Abstract
Immune checkpoint inhibitor (ICI)-associated cardiotoxicity is a rare immune-related adverse event with high mortality. In recent years, more and more reports were reported. It is urgent to improve understanding and management. Cardiac toxicity often occurs in the early stage after ICI treatment, and its clinical manifestations are diverse and nonspecific, and its pathogenesis is still unclear. Among them, the incidence of immune myocarditis is more than 1%, which can be manifested as fulminant, acute or chronic. Some asymptomatic patients may experience an incubation period to develop acute or fulminant myocarditis, and the mortality of myocarditis can be as high as 50%. Regular monitoring of cardiac biomarkers and ECG is helpful for early diagnosis. Myocardial and endocardial biopsy is the gold standard for diagnosis. Immune myocarditis is sensitive to glucocorticoid. The use of glucocorticoid should be early and sufficient. Asymptomatic myocarditis often has a good outcome if treated in time. The cardiologist’s assistance in diagnosis and treatment is helpful to improve the prognosis.
Keywords
Immune checkpoint inhibitor; Myocarditis; Cardiac biomarker; Glucocorticoid
Introduction
Immune checkpoint inhibitors (ICIs) are a new type of broad-spectrum antitumor drugs, which mainly include cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) inhibitors, programmed cell death protein-1 (PD-1) and its ligand PD-L1 inhibitors. Since 2011, ICIs have been approved for more than 20 kinds of malignant tumors. Since 2018, China has approved the listing of 8 ICIs (PD-1 / PD-L1 inhibitors), including nivolumab, pembrolizumab, toripalimab, sintilimab, camrelizumab, durvalumab, tislelizumab and atezolizumab. The approved tumors in China include non-small cell lung cancer [1,2], melanoma [3,4], Hodgkin’s lymphoma [5,6], liver cancer [7], etc. With the development of clinical studies, the indications of ICIs are increasing.
However, ICIs may overstimulate the immune function of the body, resulting in multiple systemic immunerelated adverse events (irAEs) including rash, colitis, hepatitis, endocrinopathies, and pneumonitis [8]. Immunocardiotoxicity is rare but severe, accounting for a remarkably high mortality rate in clinical practice. With the widespread use of ICIs and the improvement of the cognitive level, the number of reports has gradually increased.
In 2013, Voskens et al. [10] reported the first death case of ipilimumab-induced cardiac toxicity. The drugs reported in the literature so far include: ipilimumab [11,12], nivolumab [13,14], pembrolizumab [15,16], camrelizumab [17], and the combination of PD-1 inhibitor and CTLA- 4 inhibitor [18,19]. A retrospective study analyzed the irAEs through the FDA Adverse Events Reports System (FAERS) public dashboard in 2017–2018. A total of 36,848 toxicities of immunotherapies were reported. Out of these, 2,316 (6.3%) were cardiovascular toxicities, and 816 were ultimately fatal. The most common cardiac complications were as follows: myocarditis (15%), atrial fibrillation (13%), pericardial disease including pericardial effusion (13%), cardiac failure (17%), and coronary artery disease (19%). The mortality rate of myocarditis was as high as 50% [20]. Therefore, it is necessary to improve the cognition and management of ICI-related cardiotoxicity.
This paper reviews the related literature at home and abroad, and summarizes the diagnosis and treatment of ICI-related cardiotoxicity as follows.
Mechanism of Action
The cardiotoxicity caused by traditional anti-tumor drugs, such as chemotherapy and molecular targeted drugs, has attracted great attention, and its mechanism and treatment methods are under constant exploration and research [21,22]. The mechanism of ICIs is completely different from that of traditional chemotherapy and molecular targeted drugs. It mainly recovers the anti-tumor immune response by blocking the immune checkpoints (CTLA-4, PD-1, PD-L1, etc.) and releases cytolytic molecules (such as tumor necrosis factor-α, Granzyme B, interferon-γ), leading to tumor cell death.
