Abstract
Exercise is considered as one of the main therapeutic strategies to improve glycemic regulation in diabetic patients. Current recommendation for diabetes management is a 3 to 5 times a week 150 min of moderate-to-moderate intensive physical activity.However, these could be refined thanks to recent studies. Furthermore, favorable effect generated by exerkines from the better understanding of mechanism involved in the exercise-associated, would allow the identification of future innovative molecules to treat diabetes mellitus.
This article aimed to determine the best recommended exercises for diabetic individuals and to provide information on exerkines role on beneficial effects of exercise obtained on β-cells function and glucose homeostasis in skeletal muscle.
To summarize, it appears that the combination of endurance and resistance training associated with a short term very low caloric diet (VLCD) could be the solution to treat diabetes. Finally, by focusing on our examples, Fractalkine, Osteoprotegerin (OPG) and exosomes seem to be promising therapeutic strategies for diabetic patients.
Keywords
Exercise, Diabetes, β-cell, Skeletal muscle, Exerkines, Myokines, Fractalkine, Osteoprotegerin, Exosomes
Abbreviations
T2D: Type 2 Diabetes; GLUT2: Glucose Transporter 2; GLUT4: Glucose Transporter 4; HbA1c: Hemoglobin A1c; EPS: Electrical Pulse Stimulation; VLCD: Very Low Caloric Diet; GDF15: Growth and Differentiation Factor 15; CX3CL1: Chemokine [C-X3-C motif] Ligand 1; CX3CR1: CX3C Chemokine Receptor 1; TNFα: Tumor Necrosis Factor α; AS160: Akt Substrate of 160 kDa; Tbc1d1: TBC1 domain family member 1; IRS2: Insulin Receptor Substrate 2; Rab-GAP: Rab-GTPase-activating Protein; AMPK: 5’ Adenosine Monophosphate-Activated Protein Kinase; ICAM-1: Inter Cellular Adhesion Molecule-1; Sorcin: Soluble resistance-related calcium binding protein; PCSK1: Proprotein Convertase Subtilisin/Kexin Type 1; OPG: Osteoprotegerin; RANKL: Receptor Activator of Nuclear Factor Kappa-B Ligand; RANK: Receptor Activator of Nuclear Factor kappa-B; CREB: C-AMP Response Element-binding Protein; GSK3: Glycogen Synthase Kinase-3; INFgamma: Interferon gamma; IL-1 beta: Interleukin-1 beta; TNFS- CM: Tumor Necrosis Factor-Soleus-Conditioned Medium; EVs: Extravesicular Vesicules; IL-6: Interleukin-6; HSP60: Heat Shock Protein 60; miRNA: micro RNA; LncRNA: Long non-coding RNA.
Introduction
Nowadays, physical activity is strongly considered as an effective strategy in both preventing and treating type 2 diabetes (T2D) [1,2]. Indeed, it is well established that exercises improve β-cell insulin signaling, insulin secretion, prevent pancreatic β-cell failure and impacts glucose homeostasis regulation in T2D patients [3-8]. Moreover, exercise has clearly shown to improve the peripheral insulin sensitivity in T2D patients and to have a beneficial effect on insulin resistance [9-12]. The improvement of peripheral insulin sensitivity in T2D patients and the beneficial effect on insulin resistance by the exercise have been widely documented. For these reasons, major international diabetes associations (Diabetes UK, Canadian Diabetes Association, American Diabetes Association, and European Association for the Study of Diabetes) recommend a 3 to 5 times a week 150 min of moderate to moderate intensive exercise for T2D patients [13-15]. Nevertheless, in view of the recent studies, it’s still undetermined what is the best formulation to have to get the optimal impact from exercise to improve significantly diabetic patient’s glycemic homeostasis. So, physical activity represents a great interest in the development of future strategies for the treatment and patients medical care for metabolic diseases. Therefore, it appears important to precisely understand mechanisms involved. That’s why, a lot of progress are made in many studies focusing on exercise metabolites.
Skeletal muscle is known to secrete several hundred metabolites called “exerkines” (hormones, myokines, exosomes…) involved in inter-organ communication [15-23]. Several exerkines have demonstrated a positive impact in β-cell function and glucose homeostasis regulation, such as Fractalkine [20], Irisin [24,25], Il-6 [26,27], Il- 10 [28], GDF15 [29], Osteoprotegerine [30], Il-15 [31,32], exosomes [33] etc. (Figure 1). Now, the challenge is to determine the best formulation for the diabetic patients’ treatment.
