Keywords
Allergy, Basophil activation test, Diagnosis, Inhibitory immunoglobins, Peanut allergy
Abbreviations
BAT: Basophil Activation Test; BA-BAT: Blocking Antibodies-Basophil Activation Test; OFC: Oral Food Challenge
To the Editor,
The direct and indirect Basophil Activation Test (BAT) are well known for their capacity to predict the presence of an IgEmediated peanut allergy resulting in a reduction of expensive and time-consuming oral food challenge (OFC) tests. Although the BAT is a promising test, false-positive and false-negative outcomes are observed [1-3].
These false-positive outcomes can be a consequence of IgE binding to an epitope in the food protein used in these tests that may be inactivated after consumption due to food processing or digestion. Alternatively, the BAT protocol can be less sensitive to interference of inhibiting factors such as allergen specific IgG4 and IgA produced during tolerance development due to e.g. consumption of trace amounts of allergen. However, the influence of inhibiting factors can be detected when allergens are pre-incubated with serum, allowing IgG/IgA binding to allergens, before addition to basophils, as shown by the fact that a decrease in basophil sensitivity to Ara h2 predicted sustained unresponsiveness after peanut oral immunotherapy [4].
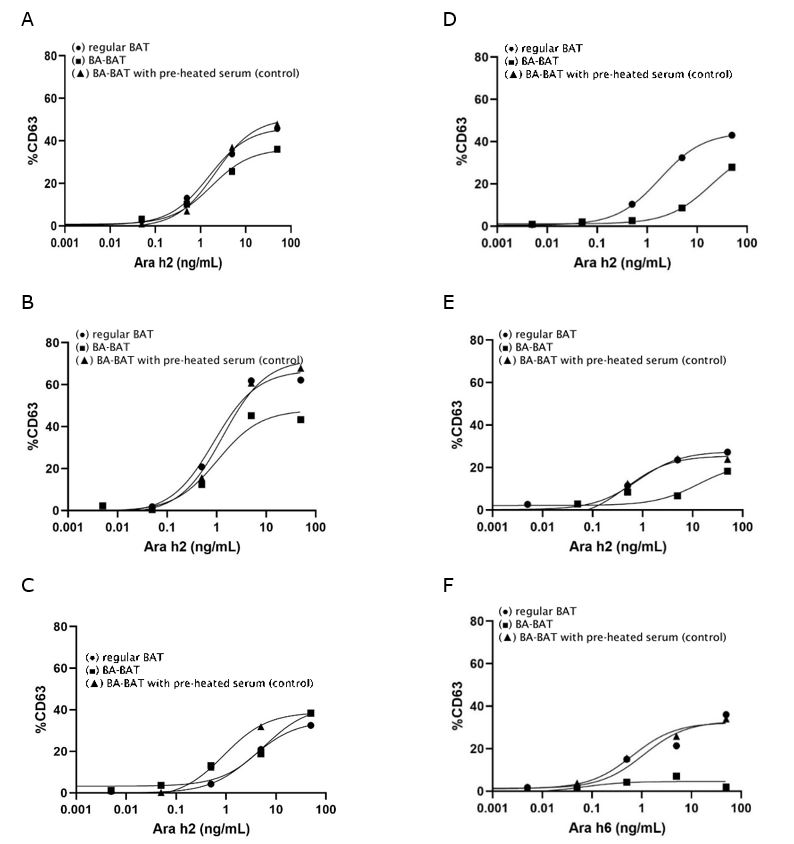
In our recent study on the indirect BAT for the diagnosis of peanut allergy we observed a few false-positive BAT outcomes [3]. We hypothesized that these false-positive BAT outcomes might among others be due to inhibiting factors, like IgG4 and IgA, produced during natural tolerance development which effect on basophil activation can’t be adequately detected in the regular BAT protocol. Therefore, we re-analysed with a modified indirect BAT protocol (called “BA(blocking antibodies)-BAT”) the serum samples (n=3) from our indirect BAT peanut study with a false-positive BAT Ara h2 or Ara h6 outcome.
In this BA-BAT Ara h2 or Ara h6 were pre-incubated with patient serum before addition to basophils (Appendix S1). We compared these results with 1) the regular (indirect) BAT and 2) the BA-BAT with addition of pre-heated serum to Ara h2 or Ara h6, to check whether denaturation of immunoglobulins abolishes the inhibition of serum on basophil activation. We also re-analysed the serum of three patients from the BAT study with a true positive BAT to verify if addition of serum can significantly change a positive outcome of a regular BAT.
