]. Subsequently, the CD8+ T cell/adipocyte co-culture conditioned media was used in a follow-up experiment with RAW264.7 macrophages in a chemotaxis assay to demonstrate that the number of macrophages migrating towards the chemotactic signals in the conditioned media was reduced compared to control [37,39], indicating a functional outcome of reduced macrophage trafficking as a result of n-3 PUFA attenuating the paracrine interactions between CD8+ T cells and adipocytes. The anti-inflammatory and anti-macrophage chemotactic secretory profile of n-3 PUFA-enriched CD8+ T cells/adipocyte co-cultures was later confirmed in both the cell-contact dependent and cell contact-independent (trans-well) co-culture models, which was mechanistically attributed, in part, to the effects of TNFα [31]. The anti-inflammatory and anti-chemotactic secretory profile in n-3 PUFA co-cultures could be reproduced in control n-6 PUFA-enriched CD8+ T cell/ adipocytes co-cultures treated with a TNFα neutralizing antibody [31].
CD4+ T cell and Adipocyte Co-Culture
CD4+ T cells subsets have been shown to change in obese AT prior to the infiltration and accumulation of macrophages [14], wherein IFNγ-secreting Th1 cells have been shown to promote macrophage M1 polarization and contribute to AT metabolic dysfunction [14,18,50,52]. Additionally, IL-17-secreting Th17 cells have also been shown to contribute to obese AT inflammation [53].
In our co-culture model (using a physiologically relevant cellular ratio and LPS concentration) adipocytes were co-cultured in direct cell contact with primary splenicderived purified CD4+ T cells from lean mice fed isocaloric diets enriched with either n-3 or n-6 PUFA. Using this approach, n-3 PUFA increased mRNA expression and/ or secreted protein of Th2 polarization markers (GATA3, IL-4) and reduced expression of Th1 polarization markers (Tbet, IFNγ), in addition to reducing the secretion of other inflammatory and macrophage chemotactic mediators (IL-1β, IL-6, MCP-1, MCP-3 and MIP-1α) [32]. These effects were reproduced in contact-dependent co-cultures containing adipocytes and splenic CD4+ T cells from HF diet-induced obese mice consuming either an n-3 or n-6 PUFA-supplemented isocaloric diet (containing equal amounts of lard/saturated fatty acids) [29]. Collectively, our findings demonstrate the ability of adipocyte/CD4+ T cell cross-talk to influence the local inflammatory and chemotactic microenvironment, which can impact subsequent macrophage chemotaxis and ultimately contribute to AT metabolic dysfunction. Future studies utilizing contact-independent conditioned media and the trans-well (cell contact-independent) models can help discern the inflammatory and chemotactic mediator cellular source to mechanistically identify the contribution of each cell type in co-culture to help direct targeted interventions to improve AT function.
Macrophages and Adipocyte Co-Culture
As obesity progresses, the infiltration of macrophages into the AT increases from 3% to approximately 20% of total non-adipocyte cells [54], wherein they exhibit an inflammatory M1 phenotype and increase inflammatory mediator production contributing substantially to obesityassociated inflammation and IR [2,9,12,14,55]. We [28,38] and others [43-47] have used 3T3-L1 adipocyte and RAW264.7 macrophage co-culture models [i.e., in cell contact-dependent and cell contact-independent (trans-well)] to study the paracrine signaling mechanisms that underlie AT dysfunction using different dietary bioactive interventions. Our initial co-culture work utilizing cell lines (3T3-L1 adipocytes and RAW264.7 macrophages) demonstrated that n-3 PUFA (EPA and DHA) could attenuate the intensity of the inflammatory cross-talk between cell types (e.g. reduced secretion of IL-6 and MCP-1) in both the cell contact-dependent and cell contact-independent (i.e., trans-well) co-culture models, which was associated with increased expression of non-inflammatory M2 macrophage markers [38]. Co-culture of 3T3-L1 adipocytes with primary splenic CD11b+ macrophages from obese mice consuming a HF diet supplemented with or without n-3 PUFA confirmed the attenuated inflammatory mediator secretory profile and reduced expression of M1 macrophage markers in n-3 PUFA cultures [28]. This outcome was partially attributable to the effects of adiponectin [28], wherein n-3 PUFA have been shown to stimulate adiponectin secretion from adipocytes [56]. Finally, using a conditioned media contact-independent model in which RAW264.7 macrophage cultures were treated with conditioned media derived from intact primary AT from n-3 PUFA-fed mice, we demonstrated that macrophage-derived inflammatory and chemotactic mediator secretion (IL-6, MCP-1, MCP- 3 and RANTES) and M1 macrophage markers were reduced, again in part, through an adiponectin-dependent mechanism [27].