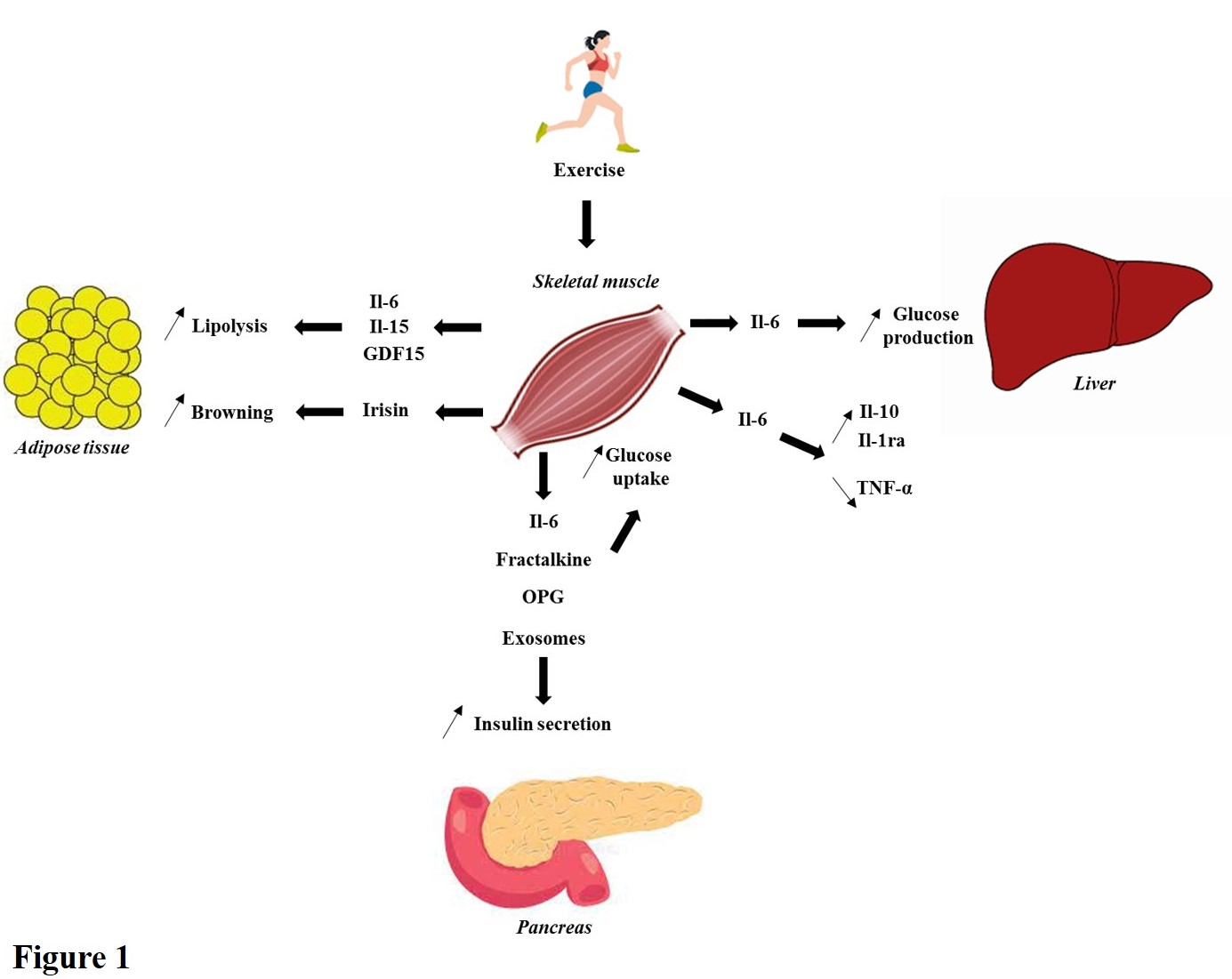
Figure 1. Brief summary of the endocrine properties of skeletal muscle. During physical activity, numerous exerkines are secreted and positively impact insulin secretion and glucose homeostasis regulation. In this figure, a brief illustration of what it known is summarized. For instance, Il-6 stimulates lipolysis in white adipose tissue, increases insulin secretion, enhances glucose uptake in skeletal muscle and trigger glucose production in liver during exercise. Moreover, Il-6 has anti-inflammatory properties stimulating Il-10 and Il-1ra release and decreasing TNF-α production. Then, Il-15 and GDF15 stimulate lipolysis. Furthermore, irisin increases browning of white adipose tissue. Finally, Fractalkine, OPG and exosomes improve insulin secretion and glucose uptake in skeletal muscle.