The mechanism of immune cardiotoxicity is not clear. The upregulated T cells of CTLA-4-deficient mice can infiltrate the heart tissue [23]. PD-1 and CTLA4-deficient mice may develop fatal myocarditis [24]. PD-1-deficient mice produce a high titer autoantibody against troponin, which causes dilated cardiomyopathy [25,26]. Preclinical studies showed that PD-1 and PD-L1 could be expressed in human cardiomyocytes after injury [27]. Lymphocytes in myocardium and tumors showed clonality of TCR, suggesting that heart and tumors can share antigens recognized by the same T-cell clones [28,29]. The myocardial biopsies of patients with myocarditis showed a large number of lymphocytic infiltration, usually involving the atrioventricular node and sinoatrial node, with or without myocardial fibrosis [18]. In the future, we need to study the specific antigen and molecular mechanism of cardiotoxicity.
Incidence
The incidence of immunomyocarditis is less than 1% [18] reported in the literature, but due to the lack of recognition, cardiac biomarkers are not used as routine detection, and the clinical manifestations are mostly nonspecific, which is easy to be missed or misdiagnosed, so the actual incidence may be underestimated.
Myocarditis often occurs in the early stage of ICI treatment, and the incidence and mortality of myocarditis significantly increase with CTLA-4 and PD-1 inhibitor combination therapy. In a retrospective study, 76% of myocarditis occurred within the six weeks after treatment, with a median of 27 days (5–155 days). The rate of myocarditis caused by PD-1 or PD-L1 inhibitor combined with CTLA-4 inhibitor was significantly higher than that of PD-1 or PD-L1 inhibitor alone (67% vs. 36%; P=0.008) [30].
Of 613 fatal toxic events caused by ICIs reported in VigiBase (WHO database) from 2009 to 2018: 333 deaths were related to PD-1/PD-L1 inhibitors, including 27 deaths due to myocarditis, accounting for 8%; and 87 deaths were related to the combination of PD-1/PD-L1 and CTLA-4 inhibitors, including 22 deaths caused by to myocarditis, accounting for 25% [9]. In a retrospective study up to 2018, of 31,321 irAEs, 122 cases were myocarditis (122/31,321, 0.39%), including 84 cases of PD-1/PD-L1 inhibitors, 6 cases of CTLA-4 inhibitors, and 32 cases of PD-1/PDL1 and CTLA-4 combination therapy; and 95 cases were pericardial diseases, including 74 cases of PD-1/PD-L1 inhibitors, 13 cases of CTLA-4 inhibitors, and 8 cases of combination therapy. The median time of occurrence of myocarditis and pericarditis was 30 days after treatment. The mortality of myocarditis and pericarditis was 50% (61/122) and 21% (20/95), respectively. Pericardial diseases were reported more often in patients with lung cancer, while myocarditis was more commonly reported in patients with melanoma [31].
A retrospective study from eight centers [32] showed that the incidence of myocarditis was 1.14% (11/964), and the incidence of myocarditis was 0.5%, 2.4%, 3.3%, 2.4%, and 1% for PD-1 inhibitor, PD-L1 inhibitor, CTLA-4 inhibitor, PD-1 combined CTLA-4 inhibitor, and PD-L1 combined CTLA-4 inhibitor, respectively. The median time of onset was 34 days (21-75 days) after ICI treatment. The age of onset was 65 ± 13 years old. Most patients had no history of heart disease, and combination therapy (34% vs. 2%; P<0.001) and diabetes (34% vs. 13%; P=0.01) were more common. A retrospective study of 12 3A-grade hospitals in China showed that the incidence of myocarditis was 1.05% (25/2373), of which 81.2% (13/16) occurred in 1-2 times of ICI treatment, the median time was 38 days (2-420) after treatment, the mortality rate was 37.5% (6/16), and 66.7% (4/6) of the elderly over 70 years old [33].