Skeletal Muscle and Macrophage Co- Culture
Moving beyond co-culture studies focused on AT in obesity is relevant given that other tissues also contribute to systemic inflammation and whole-body IR in obesity, in part, through inflammatory immune cell-tissue crosstalk, thereby, providing other targets for the systemic impacts of n-3 PUFA on the obese phenotype [26]. Skeletal muscle is the primary site for insulin-simulated glucose uptake; however, in obesity, increased circulating free fatty acids and inflammatory cytokines interfere with insulin signaling in skeletal muscle to promote development of whole-body IR [57-59]. Similar to AT, obese skeletal muscle is characterized by the infiltration and accumulation of immune cells, particularly M1 macrophages, which contribute to local inflammation and IR through immune cell-myocyte cross-talk [60-63]. Therefore, our research group [64-66] and others [67-69], have utilized a contact-independent conditioned media coculture model to examine the inflammatory macrophagemyocyte cross-talk that contributes to obese metabolic dysfunction. Importantly, attenuating macrophage-muscle inflammatory cross-talk represents another potential target for n-3 PUFA to improve obesity-associated insulin sensitivity. In this connection, RAW264.7 macrophages were stimulated with fatty acids [DHA (n-3 PUFA) versus palmitic acid (saturated fatty acid control)] and a physiologically relevant LPS dose (described above) to generate macrophage conditioned media (MCM), which contained secreted cytokines and chemokines that could impact muscle cell function in a cell contact-independent manner. When MCM was collected and transferred to cultures of differentiated L6 myotubes we demonstrated that DHA-derived MCM improved insulin stimulated L6 myotube function by increasing the phosphorylation status of mediators in the insulin signaling cascade and subsequent glucose uptake [64]. A later co-culture study using MCM generated from RAW264.7 macrophages and L6 myotubes demonstrated that n-3 PUFA-mediated attenuation of inflammatory macrophage-myocyte crosstalk is attributable, in part, to a PPAR-γ-dependent mechanism [65].
Most recently, to increase the translational relevance of the macrophage-myocyte co-culture model to recapitulate more accurately the obese skeletal muscle microenvironment, rats were fed a HF diet enriched with either n-3 or n-6 PUFA and primary purified splenic CD11b+ macrophages were isolated and co-cultured in direct contact with L6 myotubes stimulated with LPS, wherein n-6 PUFA increased inflammatory cytokine production compared to n-3 PUFA co-cultures [66]. Subsequently, in a contact-independent experiment to determine the response of macrophages to mediators secreted from myocytes, purified CD11b+ cells from obese rats (consuming n-3 PUFA and n-6 PUFA-enriched HF diets) were cultured alone in conditioned media collected from LPS-stimulated L6 myocytes. This resulted in n-3 PUFA-enriched macrophages reducing expression of inflammatory cytokines and M1 polarization markers compared to n-6 PUFA [66]. Collectively, skeletal muscle cell (myotube)/macrophage co-culture models represent a relevant future direction to elucidate the underlying mechanisms contributing to obesity-associated IR.