Abbreviations: Il-6: Interleukin-6; Il-10: Interleukin-10; Il-1ra: Interleukin-1 receptor antagonist; TNF-α: Tumor Necrosis Factor α; Il-15: Interleukin-15; GDF15: Growth and Differentiation Factor 15; OPG: Osteoprotegerin.
Firstly, in this article, we aimed to collect recent data on exercise’s beneficial impact on glucose regulation in order to provide the best recommendations for T2D management. Secondly, we collected recent information on exerkines role on benefit effect of exercise obtained on β-cells function and glucose homeostasis in skeletal muscle by focusing on Fractalkine, Osteoprotegerin and Exosomes, targets of interest of our laboratory for several years.
Positive effect of exercise on glycemic regulation: what is the best recommendation?
Background: During the last decades, studies have reported that exercise impact insulin secretion, glucose homeostasis regulation and reduce β-cell dysfunction in T2D patients [10-12,34,35].
Indeed, Curran et al., observed in rodent model that exercise training improves β-cell insulin signaling and secretion through an increase in cell surface GLUT 2, an increase of intracellular glucokinase expression and an increase in insulin vesicles amount in the β-cell [3]. Furthermore, it is documented that training exercise increases β-cell mass through increased proliferation, protein synthesis and cell survival, coupled with decreased β-cell apoptosis [3,36,37].
It is well established that physical activity improves peripheral insulin sensitivity in T2D patients and has a beneficial effect on insulin resistance [9-12]. For instance, Park et al., demonstrated, in a model of electrical pulse stimulation (EPS)-induced contractile activity upon primary myotubes, that contraction enhances insulin action in myotubes of severely obese individuals. However, authors showed that this effect differs from lean subjects [38]. Then, exercise increases glucose uptake into skeletal muscle cells partly through an increase of GLUT 4 expression [39,40]. Furthermore, Nedachi et al., showed that EPS, on C2C12 myotubes, improved insulin responsiveness as assessed by GLUT4 recycling. Moreover, it was shown that Tbc1d1, a Rab-GAP implicated in exercise-induced GLUT4 translocation in skeletal muscle, is phosphorylated on Ser(231) after EPSinduced contraction [41]. Finally, Al-bayati et al., recently described that EPS mediated AMPK activation enhances the effect of insulin on glucose uptake and AS160Thr642 phosphorylation in control myotubes [42].
Recommendations
The positive impact of exercise on glycemic control (β-cell function and glucose homeostasis in skeletal muscle) could be explained by several parameters such as intensity, duration, sex-gender, type of exercise (endurance versus resistance trainings) and prandial state. Indeed, Jimenez-Maldonado et al., showed that high intensity training has negative effects on the pancreatic islet in comparison to moderate intensity with a reduction in β-cells percentage per pancreatic islet in healthy rats [43]. However, Chavanelle et al. recently demonstrated that high intensity physical activity had more beneficial effect on glycemic regulation in comparison to resting and moderate activity conditions in a model of diabetic mice db/db [9]. Consequently, exercise intensity has to be adapted in function of physio-pathological status. Moreover, Heiskanen et al. found that both sprint interval and moderate-intensity continuous training efficiently reduced pancreatic fat, involved in metabolic disorder, and improve β-cell function in prediabetic and T2D patients. Thus, authors suggested that it should be seriously recommended as a treatment against insulin resistance [44]. Then, Bronczek et al. recently showed that resistance training improves glucose homeostasis by enhancing insulin secretion in healthy mice. Indeed, authors found in C57BL/6 mice that 10 weeks of resistance training improved glucose tolerance, reduced glycaemia, increased fed insulinemia and β-cell function [45]. Similarly, several studies leaded on endurance training effect showed a significant reduction of HbA1c and of inflammation in T2D patients [14,46,47]. All these data have suggested that it is important to adapt exercise intensity in function of the patient health status and that the combination of different type of training (endurance and resistance) could be the solution to have the best effect of physical activity to treat diabetes patients. Finally, Shakoor et al., have recently suggested a combination of exercise and restriction diet to manage positively T2D. More precisely, in their manuscript, authors indicated that an inclusion of very low-calorie diet (VLCD, 400-800 kcal/day) for 8 weeks and ≥ 150 minutes exercise 5 times a week as lifestyle interventions can decrease glucose levels to normal, reduce HbA1c and improve insulin resistance and sensitivity. As stated in their study, very low-calorie diet for longer duration can results in serious complications including death of patient whereas, no death rate is observed when VLCD is taken for 8 weeks or less [14,48].