Clinical Characteristics
Clinical symptoms
There are various clinical manifestations of ICI related cardiotoxicity. According to the literature, immunomyocarditis is usually acute or fulminant; that is, obvious cardiac related symptoms appear shortly after medication, cardiac biomarkers rise sharply and continuously, and finally cause cardiogenic shock, conduction abnormality, malignant arrhythmia or sudden death [18,34,35]. However, some patients have no symptom at the beginning, accompanied by the increase of some cardiac biomarkers. It is worth noting that such patients may develop into acute or fuliminant shortly without intervention, thus endangering life. Most of the clinical symptoms of ICI-related cardiotoxicity are non-specific. The common clinical manifestations are palpitation, chest distress, chest pain, etc., which are often combined with immune related adverse reactions of other systems. About 1/4 of the patients show myasthenia gravis or myositis. Some patients show respiratory related symptoms such as shortness of breath, cough, respiratory failure, etc., and some patients may also have rash, fever, thyroid gland, etc. Functional decline, renal dysfunction, liver dysfunction, anorexia, depression, etc, need to be carefully observed and carefully identified clinically.
Laboratory and ECG examination
Mahmood et al. [31] reported that almost all cases of myocarditis had increased troponin (94%) and abnormal ECG (89%). Evaluation of cardiac biomarkers usually includes troponin T or troponin I, creatine kinase (CK), creatine kinase (CK-MB) and myoglobin (MYO). Troponin T is more than 1.5 ng/ml, which is significantly related to severe cardiac events, but the rise of troponin cannot determine the diagnosis of myocarditis [34]. In some patients with subclinical myocarditis, troponin initially increased from baseline, and may still be in the normal range, but it increased significantly once it developed into acute or explosive myocarditis. The increase of CK and CK-MB also needs to consider cardiotoxicity, especially myocarditis [18], which is more evident in combination with myositis [35,36]. Increased BNP and NT proBNP levels or serum antistriated muscle antibodies also support the diagnosis of ICIs cardiotoxicity [37,38]. After treatment, the decrease of cardiac biomarkers indicates the improvement of the condition, while the continuous increase of MYO often indicates a poor prognosis [17].
ECG abnormality is usually nonspecific, which can be manifested as sinus arrhythmia, abnormal QRS axis, QT interval prolongation (>440 ms), atrioventricular or indoor conduction block, atrial fibrillation, T wave change, abnormal Q wave, ventricular arrhythmia, similar myocardial infarction like localization or extensive ST segment elevation.
Imaging and pathological examination
Patients suspected of ICI-related cardiotoxicity should be examined by TTE or CMR. TTE can be used as the first-line examination, most of which show that the whole or local wall motion is weakened, there is no obvious enlargement of cardiac cavity, the ejection fraction is reduced, with or without valve insufficiency, or the heart failure with normal ejection fraction. CMR is the gold standard for noninvasive examination of myocarditis, mainly including T2 weighted imaging, relative enhancement ratio of whole myocardium and gadolinium delayed enhancement sequence. When any two sequences are positive, the diagnostic performance is the best, the sensitivity is 76%, the specificity is 96%, and the diagnostic accuracy is 85%. However, it is worth noting that many patients diagnosed with explosive immune myocarditis have a normal left ventricular function on TTE and CMR [18,32].
When clinically suspected of ICI-related myocarditis, endocardial and myocardial biopsy is the gold standard for diagnosis. Histopathological analysis often finds interstitial fibrosis, lymphocyte infiltration, usually involving atrioventricular node and sinoatrial node, mainly composed of CD8+ T cells and macrophages. B lymphocytes and plasma cells are usually rare [36,39]. However, endocardial and myocardial biopsy is not the first choice because of its risk of invasive, transient, focal, and cardiac perforation. And because myocarditis can be seen as lymphocyte infiltration with a patchy distribution in the adjacent myocardial necrosis area, false negative pathological cases may also appear.