Conclusion
Through the use of appropriately crafted adipocyte/ immune cell (CD8+ and CD4+ T cell, macrophage) coculture models [1], the critical features of obese AT can be recapitulated by including physiologically relevant cellular ratios of adipocytes to immune cell populations [9,49] and LPS stimulation conditions [40-42]. Using this approach, we have shown that n-3 PUFA can attenuate the severity of the inflammatory and chemotactic paracrine interactions between adipocytes and macrophages [27,28], CD8+ T cells [31,37,39] and CD4+ T cells [29,32], which collectively contribute to obese AT dysfunction [2]. Moreover, we have expanded this co-culture model to study paracrine interactions in another metabolically active tissue, namely skeletal muscle/immune cell cross-talk [64-66] to better understand the contribution of immune cells in various tissues towards the severity of the obese phenotype and identify immune-centric intervention strategies to improve obesity-associated metabolic dysfunction.
Conflicts of Interest
The authors state that there are no conflicts of interest.
Funding Sources
This work is supported by NSERC Discovery Grants awarded to L.E.R. and J.M.M. A.L.H is supported by a NSERC Graduate Scholarship and J.L.A.M is supported by a Graduate Tuition Scholarship from the College of Biological Sciences, University of Guelph.
Author Contributions
J.M.M. – original draft preparation, concept development, review and editing; A.L.H – figures, review and editing; J.L.A.M. – review and editing; L.E.R. – concept development, review and editing.
References
2. Liddle DM, Hutchinson AL, Wellings HR, Power KA, Robinson LE, Monk JM. Integrated Immunomodulatory Mechanisms through which Long-Chain n-3 Polyunsaturated Fatty Acids Attenuate Obese Adipose Tissue Dysfunction. Nutrients. 2017;9(12).
3. Winer S, Winer DA. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol Cell Biol. 2012;90(8):755-62.
4. McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637- 43.
5. Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37(5):365-71.
6. Ramakrishnan VM, Boyd NL. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng Part B Rev. 2018;24(4):289-99.
7. Lolmède K, Duffaut C, Zakaroff-Girard A, Bouloumié A. Immune cells in adipose tissue: key players in metabolic disorders. Diabetes Metab. 2011;37(4):283-90.
8. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648-56.
9. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914-20.
10. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574-82.
11. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16-23.
12. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175-84.
13. Nakajima S, Koh V, Kua LF, So J, Davide L, Lim KS, et al. Accumulation of CD11c+CD163+ Adipose Tissue Macrophages through Upregulation of Intracellular 11ß-HSD1 in Human Obesity. J Immunol. 2016;197(9):3735-45.
14. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921-9.
15. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930-9.
16. Núñez Ruiz A, Cortés-Garcia JD, Cortez-Espinosa N, Herrera-Rojas PI, Ruíz-Rodríguez VM, Salgado- Bustamante M, et al. Diminished levels of regulatory T cell subsets (CD8+Foxp3, CD4+Foxp3 and CD4+CD39+Foxp3) but increased Foxp3 expression in adipose tissue from overweight subjects. Nutrition. 2016;32(9):943-54.
17. Gyllenhammer LE, Lam J, Alderete TL, Allayee H, Akbari O, Katkhouda N, et al. Lower omental t-regulatory cell count is associated with higher fasting glucose and lower ß-cell function in adults with obesity. Obesity (Silver Spring). 2016;24(6):1274-82.
18. Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring). 2010;18(10):1918-25.
19. Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30(2):193-9.
20. O’Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, et al. Systemic inflammation and insulin sensitivity in obese IFN-? knockout mice. Metabolism. 2012;61(8):1152-61.
21. Wensveen FM, Jelencic V, Valentic S, Šestan M, Wensveen TT, Theurich S, et al. NK cells link obesityinduced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16(4):376-85.
22. McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q, et al. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity (Silver Spring). 2012;20(7):1372-8.
23. Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610-7.
24. Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulinresistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238-47.
25. Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, et al. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. J Immunol. 2016;197(9):3650-61.