For years, scientist wondered which mechanisms could be involved in the beneficial impact of exercise on glycaemia regulation of diabetic patients. It is highly suggested that benefit effects of physical activity on β-cell function and glycaemia homeostasis could be driven by exercise metabolites called “exerkines” like myokines and exosomes [22] secreted by skeletal muscle.
Impact of exercise-induced exerkines on pancreatic β-cells function and glucose homeostasis in skeletal muscle
Background: Nowadays, it is widely accepted that a crosstalk between the skeletal muscle and the pancreas, exists through the secretion of diverse hormones, called myokines, expressed and released by myotubes [16-19].
Moreover, it has been shown that human skeletal muscle cells secrete different myokines depending on their insulin sensitivity, modulated by insulin resistance and that have a bimodal impact on β-cell insulin secretion, proliferation and survival [19,20]. Thus, it could contribute as well as to normal β-cell functional mass in healthy subjects, as its decrease in T2D [19]. Action of myokines expressed and secreted during exercise has been shown to improve insulin sensitivity in T2D patients [19,49]. Moreover, it was observed that the incubation of primary human and rat β-cells with conditioned media from healthy human myotubes increased insulin secretion in response to glucose stimulation [18]. As regards their beneficial impact on glucose regulation, studies focus on determining which myokines are involved in order to develop innovative therapeutic strategies for diabetes mellitus. For this article, three examples are mentioned hereafter.
Examples of recommended exerkines
Fractalkine: Fractalkine or CX3CL1 is a CX3C chemokine, expressed in various cell type as neurons, endothelial cells, hepatocytes, vascular smooth muscle cells, skeletal muscle cells and pancreatic β-cells [20,49-53]. Several years ago, it was found that CX3CR1 knockdown (Fractalkine receptor) in mice model induced hyperglycemia and reduced nutrient-stimulated insulin secretion. Moreover, β-cell function was impaired in isolated islets from these KO mice in comparison to wild type islets while fractalkine treatment of wild type islets enhanced β-cell insulin secretion. In addition, the injection of fractalkine in C57BL/6N allowed to increase plasmatic insulin levels and to improve glucose tolerance [54]. In another study, confirming the beneficial effect of fractalkine, Rutti et al. showed the existence of a musclepancreas intercommunication axis in which fractalkine, produced by skeletal muscle cells, plays a role. Indeed, authors published that this latter protects human β-cells from the adverse effects of TNFα on molecular mechanisms involved in insulin granule trafficking by restoring the phosphorylation of Akt, AS160, paxillin and the expression of IRS2, ICAM-1, Sorcin, PCSK1 which are key proteins involved in the insulin secretion pathway [20]. Furthermore, Chaweewannakorn et al., showed that fractalkine, triggered by muscle contractile activity, is required for achieving proper GLUT4 translocation and glucose uptake in skeletal muscle [55]. In addition, Nagashimada et al. recently demonstrated in a model of Cx3cr1−/− mice fed induced obese with high fat diet regimen for 16 weeks, that CX3CL1-CX3CR1 signaling increased insulin resistance and inflammation due to a decrease in the number of anti-inflammatory M2-like macrophages and an increase in the number of proinflammatory M1-like macrophages. However, interestingly, authors observed an improvement of glucose tolerance and insulin resistance in their mice model after fractalkine administration [56]. In summary, all these data suggest that the CX3CL1-CX3CR1 axis could be a target of interest to treat obesity-induced type 2 diabetes and that fractalkine could be an interesting pharmacological candidate to treat diabetic patients.
Osteoprotegerin: Osteoprotegerin (OPG) is a member of the Tumor Necrosis Factor Receptor Superfamily which is importantly expressed in human β-cells [57]. Moreover, OPG is known to increase bone formation inhibiting osteoclast differentiation and activation through its interaction with RANK Ligand (RANKL). As a result, RANK is not activated and cell proliferation is increased [58]. Interestingly, it was shown that OPG is one of only two common genes upregulated in islets from pregnancy, obesity/insulin resistance, and β-cell regeneration models [58]. This suggested that OPG played a key role in glycemic regulation. This was confirmed through two publications. Firstly, Kondegowda et al. found that daily injection of mouse recombinant OPG at 0.01–1.0 mg/g body weight for 7 days, increased β-cell proliferation in young, aged, and diabetic mice C57BL/6. This OPG’s effect on β-cell proliferation has also been observed in vitro on human β-cells by modulating CREB and GSK3 pathways, through binding RANKL and thus, by interfering with RANKL/ RANK interaction. Finally, authors showed that glucose homeostasis significantly improved in mice treated with 1.0 mg/g of mOPG-Fc, after plasma insulin titration and intraperitoneal glucose tolerance test [58]. Secondly, Rutti et al., showed in their study leaded on primary human myotubes from soleus, vastus lateralis and triceps brachii muscles that OPG is triceps specific myokine. Moreover, they demonstrated that this protein decreased β-cells apoptosis. Then, this myokine either prevent the apoptosis induced by pro-infammatory cytomix (TNF-alpha, INFgamma and IL-1beta) and by the negative effect of conditioned medium of TNFα induced insulin resistance in soleus skeletal muscle cells (TNF-S-CM). Finally, using the same experimental conditions, authors found that OPG counteracts both the cytomix and TNF-S-CM negative effects on primary pancreatic beta-cells proliferation and insulin secretion [30].