Diagnosis
The diagnosis of ICI-related cardiotoxicity is mainly based on the history of ICIs, clinical manifestations, cardiac biomarkers and ECG. Cardiac toxicity mostly occurs in the early stage of ICIs treatment. It is suggested to conduct cardiac biomarkers and ECG examination at baseline, and conduct continuous monitoring of cardiac biomarkers and ECG within 2 months (every 1-2 weeks) after treatment, and appropriately extend the examination interval after 2 months. Because the clinical manifestations and examination results are mostly non-specific, it is necessary to exclude other causes of cardiomyopathy, such as viral, autoimmune heart disease, infectious myocarditis or myocardial infarction. Imaging and histopathological examination are helpful for the diagnosis.
Mahmood et al. suggested that if the patient has new cardiovascular symptoms, ECG abnormality and troponin elevation can be combined to diagnose ICI related myocarditis [31]. However, there was a patient in our center who had an increase in CK, CK-MB and MYO on the 13th day after the administration of nivolumab. Troponin I was higher than the baseline, but in the normal range. The patient had no symptoms or signs, and no abnormality was found in ECG and echocardiography. Therefore, the second PD-1 inhibitor treatment was postponed without special treatment. On the 19th day, the patients suddenly appeared palpitation, chest distress, fatigue and other symptoms. CK, CK-MB, MYO and troponin I were significantly increased. ECG showed three degrees of atrioventricular block and frequent ventricular premature contraction. [17]. It indicates that the rise of cardiac biomarker may be earlier than the occurrence of clinical symptoms, and the rise of troponin has a lag in a subclinical state. Some patients have a short “incubation period” from the rise of asymptomatic enzyme spectrum to the acute attack of myocarditis. If the intervention is not given in time, the patients are likely to develop acute or fulminant myocarditis and endanger their lives. Therefore, the concept of “asymptomatic myocarditis” is put forward, that is, the patients have no cardiac related clinical symptoms or signs after receiving ICI treatment, ECG and TTE examination have no change compared with the baseline, but CK, CK-MB and MYO have reached more than 2.5 times of the normal value, and troponin has significantly increased compared with the baseline meanwhile, which can be diagnosed. Diagnosed as asymptomatic myocarditis, two patients in our center were treated with glucocorticoids in time, and the cardiac biomarkers were reduced to normal within 2 weeks, which successfully avoided the development of acute or fulminant myocarditis. For the patients with a slight increase of CK, CK-MB, MYO and troponin but without symptoms, we should closely observe the changes of the condition and appropriately shorten the interval of myocardial biomarkers monitoring.
Treatment and Prognosis
The commonly used therapeutic drugs for ICIassociated cardiac toxicity include glucocorticoids and immunosuppressants such as infliximab, antithymocyte globulin (ATG). Glucocorticoids are the first choice of drugs [40,41]. Most of them use high dose glucocorticoids, such as methylprednisolone. The first dose is 1-2 mg/kg, which is then gradually reduced. It has been reported that the first dose of methylprednisolone 1,000 mg is related to the lower MACE [31]. However, several studies have shown that corticosteroids alone may not be enough to solve the immune-mediated side effects. Even after steroid treatment, many patients still have malignant arrhythmias and heart failure symptoms worsening [11,31]. One report [42] showed that two patients began to infuse infliximab after a relapse of steroid refractory myocarditis, showing significant clinical and biochemical improvement, and troponin I decreased to normal. Other studies have shown that infliximab has been used in severe steroid refractory myocarditis [11,37,41,43]. A study [42] showed that two patients received ATG treatment after their condition worsened while on steroids, and they responded well to ATG treatment with resolution of malignant arrhythmias and cardiogenic shock. After three doses of ATG treatment, one patient’s conduction abnormality was improved from complete heart block to sinus rhythm with a 2-degree heart block only occasional dropped beats. Another patient had refractory hypotension and cardiogenic shock. After taking a single dose of ATG, the blood pressure was significantly improved and cardiogenic shock was relieved.