26. Monk JM, Liddle DM, Hutchinson AL, Wu W, Lepp D, Ma DWL, et al. Fish oil supplementation to a highfat diet improves both intestinal health and the systemic obese phenotype. J Nutr Biochem. 2019;72:108216.
27. De Boer AA, Monk JM, Liddle DM, Power KA, Ma DW, Robinson LE. Fish Oil-Derived Long-Chain n-3 Polyunsaturated Fatty Acids Reduce Expression of M1- Associated Macrophage Markers in an ex vivo Adipose Tissue Culture Model, in Part through Adiponectin. Front Nutr. 2015;2:31.
28. De Boer AA, Monk JM, Liddle DM, Hutchinson AL, Power KA, Ma DW, et al. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J Nutr Biochem. 2016;34:61-72.
29. Liddle DM, Hutchinson AL, Monk JM, Power KA, Robinson LE. Dietary n-3 polyunsaturated fatty acids modulate CD4+ T-cell subset markers, adipocyte antigen presentation potential, and NLRP3 inflammasome activity in a coculture model of obese adipose tissue. Nutrition. 2021:111388.
30. Cranmer-Byng MM, Liddle DM, De Boer AA, Monk JM, Robinson LE. Proinflammatory effects of arachidonic acid in a lipopolysaccharide-induced inflammatory microenvironment in 3T3-L1 adipocytes in vitro. Appl Physiol Nutr Metab. 2015;40(2):142-54.
31. Liddle DM, Monk JM, Hutchinson AL, Ma DWL, Robinson LE. CD8+ T cell/adipocyte inflammatory cross talk and ensuing M1 macrophage polarization are reduced by fish-oil-derived n-3 polyunsaturated fatty acids, in part by a TNF-alpha-dependent mechanism. J Nutr Biochem. 2020;76:108243.
32. Liddle DM, Hutchinson AL, Monk JM, DeBoer AA, Ma DWL, Robinson LE. Dietary long-chain n-3 PUFAs mitigate CD4+ T cell/adipocyte inflammatory interactions in co-culture models of obese adipose tissue. J Nutr Biochem. 2020;86:108488.
33. Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2007;18(4):250-8.
34. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulinsensitizing effects. Cell. 2010;142(5):687-98.
35. Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, et al. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175.
36. Fan R, Kim J, You M, Giraud D, Toney AM, Shin SH, et al. a-Linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation. J Nutr Biochem. 2020;76:108285.
37. Monk JM, Liddle DM, De Boer AA, Brown MJ, Power KA, Ma DW, et al. Fish-oil-derived n-3 PUFAs reduce inflammatory and chemotactic adipokine-mediated crosstalk between co-cultured murine splenic CD8+ T cells and adipocytes. J Nutr. 2015;145(4):829-38.
38. De Boer AA, Monk JM, Robinson LE. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS One. 2014;9(1):e85037.
39. Monk JM, Liddle DM, Brown MJ, Zarepoor L, De Boer AA, Ma DW, et al. Anti-inflammatory and antichemotactic effects of dietary flaxseed oil on CD8(+) T cell/adipocyte-mediated cross-talk. Mol Nutr Food Res. 2016;60(3):621-30.
40. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761-72.
41. Creely SJ, McTernan PG, Kusminski CM, Fisher f, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740-7.
42. Laugerette F, Furet JP, Debard C, Daira P, Loizon E, Géloën A, et al. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am J Physiol Endocrinol Metab. 2012;302(3):E374-86.
43. Kang B, Kim CY, Hwang J, Suh HJ, Choi HS. Brassinin, a phytoalexin in cruciferous vegetables, suppresses obesityinduced inflammatory responses through the Nrf2-HO-1 signaling pathway in an adipocyte-macrophage co-culture system. Phytother Res. 2019;33(5):1426-37.
44. Sakamoto Y, Kanatsu J, Toh M, Naka A, Kondo K, Iida K. The Dietary Isoflavone Daidzein Reduces Expression of Pro-Inflammatory Genes through PPARa/? and JNK Pathways in Adipocyte and Macrophage Co-Cultures. PLoS One. 2016;11(2):e0149676.