Table 1 presents the patient demographic data, sIgE Ara h2/6 values, and the BAT outcomes (see Table S1 for clinical data OFC). Figure 1 shows the results of the dose-response curves of the regular and BA-BATs for Ara h2 or Ara h6. These results show a clear decrease in basophil sensitivity (higher EC50 value) and reactivity (lower AUC value) with the BABAT in patients with a false-positive BAT compared to a true positive BAT (Table 1; AUC and EC50 ratio). In addition, the BA-BAT with pre-incubation of heated serum with Arah2/h6 resulted in similar dose-response curves as the regular BAT. This indicates that inhibition by serum is not caused by nonspecific factors but most probably by immunoglobulins which lose their antigen-binding capacity at high temperature.
| Patient | A | B | C | D | E | F |
| age (year) | 7.5 | 2.8 | 4.9 | 4.3 | 1.3 | 0.7 |
| m/f | m | m | f | f | m | f |
| sIgE (kU/L) | ||||||
| Peanut extract | 1.0 | 2.8 | 1.8 | 4.8 | 4.7 | 2.2 |
| Ara h2 | 1.4 | 2.5 | 1.5 | 3.1 | 1.7 | 0.2 |
| Ara h6 | 0.1 | 0.6 | 3.5 | 2.9 | 3.3 | 1.5 |
| Oral Food Challenge | pos | pos | pos | neg | neg | neg |
| BAT sensitivity; EC50 (ng/mL) | ||||||
| regular BAT | 1.5 | 0.9 | 3.4 | 1.8 | 0.8 | 1.1 |
| BA-BAT | 1.7 | 1.0 | 5.9 | 19.3 | 13.1 | nd† |
| BA-BAT (pre-heated serum) | 2.1 | 1.3 | 0.9 | nd‡ | 0.5 | 0.7 |
| BAT reactivity; AUC | ||||||
| regular BAT | 2066 | 3100 | 1259 | 1953 | 1288 | 1515 |
| BA-BAT | 1607 | 2204 | 1360 | 992 | 698 | 228 |
| BA-BAT (pre-heated serum) | 2186 | 3249 | 1687 | nd | 1210 | 1528 |
| AUCshift | ||||||
| AUC ratio (BA-BAT/regular BAT) | 0.8 | 0.7 | 1.1 | 0.5 | 0.5 | 0.2 |
| AUC ratio (BA-BAT pre-heated serum/regular BAT) | 1.1 | 1.0 | 1.3 | nd | 0.9 | 1.0 |
| EC50 shift | ||||||
| EC50 ratio (BA-BAT/regular BAT) | 1.1 | 1.1 | 1.7 | 10.7 | 16.4 | nd |
| EC50 ratio (BA-BAT preheated serum/regular BAT) | 1.4 | 1.4 | 0.3 | nd | 0.6 | 0.6 |
| † EC50 value could not be reliably determined due to very low basophil activation (<10%). ‡ not detected due to insufficient serum available. |
||||||
In conclusion, these preliminary results show that the specificity of the (indirect) BAT for peanut allergy diagnosis might be improved by pre-incubating serum with allergen before addition to basophils (BA-BAT) after a positive regular BAT in patients with Arah2/6 values in the lower range (i.e. 0.1- 5 kU/L). The BA-BAT can determine the effect of interfering immunoglobulins which is similar to the effects of allergen immunotherapy-induced IgG/IgA on IgE-mediated basophil and T-cell activation as reported previously [4,5]. This means that for new studies on the validation of the BAT for allergy diagnosis this phenomenon should be taken into account when developing BAT protocols. Furthermore, it might be valuable to determine whether the BA-BAT protocol compared to the regular protocol has a higher diagnostic capacity to predict threshold dose and severity of an allergic reaction [6].
Acknowledgements
We thank the technicians, Yvonne Schmidt-Hieltjes, Rene Mollink and Clemens Elshof, for their excellent contribution.
Conflict of Interest
The authors declare no conflict of interest.
Contributions
J Ruinemans-Koerts: conceptualization (lead); writing – original draft (lead); formal analysis (lead). M van Uum-Otters: investigation. D van Baar: investigation; methodology; formal analysis. J van Neerven: conceptualization; writing-original draft. J Wesseling: supervision; resources; writing- review and editing.
Funding
The work was supported by Vriendenfonds Rijnstate (no. 20210712-RVF18-a7).
References
2. Santos AF, Bergmann M, Brough HA, Couto-Francisco N, Kwok M, Panetta V, et al. Basophil activation test reduces oral food challenges to nuts and sesame. The Journal of Allergy and Clinical Immunology: In Practice. 2021 May 1;9(5):2016-27.
3. Ruinemans-Koerts J, Brouwer ML, Schmidt-Hieltjes Y, Stevens P, Merkus PJ, Doggen CM, et al. The Indirect Basophil Activation Test Is a Safe, Reliable, and Accessible Tool to Diagnose a Peanut Allergy in Children. The Journal of Allergy and Clinical Immunology: In Practice. 2022 May 1;10(5):1305-11.
4. Patil SU, Steinbrecher J, Calatroni A, Smith N, Ma A, Ruiter B, et al. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. Journal of Allergy and Clinical Immunology. 2019 Nov 1;144(5):1310-9.
5. Van Neerven RJ, Knol EF, Ejrnaes A, Würtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. International archives of Allergy and Immunology. 2006;141(2):119-29.
6. Santos AF, Du Toit G, O’Rourke C, Becares N, Couto-Francisco N, Radulovic S, et al. Biomarkers of severity and threshold of allergic reactions during oral peanut challenges. Journal of Allergy and Clinical Immunology. 2020 Aug 1;146(2):344-55.