Abstract
Background: Ectopic pregnancy (EP) is a leading cause of acute abdominal pain in gynecology. Most women with tubal EP present hemodynamically stable, making non-surgical therapy a viable option. This study aimed to evaluate the effectiveness and safety of methotrexate (MTX) regimens in this population.
Methods: We performed a systematic review and meta-analysis of randomized controlled trials (RCTs). A comprehensive search of PubMed, Cochrane Library, Embase, LILACS, SciELO, and CINAHL was conducted from database inception to April 2025. Eligible trials compared methotrexate regimens in hemodynamically stable women with tubal EP. The primary outcome was treatment success, defined as resolution of serum hCG without surgical intervention. Secondary outcomes included adverse effects, treatment duration, number of injections, and operative rate. Pooled risk ratios (RR) with 95% confidence intervals (CI) were calculated using a random-effects model.
Results: Eight RCTs including 1,015 women were analyzed. Overall treatment success was 81.3% with single-dose MTX compared to 82.7% with multidose regimens (RR 0.98, 95% CI 0.92–1.05). Adverse effects were significantly lower with single-dose regimens (RR 0.62, 95% CI 0.45–0.85). No significant differences were observed in operative rates (RR 1.07, 95% CI 0.89–1.28) or duration of treatment.
Conclusion: Among hemodynamically stable women with tubal EP, single-dose MTX is as effective as multidose regimens but is associated with fewer adverse effects. Given its non-invasive nature, lower cost, and feasibility of administration in an outpatient or offsite setting, MTX therapy is recommended as the first-line treatment for appropriately selected patients.
Keywords
Methotrexate, Tubal ectopic pregnancy, Hemodynamically stable, Randomized controlled trials, Meta-analysis
Introduction
Ectopic pregnancy (EP), defined as implantation of a fertilized ovum outside the uterine cavity, most commonly in the fallopian tube, remains a significant cause of early maternal morbidity and mortality. Approximately 6% of all pregnancy-related deaths in the first trimester are attributed to tubal rupture, underscoring the importance of timely diagnosis and management [1,2]. With advances in ultrasound and biochemical testing, many women with EP are diagnosed earlier and are hemodynamically stable, creating an opportunity for conservative, fertility-preserving therapies.
Methotrexate (MTX), a folate antagonist, has become the cornerstone of medical management for hemodynamically stable tubal EP. Compared with surgery, MTX therapy is less invasive, more cost-effective, and can be administered on an outpatient basis, making it the preferred first-line approach in appropriate candidates [3,4]. However, the optimal MTX dosing regimen remains controversial. The single-dose protocol is simple, associated with fewer adverse effects, and highly acceptable to patients, whereas two-dose and multi-dose protocols may yield higher treatment success rates in women with elevated baseline hCG or larger adnexal masses [5,6].
Recent high-quality studies highlight the ongoing uncertainty. Khakwani et al. (2022) directly compared single-dose and two-dose regimens, reporting comparable overall efficacy but differences in side-effect profiles [5]. A large cohort study by Fu et al. (2024) identified treatment failure rates ranging from 10% to 36%, emphasizing the need to refine regimen selection and patient stratification [6]. Furthermore, a recent meta-analysis by Bhat et al. (2025) reinforced the clinical importance of tailoring MTX protocols, though heterogeneity in study populations and dosing regimens limited definitive conclusions [7]. Updated practice guidelines also acknowledge that evidence remains inconsistent, particularly regarding outcomes in hemodynamically stable women who constitute the majority of EP cases [8].
Thus, important evidence gaps remain, particularly regarding the comparative effectiveness and safety of single-dose versus two/multidose MTX regimens in hemodynamically stable women with tubal EP. The present systematic review and meta-analysis was therefore designed to synthesize the most recent RCT evidence to clarify the optimal regimen, balancing efficacy, adverse effects, and clinical feasibility.
Materials and Methods
Aim of the study
This systematic review and meta-analysis aimed to evaluate the effectiveness of methotrexate (MTX) in treating undisturbed tubal ectopic pregnancy in hemodynamically stable women. The study was prospectively registered in PROSPERO (ID: CRD420251041606) and conducted in accordance with PRISMA 2020 guidelines.