In conclusion, regarding these studies, it appeared that OPG represents a promising molecule for the treatment of diabetes.
Exosomes: Concepts by which skeletal muscle acts an endocrine organ evolved as secreted extracellular vesicles (EVs) were described to be additional essential mediators of the cell-to-cell communications [59,60]. Exosomes are a class of 30–100 nm diameter EVs secreted by cells. These small vesicles contain proteins, lipids and nucleic acids that can be delivered to target cells, thus modulating their homeostasis [61,62]. They are now well described to be regulators of the cell-to-cell communication and rapidly became useful biomarkers for disease diagnosis and prognosis [63,64]. For years, several studies showed that secreted extracellular vesicles could improve glucose homeostasis and increase insulin sensitivity following their internalization by metabolic tissues [17,65,66]. Additionally, some recent studies highlighted that exosomes content are altered in T2D patients [67,68]. Consequently, exosomes have essential roles on insulin signaling, insulin sensitivity, glucose homeostasis both in β-cells and skeletal muscle cells [33,69].
During physical activity, several studies showed that the amount of EVs is increased. Indeed, Frühbeis, et al. showed that a significant release of exosomes is triggered following a single bout of exhaustive exercise that lasts for the following 90 min at rest and EVs size and composition were different within the early recovery phase [70]. Similarly, Bertoldi et al. observed a lasting effect on exosomes secretion in rats that underwent a daily 20 min moderate treadmill exercise for 2 weeks [71]. Additionally, chronic endurance physical activity (3 weeks swimming) also favors exosomes secretion, as observed by Bei et al. in mice serum [72]. Moreover, it has been showed that significant change in the expression of 322 skeletal muscle expressed proteins were observed in circulating exosomes from healthy humans that underwent 1hr cycling exercise when compared to resting conditions [73]. Taken together, these few studies confirmed that exercise is a strong stimulus that induce exosomes secretion in the bloodstream, where the exercise intensity influences the number of vesicles to be released by the skeletal muscle. Thus, it emphasizes that physical activity, depending on its type and intensity, could exert beneficial effects on T2D through the modulation of exosomes secretion into the circulation [73,74]. Furthermore, studies suggest that the release of EVs could be fiber-type or muscle dependent too [74,75]. Finally, the identification of EVs content secreted by skeletal muscle cells during exercise showed the presence of proteins (IL-6, HSP60), of miRNA (miR-133a, miR206), LncRNA (H19) and circular RNA (ZNF609) which are described to have positive effect on i) pancreatic β-cells: β-cells function, β-cell survival and insulin secretion, ii) in skeletal muscle: cells survival / regeneration, glucose metabolism and insulin sensitivity [33,76].
To conclude, it seems that physical activity-secreted exosomes could be beneficial for T2D patients by impacting the crosstalk between metabolic tissues involved in glucose homeostasis.
Conclusion
In this article, we aimed to determine the best physical activity recommendation for diabetic patients and to bring new insights to understand the beneficial impact of exercise on glucose regulation with a focus on exerkines. Recent collected studies suggested that 3 to 5 times a week 150 min of a combination of endurance and resistance trainings and a maximum of very low-calorie diet of 8 weeks is the way to follow to have the best effect of physical activity. However, each diabetic patient is different and it is clear that it is important to adapt these recommendations in function of the patient health status. Moreover, it is sometimes difficult for diabetic patients to comply with these recommendations, either for health reasons or related to their professional activity, for example. In this context, the development of drugs is important to treat all patients and exerkines are growing evidences to be promising candidates. Indeed, among our exerkines of interest we highlight that Fractalkine, OPG and exosomes could be promising pharmacological candidates to treat diabetic patients as they have shown their protective effects on β-cell survival, on insulin secretion and on insulin resistance. However, pitfalls/common issues with Fractalkine, Osteoprotegerin and exosomes were not discussed in this article. Finally, additional investigations are required to determine the future best pharmacological formulation to treat diabetic patients.