One patient developed immune myocarditis after receiving 3 doses of nivolumab, and the high dose of methylprednisolone (500 mg/day for 3 days) and plasma exchange were ineffective. Five doses of Abatacept (a CTLA-4 agonist) were repeated every two weeks. After treatment, troponin T decreased rapidly, ventricular premature contraction was relieved within three weeks, and the symptoms related to myocarditis and myositis were improved [44]. In refractory myocarditis, glucocorticoids can be combined with immunosuppressants including immunoglobulin, ATG, mycophene ester or infliximab to improve prognosis [31]. Other adjuvant drugs include diuretics, β-blockers, ACEI drugs, antiarrhythmic drugs, etc.
In the case of cardiotoxicity of ICI, it is necessary to carry out hierarchical management and work closely with experts of relevant departments. Cardiologists can help to deal with heart- related symptoms, reduce or repair myocardial damage, and perform cardiac biopsy, pacemaker installation, ablation and other operations when necessary. In the cases of patients with respiratory failure, we should consult with the respiratory department and/or ICU doctor in time, assist in the installation of auxiliary respiratory devices, strengthen anti-infection treatment, etc. Patients with cardiotoxicity, considering the possibility of explosive and fatal myocarditis, generally will not receive ICIs treatment again [45]. However, it has been reported that if ICIs is used again in patients diagnosed with ICIs cardiotoxicity attack, there is no cardiotoxicity again. In patients with mild cardiotoxicity, the complete reversal of left ventricular systolic dysfunction is as high as 50% [32]. Therefore, individual treatment should be given according to the treatment effect and risk assessment of patients.
One patient in our center was observed and waited in a subclinical period. After 6 days, the symptoms showed an acute attack. After glucocorticoid treatment, most of the cardiac markers decreased. Pacemaker installation improved cardiac function, but the patient eventually died of heart and respiratory failure. Another patient showed explosive symptoms on the 10th day of ICI. After glucocorticoid treatment, the biomarker of heart decreased rapidly, but the arrhythmia did not improve significantly, and the patient finally died of tumor progression. It is suggested that myocarditis is sensitive to glucocorticoids, but once the patient has an acute or explosive attack, its clinical symptoms and ECG abnormalities are difficult to reverse, which may be related to irreversible immune damage. Therefore, it is emphasized that the ICI-related myocarditis should be detected, diagnosed and treated early. The subsequent 2 patients in our center were diagnosed as asymptomatic myocarditis and treated with glucocorticoid in time. The cardiac biomarkers rapidly reduced to the baseline level within 2 weeks. There was no cardiac discomfort after 9 months and 3 months follow-up. According to the experience of our center, for asymptomatic myocarditis, the initial dose of methylprednisolone is recommended to be ≤ 4 mg/kg/d lasting for 3~7days, treat until cardiac biomarkers return to baseline, and then taper over 4~6 weeks.
Summary and Prospect
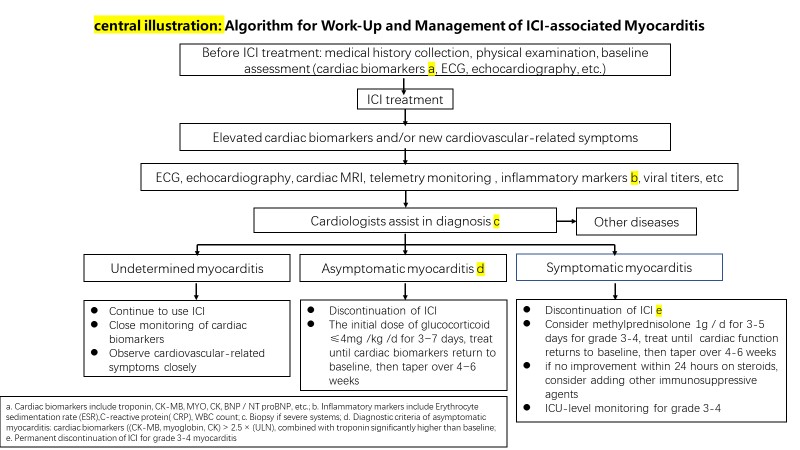
ICI-associated cardiotoxicity is rare. Among such cases, myocarditis accounts for the highest mortality rate, with most deaths occurring in the early stage after treatment. Elderly patients over 70 years old are more likely to die. The clinical manifestations of cardiotoxicity are various. Regular monitoring of cardiac biomarkers is helpful for early diagnosis. The diagnostic criteria of asymptomatic myocarditis need to be confirmed in more cases. Immune myocarditis is sensitive to glucocorticoid. And the use of glucocorticoids should be sufficient in dosage and duration. In case of cardiotoxicity, the cardiologist shall be invited to assist in diagnosis and treatment. Algorithm for work-Up and management of ICI-associated myocarditis is showed in central illustration. In addition, we should deal with other immune-related toxicity in time, actively prevent and treat glucocorticoid-related side effects such as gastric mucosal injury, hypocalcemia, hypertension, hyperglycemia, infection, etc., and actively control basic diseases, treat tumor-related symptoms, so as to improve the prognosis to the greatest extent.