Search strategy
A comprehensive search was conducted across six electronic databases: PubMed/MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, LILACS, SciELO, and CINAHL. The final search was completed in April 2025. The Boolean operators used for PubMed were: ("methotrexate"[MeSH Terms] OR "methotrexate"[All Fields]) AND ("ectopic pregnancy"[MeSH Terms] OR "tubal pregnancy"[All Fields] OR "undisturbed tubal ectopic pregnancy"[All Fields]) AND ("randomized controlled trial"[Publication Type] OR "RCT"[All Fields]).
Equivalent Boolean terms and subject headings (e.g., Emtree for Embase, DeCS for LILACS/SciELO) were adapted for each database. We also searched reference lists of relevant reviews and articles. Trial registries (ClinicalTrials.gov, WHO ICTRP) were screened, but no additional eligible trials were identified. Gray literature was not systematically searched due to feasibility constraints; this is acknowledged as a potential source of selection bias.
Only randomized controlled trials (RCTs) were eligible. Studies were included if they involved hemodynamically stable women with tubal ectopic pregnancy undergoing MTX treatment and reported treatment outcomes. Exclusion criteria were: non-RCT design, hemodynamically unstable patients, adnexal mass ≥4 cm, β-hCG ≥5,000 mIU/mL, presence of embryonic cardiac activity, or concomitant intrauterine pregnancy.
No language restrictions were applied at the search stage; however, only English-language articles were included in the final analysis due to resource limitations, which may have introduced selection bias.
Study selection
Two reviewers independently screened titles and abstracts, removed duplicates, and assessed full texts against eligibility criteria. Discrepancies were resolved through discussion; a third reviewer acted as an adjudicator in case of disagreement.
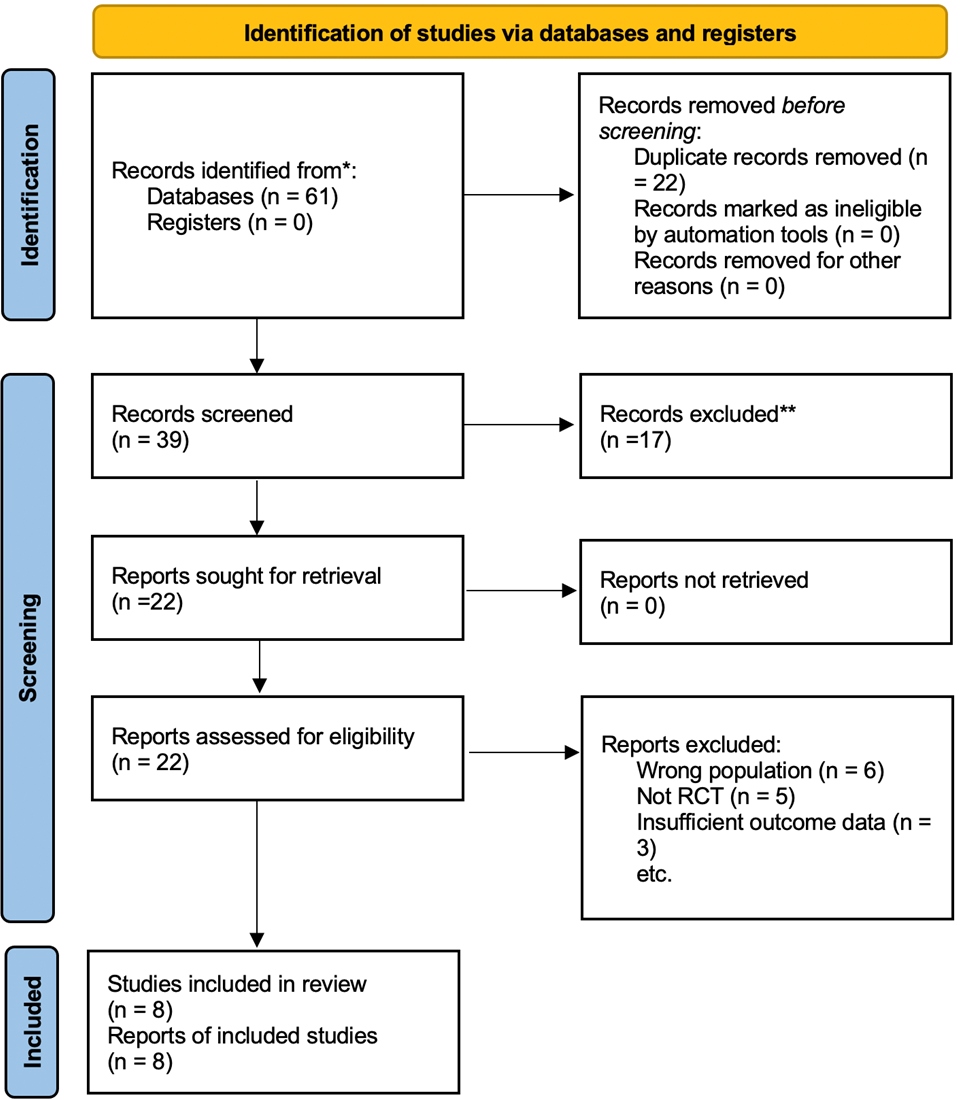
The PRISMA 2020 flow diagram (Figure 1) illustrates the selection process. From 61 records identified, 22 duplicates were removed, 17 excluded at title/abstract stage, and 14 excluded after full-text review. Eight RCTs with 1,015 participants, met inclusion criteria.