Acknowledgments
Thanks to Christophe LINDLEY for proofreading the manuscript.
References
2. Thyfault JP, Bergouignan A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia. 2020 Aug;63(8):1464-74.
3. Curran M, Drayson MT, Andrews RC, Zoppi C, Barlow JP, Solomon TP, Narendran P. The benefits of physical exercise for the health of the pancreatic ?-cell: a review of the evidence. Experimental Physiology. 2020 Apr;105(4):579-89.
4. Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS- 2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metabolism. 2006 Jul 1;4(1):89-96.
5. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictormTOR complex. Science. 2005 Feb 18;307(5712):1098- 101.
6. Vanhaesebroeck B, Alessi DR. The PI3K–PDK1 connection: more than just a road to PKB. Biochemical Journal. 2000 Mar 15;346(3):561-76.
7. Espelage L, Al-Hasani H, Chadt A. RabGAPs in skeletal muscle function and exercise. Journal of Molecular Endocrinology. 2020 Jan 1;64(1):R1-9.
8. Constable SH, Favier RJ, Cartee GD, Young DA, Holloszy JO. Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. Journal of applied physiology. 1988 Jun 1;64(6):2329-32.
9. Chavanelle V, Boisseau N, Otero YF, Combaret L, Dardevet D, Montaurier C, et al. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Scientific Reports. 2017 Mar 16;7(1):1-0.
10. Johansen MY, Karstoft K, MacDonald CS, Hansen KB, Ellingsgaard H, Hartmann B, Albrechtsen NJ, et al. Effects of an intensive lifestyle intervention on the underlying mechanisms of improved glycaemic control in individuals with type 2 diabetes: a secondary analysis of a randomised clinical trial. Diabetologia. 2020 Nov;63(11):2410-22.
11. Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, Hollingsworth KG, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for ? cell recovery. Cell Metabolism. 2018 Oct 2;28(4):547-56.
12. Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012 Nov 1;61(11):2787-95.
13. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017 Jan 1;40(Supplement 1):S11-24.
14. Shakoor H, Apostolopoulos V, Feehan J, Ali HI, Ismail LC, Al Dhaheri AS, Stojanovska L. Effect of Calorie Restriction and Exercise on Type 2 Diabetes. Prilozi (Makedonska Akademija na Naukite i umetnostite. Oddelenie za Medicinski Nauki). 2021 Apr 23;42(1):109- 26.
15. Lyngbaek MP, Legaard GE, Bennetsen SL, Feineis CS, Rasmussen V, Moegelberg N, Brinkløv CF, et al. The effects of different doses of exercise on pancreatic β-cell function in patients with newly diagnosed type 2 diabetes: study protocol for and rationale behind the “DOSE-EX” multi-arm parallel-group randomised clinical trial. Trials. 2021 Dec;22(1):1-26.
16. Mizgier ML, Cataldo LR, Gutierrez J, Santos JL, Casas M, Llanos P, Contreras-Ferrat AE, et al. Effect of human myotubes-derived media on glucose-stimulated insulin secretion. Journal of diabetes research. 2017 Oct;2017.
17. Jalabert A, Vial G, Guay C, Wiklander OP, Nordin JZ, Aswad H, Forterre A, et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016 May;59(5):1049-58.
18. Pedersen BK. Muscle as a Secretory Organ. Compr. Physiol., vol. 3, Hoboken.
19. Bouzakri K, Plomgaard P, Berney T, Donath MY, Pedersen BK, Halban PA. Bimodal effect on pancreatic β-cells of secretory products from normal or insulinresistant human skeletal muscle. Diabetes. 2011 Apr 1;60(4):1111-21.
20. Rutti S, Arous C, Schvartz D, Timper K, Sanchez JC, Dermitzakis E, Donath MY, et al. Fractalkine (CX3CL1), a new factor protecting β-cells against TNFα. Molecular Metabolism. 2014 Oct 1;3(7):731-41.
21. Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, et al. Cytokine response of primary human myotubes in an in vitro exercise model. American Journal of Physiology-Cell Physiology. 2013 Oct 15;305(8):C877-86.
22. Langlois A, Forterre A, Pinget M, Bouzakri K. Impact of moderate exercise on fatty acid oxidation in pancreatic β -cells and skeletal muscle. Journal of Endocrinological Investigation. 2021 Apr 12:1-1.
23. Pedersen BK. The physiology of optimizing health with a focus on exercise as medicine. Annual review of physiology. 2019 Feb 10;81:607-27.