Central illustration: Algorithm for Work-Up and Management of ICI-associated Myocarditis. a. Cardiac biomarkers include troponin, CK-MB, MYO, CK, BNP / NT proBNP, etc.; b. Inflammatory markers include Erythrocyte sedimentation rate (ESR),C-reactive protein( CRP), WBC count; c. Biopsy if severe systems; d. Diagnostic criteria of asymptomatic myocarditis: cardiac biomarkers ((CK-MB, myoglobin, CK) > 2.5 × (ULN), combined with troponin significantly higher than baseline; e. Permanent discontinuation of ICI for grade 3-4 myocarditis.
References
2. Mok TS, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. The Lancet. 2019 May 4;393(10183):1819-30.
3. Si L, Zhang X, Shu Y, Pan H, Wu D, Liu J, et al. A phase Ib study of pembrolizumab as second-line therapy for Chinese patients with advanced or metastatic melanoma (KEYNOTE-151). Translational Oncology. 2019 Jun 1;12(6):828-35.
4. Tang B, Chi Z, Chen YB, Liu X, Wu D, Chen J, et al. Safety, Efficacy and Biomarker Analysis of Toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial. Clinical Cancer Research. 2020 Jan 1.
5. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. The Lancet Haematology. 2019 Jan 1;6(1):e12-9.
6. Song Y, Wu J, Chen X, Lin T, Cao J, Liu Y, et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clinical Cancer Research. 2019 Dec 15;25(24):7363-9.
7. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, openlabel, parallel-group, randomised, phase 2 trial. The Lancet Oncology. 2020 Feb 26.
8. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncology. 2016 Oct 1;2(10):1346-53.
9. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and metaanalysis. JAMA Oncology. 2018 Dec 1;4(12):1721-8.
10. Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PloS one. 2013 Jan 14;8(1):e53745.
11. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir- Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. Journal for Immunotherapy of Cancer. 2016 Dec 1;4(1):50.
12. Berg DD, Vaduganathan M, Davids MS, Alyea EP, Torre M, Padera Jr RF, et al. Immune-related fulminant myocarditis in a patient receiving ipilimumab therapy for relapsed chronic myelomonocytic leukaemia. European Journal of Heart Failure. 2017 May;19(5):682-5.
13. Behling J, Kaes J, Muenzel T, Grabbe S, Loquai C. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Research. 2017 Apr 1;27(2):155-8.
14. Matson DR, Accola MA, Rehrauer WM, Corliss RF. Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. Journal of Forensic Sciences. 2018 May;63(3):954-7.
15. Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. Journal for Immunotherapy of Cancer. 2015 Dec 1;3(1):11.
16. Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti- PD-1 therapy. European Journal of Cancer. 2016 Jun 1;60:210-25.
17. Wang F, Sun X, Qin S, Hua H, Liu X, Yang L, et al. A retrospective study of immune checkpoint inhibitorassociated myocarditis in a single center in China. Chinese Clinical Oncology. 2020 Apr 7.
18. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. New England Journal of Medicine. 2016 Nov 3;375(18):1749-55.
19. Mahmood SS, Chen CL, Shapnik N, Krishnan U, Singh HS, Makker V. Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: A case report. Gynecologic Oncology Reports. 2018 Aug 1;25:74-7.
20. Master SR, Robinson A, Mills GM, Mansour RP. Cardiovascular complications of immune checkpoint inhibitor therapy. ASCO 2019 Abstract 2568.
21. Chang VY, Wang JJ. Pharmacogenetics of chemotherapy-induced cardiotoxicity. Current Oncology Reports. 2018 Jul 1;20(7):52.
22. Jerusalem G, Lancellotti P, Kim SB. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Research and Treatment. 2019 Jun 5:1-4.
23. Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995 Nov 10;270(5238):985-8.
24. Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. International Immunology. 2010 Jun 1;22(6):443-52.
25. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001 Jan 12;291(5502):319-22.
26. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1- deficient mice. Nature Medicine. 2003 Dec;9(12):1477-83.
27. Varricchi G, Galdiero MR, Tocchetti CG. Cardiac toxicity of immune checkpoint inhibitors: cardiooncology meets immunology. Circulation. 2017 Nov 21;136(21):1989-92.
28. Tocchetti CG, Galdiero MR, Varricchi G. Cardiac toxicity in patients treated with immune checkpoint inhibitors: it is now time for Cardio-Immuno-Oncology. Journal of the American College of Cardiology. 2018 Apr 24;71(16):1765-1767.
29. Salem JE, Manouchehri A, Moey M, Lebrun- Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. The Lancet Oncology. 2018 Dec 1;19(12):1579-89.
30. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. The Lancet. 2018 Mar 10;391(10124):933.
31. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. Journal of the American College of Cardiology. 2018 Apr 16;71(16):1755- 64.
32. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor–related cardiotoxicity. Circulation. 2017 Nov 21;136(21):2085-7.
33. Wang F, Qin S, Lou F, Chen FX, Shi M, Liang X, et al. Retrospective analysis of immune checkpoint inhibitorassociated myocarditis from 12 cancer centers in China. ASCO 2020 Abstract e15130.
34. Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. The Oncologist. 2018 Aug;23(8):936.
35. Touat M, Maisonobe T, Knauss S, Salem OB, Hervier B, Auré K, et al. Immune checkpoint inhibitor related myositis and myocarditis in patients with cancer. Neurology. 2018 Sep 4;91(10):e985-94.
36. Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017 Sep 12;89(11):1127-34.
37. Frigeri M, Meyer P, Banfi C, Giraud R, Hachulla AL, Spoerl D, et al. Immune checkpoint inhibitor-associated myocarditis: a new challenge for cardiologists. Canadian Journal of Cardiology. 2018 Jan 1;34(1):92-e1.
38. Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, et al. Smoldering myocarditis following immune checkpoint blockade. Journal for Immunotherapy of Cancer. 2017 Dec 1;5(1):91.
39. Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. Journal for Immunotherapy of Cancer. 2016 Dec;4(1):1-8.
40. Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2017 Jul 1;28(suppl_4):iv119-42.
41. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Current opinion in oncology. 2017 Mar 1;29(2):136-44.
42. Agrawal N, Khunger A, Vachhani P, Colvin TA, Hattoum A, Spangenthal E, et al. Cardiac toxicity associated with immune checkpoint inhibitors: case series and review of the literature. Case Reports in Oncology. 2019;12(1):260-76.
43. Tay RY, Blackley E, McLean C, Moore M, Bergin P, Gill S, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. British Journal of Cancer. 2017 Sep;117(7):921-4.
44. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor–associated myocarditis. New England Journal of Medicine. 2019 Jun 13;380(24):2377-9.
45. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Annals of Oncology. 2016 Apr 1;27(4):559-74.