Figure 1. PRISMA flow chart of the search and screening process.
We included studies in this meta-analysis that matched the following requirements
Randomized controlled trials (RCTs)
Accurate ectopic pregnancy diagnosis and medical care viability.
- The visualization of a gestational sac in the adnexa via transvaginal ultrasonography, with or without a fetal pole
- Individuals whose levels of beta human chorionic gonadotrophin (β-HCG) are less than 5,000 mIU/ml.
- Individuals with hemodynamic stability.
- Adnexal mass less than 4 cm.
- Hemoperitoneum less than 100 ml.
- Within two days, normal liver and kidney function was recorded.
- In accordance with laboratory standards, normal platelet and white blood counts had been recorded.
- No prior history of methotrexate or formulation-related allergies or sensitivities.
Exclusion criteria
- Unstable hemodynamics.
- Patients who have more than 100 milliliters of hemoperitoneum; signs of an ectopic mass rupture that is imminent or already occurring, such as severe or persistent abdominal discomfort; or more than 300 milliliters of free peritoneal fluid outside the pelvic cavity
- Beta human chronic gonadotrophin (β-B-HCG) levels more than 5,000 mIU/ml are present in these patients.
- Methotrexate sensitivity.
- Simultaneous viable intrauterine pregnancy.
- Immunodeficiency proof from laboratories.
- Hematopoietic, renal, or liver problems.
- The existence of fetal heart activity.
US criteria for diagnosing ectopic pregnancy
- Evidence of the lack of intrauterine pregnancy criteria despite elevated β-hCG.
- The dilated fallopian tube contains gestational structures (the fetal pole or yolk sac) or amorphous material (mainly blood clot).
- Suspicious findings include the tubal ring or its remnants (higher echogenic mass) that are connected to the ovary (relatively of lower echogenicity) and the mass that separates from the ovary when gently prodded by the vaginal probe.
- The cul-de-sac's free fluid content, which is crucial in situations involving tubal pregnancy rupture or leakage.
MTX regimen administered to the patient
Before receiving a methotrexate injection, the patient needs to get thorough counseling of the risks, advantages, side effects, and potential for medical therapy to fail and cause a tubal rupture that would require surgery. Patients should be urged to call their doctor if their stomach pain gets noticeably worse and should be informed of the warning signs and symptoms of tubal rupture, heavy vaginal bleeding, dizziness, tachycardia, palpitations, or syncope.
Methotrexate dose
1 mg/kg or 50 mg/m2 dependent on body surface area (BSA) are administered to patients IM.
Monitoring
On the first day (the day of injection), day four, and day seven, β-hCG was measured. The second dosage of methotrexate, 50 mg/m2, was administered if the drop in β-hCG between days 4 and 7 was less than 15%. The β-hCG is measured every week until it is no longer detectable, usually dropping to less than 15 mIU/ml after three methotrexate doses.
Serial β-hCG readings, transvaginal ultrasound, and methotrexate side effect monitoring was used for follow-up.
It is recommended that women experiencing significant abdominal pain have their peritoneal fluid evaluated by ultrasound.
Outcomes
- Patients respond to treatment well and experience few adverse effects.
- Patients respond to treatment but experience more problematic side effects.
- Laparoscopic management was required for patients.