45. Mazur-Bialy AI, Pochec E. Riboflavin Reduces Pro- Inflammatory Activation of Adipocyte-Macrophage Coculture. Potential Application of Vitamin B2 Enrichment for Attenuation of Insulin Resistance and Metabolic Syndrome Development. Molecules. 2016;21(12).
46. Kim M, Song K, Kim YS. Alantolactone improves palmitate-induced glucose intolerance and inflammation in both lean and obese states in vitro: Adipocyte and adipocyte-macrophage co-culture system. Int Immunopharmacol. 2017;49:187-94.
47. Kim CY, Kang B, Suh HJ, Choi HS. Red ginsengderived saponin fraction suppresses the obesity-induced inflammatory responses via Nrf2-HO-1 pathway in adipocyte-macrophage co-culture system. Biomed Pharmacother. 2018;108:1507-16.
48. Nitta CF, Orlando RA. Crosstalk between immune cells and adipocytes requires both paracrine factors and cell contact to modify cytokine secretion. PLoS One. 2013;8(10):e77306.
49. Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44(3):479- 86.
50. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836-45.
51. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond). 2008;32(3):451-63.
52. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116-20.
53. Qu Y, Zhang Q, Ma S, Liu S, Chen Z, Mo Z, et al. Interleukin-17A Differentially Induces Inflammatory and Metabolic Gene Expression in the Adipose Tissues of Lean and Obese Mice. Int J Mol Sci. 2016;17(4):522.
54. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494- 505.
55. Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3):797-809.
56. Oster RT, Tishinsky JM, Yuan Z, Robinson LE. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPAR? mRNA, in 3T3-L1 adipocytes. Appl Physiol Nutr Metab. 2010;35(6):783-9.
57. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 Suppl 3:14-23.
58. Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance.Nutr Rev. 2007;65(6 Pt 2):S39-46.
59. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367-77.
60. Patsouris D, Cao JJ, Vial G, Bravard A, Lefai E, Durand A, et al. Insulin resistance is associated with MCP1- mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS One. 2014;9(10):e110653.
61. Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring). 2014;22(3):747-57.
62. Khan IM, Perrard XY, Brunner G, Lui H, Sparks LM, Smith SR, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond). 2015;39(11):1607-18.
63. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43-54.
64. Hutchinson AL, Monk JM, Liddle DM, Robinson LE. Investigating the potential for n-3 and n-6 polyunsaturated fatty acids to modulate myocyte-macrophage inflammatory cross-talk and improve myocyte insulin sensitivity. Applied Physiology, Nutrition and Metabolism. 2016;41:S17-S8.
65. Hutchinson A, Liddle D, Robinson L. n-3 and n-6 polyunsaturated fatty acids attenuate macrophagemyocyte inflammatory crosstalk in a co-culture model of obese skeletal muscle. Applied Physiology, Nutrition and Metabolism. 2018;43:S17.
66. Hutchinson A, Liddle D, Ansari R, Robinson L. Dietary n-3 vs. n-6 PUFA differentially modulate macrophage-myocyte inflammation and cross-talk. Current Developments in Nutrition. 2020;4(2):1644.
67. Samokhvalov V, Bilan PJ, Schertzer JD, Antonescu CN, Klip A. Palmitate- and lipopolysaccharide-activated macrophages evoke contrasting insulin responses in muscle cells. Am J Physiol Endocrinol Metab. 2009;296(1):E37- 46.
68. Kewalramani G, Fink LN, Asadi F, Klip A. Palmitateactivated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase C ? and e. PLoS One. 2011;6(10):e26947.
69. Pillon NJ, Arane K, Bilan PJ, Chiu TT, Klip A. Muscle cells challenged with saturated fatty acids mount an autonomous inflammatory response that activates macrophages. Cell Commun Signal. 2012;10(1):30.