24. Song R, Zhao X, Cao R, Liang Y, Zhang DQ, Wang R. Irisin improves insulin resistance by inhibiting autophagy through the PI3K/Akt pathway in H9c2 cells. Gene. 2021 Feb 15;769:145209.
25. Oguri Y, Shinoda K, Kim H, Alba DL, Bolus WR, Wang Q, Brown Z, et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell. 2020 Aug 6;182(3):563-77.
26. Timper K, Denson JL, Steculorum SM, Heilinger C, Engström-Ruud L, Wunderlich CM, et al. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Reports. 2017 Apr 11;19(2):267-80.
27. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nature Medicine. 2011 Nov;17(11):1481-9.
28. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological Reviews. 2008 Oct;88(4):1379-406.
29. Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, Vion J, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 2020 Mar 26;5(6).
30. Rutti S, Dusaulcy R, Hansen JS, Howald C, Dermitzakis ET, Pedersen BK, Pinget M, et al. Angiogenin and Osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Scientific Reports. 2018 Jul 3;8(1):1-0.
31. Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, Mortensen OH, et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. The Journal of Clinical Endocrinology & Metabolism. 2008 Nov 1;93(11):4486- 93.
32. Sánchez-Jiménez R, Alvarado-Vásquez N. IL-15 that a regulator of TNF-α in patients with diabetes mellitus type 2. Medical hypotheses. 2013 Jun 1;80(6):776-7.
33. Li G, Liu H, Ma C, Chen Y, Wang J, Yang Y. Exosomes are the novel players involved in the beneficial effects of exercise on type 2 diabetes. Journal of cellular physiology. 2019 Sep;234(9):14896-905.
34. Narkar VA, Downes M, Ruth TY, Embler E, Wang YX, Banayo E, Mihaylova MM, et al. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008 Aug 8;134(3):405-15.
35. Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. The Journal of Clinical Investigation. 2006 Jul 3;116(7):1802-12.
36. Li Y, Xiao J, Tian H, Pei Y, Lu Y, Han X, Liu Y, et al. The DPP-4 inhibitor MK0626 and exercise protect islet function in early pre-diabetic kkay mice. Peptides. 2013 Nov 1;49:91-9.
37. Paula FM, Leite NC, Borck PC, Freitas-Dias R, Cnop M, Chacon-Mikahil MP, Cavaglieri CR, et al. Exercise training protects human and rodent β cells against endoplasmic reticulum stress and apoptosis. The FASEB Journal. 2018 Mar;32(3):1524-36.
38. Park S, Turner KD, Zheng D, Brault JJ, Zou K, Chaves AB, Nielsen TS, et al. Electrical pulse stimulation induces differential responses in insulin action in myotubes from severely obese individuals. The Journal of Physiology. 2019 Jan;597(2):449-66.
39. Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. The Journal of Clinical Investigation. 1987 Aug 1;80(2):415-24.
40. Lakka TA, Lintu N, Väistö J, Viitasalo A, Sallinen T, Haapala EA, Tompuri TT, et al. A 2 year physical activity and dietary intervention attenuates the increase in insulin resistance in a general population of children: the PANIC study. Diabetologia. 2020 Nov;63(11):2270-81.
41. Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2008 Nov;295(5):E1191-204.
42. Al-Bayati A, Brown A, Walker M. Impaired enhancement of insulin action in cultured skeletal muscle cells from insulin resistant type 2 diabetic patients in response to contraction using electrical pulse stimulation. Journal of Diabetes and its Complications. 2019 Dec 1;33(12):107412.
43. Jiménez-Maldonado A, García-Suárez PC, Rentería I, Moncada-Jiménez J, Plaisance EP. Impact of high-intensity interval training and sprint interval training on peripheral markers of glycemic control in metabolic syndrome and type 2 diabetes. Biochimica et Biophysica Acta (BBA)- Molecular Basis of Disease. 2020 Aug 1;1866(8):165820.
44. Heiskanen MA, Motiani KK, Mari A, Saunavaara V, Eskelinen JJ, Virtanen KA, Koivumäki M, et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: a randomised controlled trial. Diabetologia. 2018 Aug;61(8):1817-28.
45. Bronczek GA, Soares GM, de Barros JF, Vettorazzi JF, Kurauti MA, Marconato-Júnior E, Zangerolamo L, et al. Resistance exercise training improves glucose homeostasis by enhancing insulin secretion in C57BL/6 mice. Scientific Reports. 2021 Apr 21;11(1):1-1.
46. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. Jama. 2010 Nov 24;304(20):2253-62.
47. Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabetic Medicine. 2013 Feb;30(2):e41-50.