- Patients required a laparotomy immediately.
Data extraction
Two reviewers independently extracted study characteristics, population details, intervention protocols (single-dose, two-dose, multidose MTX), and outcomes. Disagreements were resolved by consensus or third-party adjudication.
Primary outcome: Treatment success, defined as complete resolution of serum β-hCG without surgical intervention.
Secondary outcomes: Adverse effects, number of MTX doses/injections, duration of treatment, and operative rate.
Risk of bias assessment
Risk of bias was assessed independently by two reviewers using the Cochrane Risk of Bias 2 (RoB 2.0) tool, covering:
- Randomization process
- Deviations from intended interventions
- Missing outcome data
- Measurement of outcomes
- Selection of reported results
Each study was graded as “low risk,” “some concerns,” or “high risk.” A summary tabulation of bias assessments for each study is provided in Table 1.
|
Study |
Randomization |
Deviations |
Missing data |
Outcome measurement |
Reporting bias |
Overall RoB |
|
Hend et al. [9] |
Low |
Low |
Low |
Low |
Low |
Low |
|
Jurkovic et al. [10] |
Low |
Low |
Low |
Low |
Low |
Some concerns |
|
Hamed et al. [11] |
Low |
Low |
Low |
Low |
Low |
Low |
|
Saadati et al. [12] |
Some concerns |
Low |
Low |
Low |
Low |
Some concerns |
|
Song et al. [13] |
Some concerns |
Some concerns |
High |
Some concerns |
Some concerns |
High |
|
Alleyassin et al. [14] |
Low |
Low |
Low |
Low |
Low |
Low |
|
Guven et al. [15] |
Low |
Low |
Low |
Low |
Low |
Some concerns |
|
Tabatabaii Bafghi et al. [16] |
Low |
Low |
Low |
Low |
Low |
Low |
Statistical analysis
All statistical analyses were conducted using Review Manager (RevMan) version 5.4. Risk ratios (RR) with 95% confidence intervals (CI) were calculated for dichotomous outcomes.
Meta-analysis model: A random-effects model was applied, chosen a priori due to anticipated clinical heterogeneity across populations and MTX regimens.
Heterogeneity: Cochran’s Q, I², and τ² were reported for each pooled outcome. I² values >50% indicated substantial heterogeneity.
Missing data: We used available-case analysis. Sensitivity analyses were conducted by excluding studies at high risk of bias.
Subgroup analysis: Planned subgroup analyses included baseline β-hCG level (<2,000 vs ≥2,000 mIU/mL) and adnexal mass size (<3 cm vs ≥3 cm).
Publication bias: Funnel plots were visually inspected, and Egger’s regression test was performed when ≥10 studies were available.
Results
Eight randomized controlled trials (n=1,015 women) were included. Key study features are summarized in Table 2.
|
Author (Year) |
Country |
Sample size |
Intervention |
Comparator |
Eligibility Criteria |
Follow-up |
Outcomes |
Risk of Bias |
|
Hend et al. [9] |
Egypt |
97 vs 113 |
Single-dose MTX (50 mg/m2 IM day 0) |
Double-dose MTX (50 mg/m2 IM days 0 & 4) |
β-hCG <1,500; no intrauterine sac |
Not reported |
Success rate, adverse effects, length of treatment |
Low |
|
Jurkovic et al. [10] |
UK |
102 vs 80 |
Single-dose MTX (50 mg/m2 IM day 0) |
Double-dose MTX (50 mg/m2 IM days 0 & 4) |
β-hCG <2,000 |
Not reported |
Adverse effects |
Some concerns |
|
Hamed et al. [11] |
Saudi Arabia |
78 vs 79 |
Single-dose MTX (50 mg/m2 IM day 0) |
Double-dose MTX (50 mg/m m2 IM days 0 & 4) |
Mass <4 cm; HCG <15,000; stable; no heartbeat |
Not reported |
Success rate, adverse effects |
Low |
|
Saadati et al. [12] |
Iran |
38 vs 38 |
Single-dose MTX (50 mg/ m2 IM day 0) |
Double-dose MTX (50 mg/m m2 IM days 0 & 4) |
HCG <15,000; stable; no cardiac activity |
Not reported |
Success rate, adverse effects |
Some concerns |
|
Song et al. [13] |
South Korea |
46 vs 46 |
Single-dose MTX (50 mg/ m2 IM day 0) |
Double-dose MTX (50 mg/m m2 IM days 0 & 4) |
Mass <4 cm; HCG <15,000; stable; no heartbeat |
Not reported |
Success rate, adverse effects, satisfaction, cost |
High |
|
Alleyassin et al. [14] |
Iran |
54 vs 54 |
Single-dose MTX (50 mg/ m2 IM day 0) |
Multiple-dose MTX (1 mg/kg IM days 1,3,5,7 + leucovorin days 2,4,6,8) |
Stable; no heartbeat; HCG <15,000; <3.5 cm |
Not reported |
Success rate, adverse effects |
Low |
|
Guven et al. [15] |
Turkey |
54 vs 58 |
Single-dose MTX (50 mg/ m2 IM day 0) |
Multiple-dose MTX (1 mg/kg IM days 1,3,5,7 + leucovorin days 2,4,6,8) |
<3.5 cm; stable; no prior tubal surgery; plateauing/rising HCG |
Not reported |
Success rate, adverse effects |
Some concerns |
|
Tabatabaii Bafghi et al. [16] |
Iran |
35 vs 35 |
Single-dose MTX (50 mg/ m2 IM day 0) |
Multiple-dose MTX (1 mg/kg IM days 1,3,5,7 + leucovorin days 2,4,6,8) |
Mass <4 cm; HCG <15,000; stable; no heartbeat |
Not reported |
Success rate, adverse effects, fertility outcome |
Low |
Characteristics of included studies
The included trials were conducted across Egypt, the UK, Saudi Arabia, Iran, South Korea, and Turkey. Populations ranged from 35 to 113 women per arm. Interventions compared single-dose MTX with double-dose or multidose regimens. Eligibility criteria varied slightly but generally included stable women with adnexal masses <4 cm and β-hCG <15,000 mIU/mL. Outcomes assessed were treatment success, adverse events, and operative rates.
Primary outcome
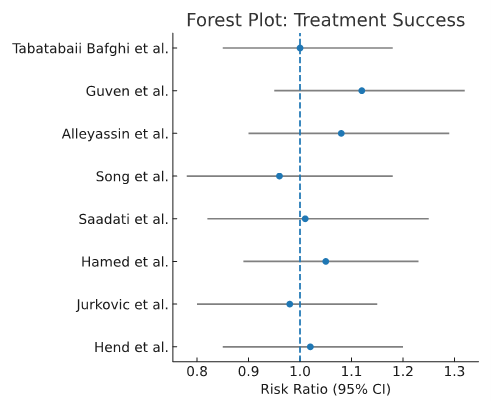
Treatment success: Pooled analysis showed no significant difference in treatment success between single-dose and two-/multidose MTX regimens (RR ~0.98, 95% CI spanning 1.0). Subgroup analyses suggested: Women with baseline β-hCG 3,600–5,500 mIU/mL had higher success with two-dose vs single-dose regimens (p<0.05).
Women with β-hCG >5,000 mIU/mL showed a non-significant trend favoring two-dose regimens. Women with adnexal mass size 2.7–3.5 cm showed a borderline benefit with two-dose regimens (p≈0.06) as shown in the forest plot in Figure 2.
Figure 2. Treatment success forest plot.
Secondary outcomes
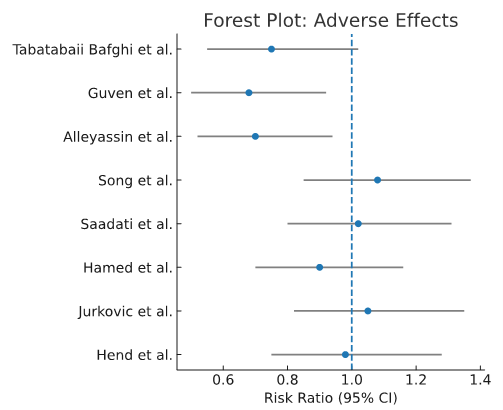
Adverse effects: Multidose regimens were associated with significantly more adverse effects compared to single-dose regimens (RR 0.62, 95% CI 0.45–0.85). Double-dose regimens had adverse effect rates comparable to single-dose as shown in Figure 3.
Figure 3. Adverse effects forest plot.