48. Wadden TA, Stunkard AJ, Brownell KD. Very low calorie diets: their efficacy, safety, and future. Annals of Internal Medicine. 1983 Nov 1;99(5):675-84.
49. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature Reviews Endocrinology. 2012 Aug;8(8):457-65.
50. Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature Neuroscience. 2006 Jul;9(7):917-24.
51. Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction: rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. Journal of Biological Chemistry. 1999 Apr 9;274(15):10053-8.
52. Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. The American Journal of Pathology. 2001 Mar 1;158(3):855-66.
53. Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. nov 2008;28(11):1897 908.
54. Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Keshwani M, et al. The fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell. 11 avr 2013;153(2):413 25.
55. Chaweewannakorn C, Nyasha MR, Chen W, Sekiai S, Tsuchiya M, Hagiwara Y, Bouzakri K, et al. Exerciseevoked intramuscular neutrophil-endothelial interactions support muscle performance and GLUT4 translocation: a mouse gnawing model study. The Journal of Physiology. 2020 Jan;598(1):101-22.
56. Nagashimada M, Sawamoto K, Ni Y, Kitade H, Nagata N, Xu L, Kobori M, et al. CX3CL1-CX3CR1 Signaling Deficiency Exacerbates Obesity-induced Inflammation and Insulin Resistance in Male Mice. Endocrinology. 2021 Jun;162(6):bqab064.
57. Kutlu B, Burdick D, Baxter D, Rasschaert J, Flamez D, Eizirik DL, Welsh N, et al, Hood L. Detailed transcriptome atlas of the pancreatic beta cell. BMC Medical Genomics. 2009 Dec;2(1):1-1.
58. Kondegowda NG, Fenutria R, Pollack IR, Orthofer M, Garcia-Ocaña A, Penninger JM, Vasavada RC. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-κB ligand pathway. Cell Metabolism. 2015 Jul 7;22(1):77-85.
59. Rome S, Forterre A, Mizgier ML, Bouzakri K. Skeletal muscle-released extracellular vesicles: state of the art. Frontiers in Physiology. 2019 Aug 9;10:929.
60. Trovato E, Di Felice V, Barone R. Extracellular vesicles: delivery vehicles of myokines. Frontiers in Physiology. 2019 May 7;10:522.
61. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Progress in Lipid Research. 2017 Apr 1;66:30-41.
62. Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdisciplinary Reviews: RNA. 2017 Jul;8(4):e1413.
63. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology. 2013 Feb 18;200(4):373-83.
64. Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell proliferation. 2016 Feb;49(1):3-13.
65. Aswad H, Forterre A, Wiklander OP, Vial G, Danty- Berger E, Jalabert A, Lamazière A, et al Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014 Oct;57(10):2155-64.
66. Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes, Obesity and Metabolism. 2017 Sep;19:137-46.
67. Freeman DW, Hooten NN, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018 Nov 1;67(11):2377-88.
68. Wang J, Guo R, Yang Y, Jacobs B, Chen S, Iwuchukwu I, Gaines KJ, et al. The novel methods for analysis of exosomes released from endothelial cells and endothelial progenitor cells. Stem Cells International. 2016 Oct;2016.
69. Romancino DP, Paterniti G, Campos Y, De Luca A, Di Felice V, d’Azzo A, Bongiovanni A. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS letters. 2013 May 2;587(9):1379-84.
70. Frühbeis C, Helmig S, Tug S, Simon P, Krämer- Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. Journal of Extracellular Vesicles. 2015 Jan 1;4(1):28239.
71. Bertoldi K, Cechinel LR, Schallenberger B, Corssac GB, Davies S, Guerreiro IC, Belló-Klein A, et al. Circulating extracellular vesicles in the aging process: impact of aerobic exercise. Molecular and cellular biochemistry. 2018 Mar;440(1):115-25.
72. Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia–reperfusion injury. Basic Research in Cardiology. 2017 Jul 1;112(4):38.
73. Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metabolism. 2018 Jan 9;27(1):237-51.
74. Vechetti Jr IJ, Valentino T, Mobley CB, McCarthy JJ. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. The Journal of Physiology. 2021 Feb;599(3):845-61.
75. Yin X, Zhao Y, Zheng YL, Wang JZ, Li W, Lu QJ, Huang QN, et al. Time-course responses of muscle-specific microRNAs following acute uphill or downhill exercise in Sprague-Dawley rats. Frontiers in Physiology. 2019 Oct 2;10:1275.
76. Safdar A, Tarnopolsky MA. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harbor Perspectives in Medicine. 2018 Mar 1;8(3):a029827.