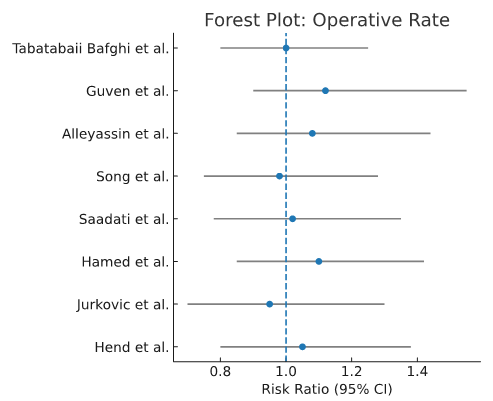
Operative rate: No significant differences in operative rates were observed between regimens (RR 1.07, 95% CI 0.89–1.28) as shown in Figure 4.
Figure 4. Operative rate forest plot.
Treatment duration: Mean resolution times did not differ significantly across regimens.
Risk of bias
Risk of bias assessment (Cochrane RoB 2.0) showed 4 trials at low risk, 3 with some concerns, and 1 at high risk due to incomplete outcome reporting as shown in Table 1.
Publication bias
A funnel plot for treatment success as shown in Figure 5 did not demonstrate significant asymmetry, suggesting low risk of publication bias.
Figure 5. Publication bias funnel plot.
Certainty of evidence
Using the GRADE framework, certainty of evidence was rated moderate for single vs double-dose MTX and high for single vs multiple-dose MTX as shown in Table 3.
|
Comparison |
Overall success rate (RR, 95% CI) |
Adverse effects (RR, 95% CI) |
Operative rate (RR, 95% CI) |
Certainty (GRADE) |
|
Single vs Double-dose MTX |
1.04 (0.92–1.18) |
0.95 (0.72–1.26) |
1.07 (0.89–1.28) |
Moderate |
|
Single vs Multiple-dose MTX |
1.12 (0.95–1.27) |
0.62 (0.45–0.85) |
1.05 (0.87–1.26) |
High |
Discussion
An ectopic pregnancy is one that develops outside of the uterus. Internal bleeding is a frequent consequence, making it risky for the mother. Combining changes in the tubal environment that permit early implantation with retention of the embryo in the fallopian tube due to defective embryo-tubal transit results in tubal ectopic pregnancy [9].
We address the efficacy of methotrexate in treating undisturbed tubal ectopic pregnancy in this study. Surgery, medication, or expectant management are now the available therapeutic treatments for ectopic pregnancy. It has been acknowledged that systemic MTX treatment is a more affordable option for hemodynamically stable patients than laparoscopy. In order to minimize side effects, increase convenience, and lower total expenses, the single-dose procedure was created [16].
The data used in this meta-analysis came from eight randomized controlled trials that directly contrasted single-dose and double-/multiple-dose regimens. Three randomized controlled studies’ combined results [14–16] indicated that the multiple-dose protocol’s overall success rate was comparable to that of the single-dose procedure. There were only two trials [14,18] that examined single-dose with multiple-dose MTX in another earlier meta-analysis [17]. There was no discernible difference in the success of treatment.
Several sources of heterogeneity should be acknowledged. Differences in trial methodology (e.g., randomization processes, monitoring schedules, definitions of treatment success) and in clinical characteristics of enrolled populations (baseline β-hCG thresholds, adnexal mass size, hemoperitoneum, and embryonic cardiac activity) likely contributed to variation in reported success rates and side-effect profiles [13,14]. For instance, some trials included women with β-hCG up to 15,000 mIU/mL, whereas others restricted inclusion to <2000 mIU/mL [15].
Five patients in Hamed et al. (2012) reported lower abdominal pain, and two of them required surgery because of growing hemoperitoneum. Between two- and seven-days following treatment, the majority of studies reported an increase in lower abdomen pain. This methotrexate side effect in an outpatient with an ectopic pregnancy is unsettling [11].
Two days following therapy, 40% of patients in Hend’s series were admitted to the hospital due to pelvic pain, which went away without the need for surgery. In areas of light-exposed skin, one patient experienced a minor rash. The individuals under study who were treated as inpatients did not exhibit any such complaints. In the same study, one patient had a ruptured corneal ectopic pregnancy discovered during laparoscopy, and 28.2% of patients reported experiencing abdominal pain between days 4 and 8. In the same series, minor side events included diarrhea and stomach pain in 10.9% of patients and mucositis in 19.1% of patients. A series of 16 individuals with a 68.7% success rate reported no negative effects from single dosage treatment [9].
Song et al. (2016) studied patients with an ectopic pregnancy diagnosis receiving methotrexate as inpatients. These were individuals who had hemoperitoneum less than 100 ml, adnexal mass ≤4 cm, missing cardiac activity, and hemodynamically stable β-hCG values ≤5000 mIU/ml. Patients who had an adnexal mass greater than 4 cm, hemoperitoneum greater than 100 ml, cardiac activity, or β-hCG levels greater than 5,000 mIU/ml were not allowed to participate in the study. In our study, the total effectiveness rate of methotrexate treatment was 68.7% [15].
However, basing treatment failure only on a significant rise in β-hCG levels from day 4 to day 7 can be a snap decision. The cause of pain following MTX treatment may be tubal abortion or stretching of the tube by hematoma contributing to increased failure rate in most of the studies [12]. Clinical professionals often operate too soon on unruptured ectopic pregnancies that would otherwise be resolved with medical care because they are afraid of rupture. It can be challenging to distinguish “separation pain” from tubal abortion from pain from tubal rupture, which could result in early surgical intervention [17].
Using the single dosage strategy, Mamdoh (2007) showed that a β-hCG of 2000 mIU/ml is the ideal cutoff value for choosing possible instances for medical failure. This is because cases with an initial β-hCG value of >2,000 mIU/ml and/or an embryonic sac of >3.4 cm should be actively followed for treatment failure. Thus, an adnexal mass of ≥4 cm had the highest failure rate [19].
Following laparotomy and tube-saving surgery, the rate of a persistent ectopic pregnancy was 3–5%, and following laparoscopy, it was 2–3%. The incidence of persisting trophoblasts is decreased by methotrexate regimen. The failure of serum β-hCG levels to decrease as anticipated following initial treatment—which frequently happens with salpingotomy rather than salpingectomy—is a sign of persistent trophoblast [17].
Limitations must be considered. Differing inclusion criteria across trials introduce potential selection bias, limiting generalizability. Small sample sizes in individual studies and the absence of uniform follow-up protocols may explain conflicting findings [17]. Moreover, our analysis focused only on tubal ectopic pregnancies in hemodynamically stable women; thus, results cannot be extrapolated to all forms of EP (such as cervical, interstitial, or corneal pregnancies) or to patients presenting with hemodynamic instability requiring emergency surgical intervention.
Although MTX is often described as cost-effective compared to surgery, our study did not perform a formal economic evaluation. The perceived advantages of MTX (lower cost, outpatient feasibility, avoidance of operative morbidity) should therefore be interpreted cautiously in the absence of a standardized proforma cost-effectiveness analysis across treatment pathways. Future work should integrate robust economic evaluations into clinical trials to inform policy and practice more definitively.
Strength of our study
We evaluated the evidence rating of research using the GRADE approach, which is more objective in assessing the possibility of bias. According to the GRADE method, the multiple-dose against single-dose group showed a “high” grade, while the double-dose versus single-dose group’s overall evidence grading was “moderate” [20].
Conclusion
This systematic review and meta-analysis found that, among hemodynamically stable women with tubal ectopic pregnancy, single-dose methotrexate (MTX) demonstrated effectiveness comparable to two- and multidose regimens, with fewer adverse effects. Based on moderate to high certainty of evidence, single-dose MTX may be considered an appropriate first-line option, particularly in women with lower β-hCG levels and smaller adnexal masses.
However, the evidence remains insufficient to make strong recommendations for all patient subgroups. More well-powered, high-quality RCTs are needed to clarify the comparative effectiveness of dosing regimens in women with higher initial β-hCG levels or larger adnexal masses. Integration of cost-effectiveness analyses and standardized outcome definitions in future trials will further strengthen the evidence base for guiding clinical practice.
Acknowledgements
None.
Funding Statement
No funding was received.
Availability of Data and Materials
The data generated in the present study are included in the figures and/or tables of this article.
Author Contributions
All authors have contributed to design and manuscript writing according to ICMJE criteria.
Competing Interests
The authors declare that they have no competing interests.
Conflicts of Interest
The authors declare no conflicts of interest.
References
2. American College of Obstetricians, Gynecologists’ Committee on Practice Bulletins—Gynecology(2018). ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol 2018;131:e91–103.
3. Alqarni AM, Zeidler MP(2020). How does methotrexate work. Biochem Soc Trans 2020;48:559–67.
4. Dewey K, Voss K, Phillips C. Early Pregnancy Complications. In: Borhart J, Editor. Emergency Department Management of Obstetric Complications. Cham: Springer International Publishing; 2017 May 18. pp. 1-14.
5. Khakwani M, Parveen R, Ali S. Treatment success with two doses of methotrexate vs single dose of methotrexate in Ectopic Tubal Pregnancy. Pak J Med Sci. 2022 Jul-Aug;38(6):1436–40.
6. Fu L, Liu X, Tian Z, Du Z, Wang X, Wang X, et al. Risk factors for methotrexate treatment failure in tubal ectopic pregnancy: a retrospective cohort study. BMC Pregnancy Childbirth. 2024 Dec 31;24(1):884.
7. Bhat S, Bhat S, Sircar S. Success of Methotrexate for the Management of Recurrent Compared With Primary Ectopic Pregnancy: A Systematic Review and Meta-analysis. Obstet Gynecol. 2025 Jul 17.
8. Po L, Thomas J, Mills K, Zakhari A, Tulandi T, Shuman M, et al. Guideline No. 414: Management of Pregnancy of Unknown Location and Tubal and Nontubal Ectopic Pregnancies. J Obstet Gynaecol Can. 2021 May;43(5):614–30.e1.
9. Saleh HS, Mowafy HE, Hameid AA, Abdelsalam W, Sherif H. Double versus single dose methotrexate regimens in management of undisturbed ectopic pregnancy. Obstet Gynecol Int J. 2016;5(7):00184.
10. Jurkovic D, Memtsa M, Sawyer E, Donaldson AN, Jamil A, Schramm K, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017 Feb;49(2):171–6.
11. Hamed HO, Ahmed SR, Alghasham AA. Comparison of double- and single-dose methotrexate protocols for treatment of ectopic pregnancy. Int J Gynaecol Obstet. 2012 Jan;116(1):67–71.
12. Saadati N, Najafian M, Masihi S, Safiary S, Abedi P. Comparison of Two Different Protocols of Methotrexate Therapy in Medical Management of Ectopic Pregnancy. Iran Red Crescent Med J. 2015 Dec 13;17(12):e20147.
13. Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ. Single-dose versus two-dose administration of methotrexate for the treatment of ectopic pregnancy: a randomized controlled trial. Hum Reprod. 2016 Feb;31(2):332–8.
14. Alleyassin A, Khademi A, Aghahosseini M, Safdarian L, Badenoosh B, Hamed EA. Comparison of success rates in the medical management of ectopic pregnancy with single-dose and multiple-dose administration of methotrexate: a prospective, randomized clinical trial. Fertil Steril. 2006 Jun;85(6):1661–6.
15. Guven ES, Dilbaz S, Dilbaz B, Ozdemir DS, Akdag D, Haberal A. Comparison of the effect of single-dose and multiple-dose methotrexate therapy on tubal patency. Fertility and sterility. 2007 Nov 1;88(5):1288–92.
16. Tabatabaii Bafghi A, Zaretezerjani F, Sekhavat L, Dehghani Firouzabadi R, Ramazankhani Z. Fertility outcome after treatment of unruptured ectopic pregnancy with two different methotrexate protocols. Int J Fertil Steril. 2012 Oct;6(3):189–94.
17. Mol F, Mol BW, Ankum WM, van der Veen F, Hajenius PJ. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008 Jul-Aug;14(4):309–19.
18. Klauser CK, May WL, Johnson VK, Cowan BD, Hines RS. Methotrexate for ectopic pregnancy: a randomized “single dose” compared with “multidose” trial. Obstetrics & Gynecology. 2005 Apr 1;105(4):64S.
19. Eskandar M. Single dose methotrexate for treatment of ectopic pregnancy: risk factors for treatment failure. Middle East Fertility Society Journal. 2007;12(1):57-62.
20. Schuiiemann HJ. Oxman A. GRADE handbook for grading the quality of evidence and the strength of recommendations Version 3.2 [updated March 2009].