Abstract
Background: Chronic Spontaneous Urticaria (CSU) is a multifactorial disease with an incompletely understood etiology. COVID-19 vaccines can influence the immune system. This study evaluates the risk factors and comorbidities associated with CSU in patients who developed CSU following COVID-19 vaccination or infection.
Methods: This cross-sectional study was conducted in Shiraz, Iran, and enrolled thirty-six adult patients with new-onset CSU developing within two months of COVID-19 vaccination or infection, persisting for at least six weeks. Diagnosis of CSU was confirmed by allergy and clinical immunology subspecialists based on EAACI guidelines. Clinical history, physical examination, laboratory findings, and skin prick test (SPT) results were collected. Associated diseases, allergy history, and inflammatory markers were also assessed.
Results: The mean age of participants was 38.17 ± 15.50 years. The median onset of CSU symptoms was 7.0 days (5.25, 12.25) for the AstraZeneca group and 7.0 days (2.50, 10.50) for the COVID-19 infection group, earlier than in other vaccine groups. CSU occurred more frequently after the first vaccine dose (P≤0.001) and more commonly at night. Most patients (66.7%) had a pruritus score of 3; 47.2% had a wheal score of 2. A history of allergy, atopy, or comorbidities was present in most patients. Positive SPT to aeroallergens was found in 63.9%. Laboratory data for autoinflammatory markers (e.g., ESR, CRP) were collected but did not show significant elevation. Daily use of second-generation H1 antihistamines controlled symptoms in 75% of patients.
Conclusion: CSU was most likely to develop within 6.5 days following the first dose of COVID-19 vaccination, especially AstraZeneca and Sinopharm. The high rate of positive SPT and allergy history suggests a predisposing allergic background. Most cases were not severe and responded well to antihistamine therapy.
Keywords
COVID-19, COVID-19 vaccines, Chronic spontaneous urticaria, Skin prick test, Hypersensitivity, Antihistamines, SARS-CoV-2, Inflammatory markers, Allergy history
Introduction
Since late 2019, COVID-19 has become a global pandemic; the SARS-CoV-2 virus causes respiratory and non-respiratory symptoms, including a spectrum of skin manifestations such as urticaria, macular-papular rash, vesicular lesions, and vaso-occlusive skin findings [1,2]. Vaccines have been critical in preventing COVID-19 infection, with multiple platforms such as inactivated microbe vaccines (Sinopharm, Sinovac, Covaxin), viral vector vaccines (AstraZeneca, Johnson & Johnson), protein subunit vaccines (Covovax, Novavax), and mRNA vaccines (Moderna, Pfizer-BioNTech) in use worldwide [3,4]. In Iran, the primary WHO-approved vaccines are Sinopharm, Covaxin, and AstraZeneca. Although these vaccines are generally safe, rare adverse reactions have been documented, and certain risk factors—including vaccine type, dosage, younger age, and female sex—are associated with greater odds of side effects [5].
Urticaria is a complex skin disorder with infectious, allergic, and autoimmune etiologies, manifesting as acute or chronic forms. Chronic urticaria is defined as the presence of wheals for more than six weeks [6]. Emerging evidence describes both acute and chronic urticaria after COVID-19 infection or vaccination, with delayed skin reactions such as chronic urticaria being reported [7]. The overall prevalence of urticaria post-COVID-19 vaccination is approximately 1.1%; chronic cases are rare, with an incidence of 20 per 100,000 [8]. The proposed mechanisms include immune modulation via vaccine-induced adaptive immunity and possible type IIb autoimmunity [9,10].
Recent studies suggest that patients with a history of allergy or atopy may be at greater risk for post-vaccination urticaria. However, there is a lack of region-specific data, especially in Iran, on CSU risk factors and prevalence after COVID-19 vaccination. The long-term effects and immunologic profiles, including inflammatory markers, remain understudied. Therefore, this study aims to evaluate the clinical and immunological risk factors for new-onset CSU following COVID-19 vaccination or infection, with particular attention to allergy history, comorbidities, and autoinflammatory profiles.
Methods
Study design and setting
This cross-sectional study was conducted from January 2022 to March 2023 at a tertiary referral center in Shiraz, Iran. Patients aged 18 or older presenting to the asthma, allergy, and immunology clinic were screened for eligibility.
Inclusion criteria
- New-onset chronic spontaneous urticaria, developing within 2 months after COVID-19 infection or at least one dose of COVID-19 vaccine, and persisting for at least six weeks
- Age >18 years
Exclusion criteria
- Urticaria lasting less than six weeks
- Age <18
- Flares of pre-existing CSU
- Patients declining participation
- Other urticaria types (e.g., physical, acute recurrent, vasculitis-associated, autoimmune)
- Prior urticaria therapy
Diagnosis of CSU
All patients were evaluated by certified allergy and clinical immunology subspecialists, using the EAACI/GA [2] LEN/EDF/WAO guidelines [11]. Diagnosis was based on a detailed clinical history, physical examination, and, where indicated, laboratory or skin tests. Only cases with no identifiable acute triggers and symptoms persisting >6 weeks were classified as CSU.
Data collection
Collected data included demographic details (age, sex, BMI, residence), timing of urticaria onset after vaccination/infection, vaccination details (type, dose), clinical features (diurnal variation, severity, frequency, urticaria activity score), history of allergies (including atopy, drug/food allergy, allergic rhinitis, asthma, atopic dermatitis), comorbidities, family history, medication use, weight changes, pet ownership, and smoking status.
Skin prick testing (SPT) was performed for indoor (mites, molds, cockroach) and outdoor aeroallergens (grass, weed, tree pollens), following EAACI protocols. Wheal and pruritus scores were assigned according to standardized scales.
Autoinflammatory and inflammatory markers (e.g., ESR, CRP) were measured where possible; however, results were within normal limits and are detailed in Table 1. All data were collected confidentially after obtaining informed consent. The study protocol was approved by the Shiraz University of Medical Sciences ethics committee (IR.SUMS.MED.REC.1401.132).
Statistical analysis
Categorical variables are reported as frequencies and percentages. The Kolmogorov-Smirnov test was used for normality assessment. Means±SD were used for normally distributed variables; medians and IQR for non-normal data. Chi-square, ANOVA, or Kruskal-Wallis tests were applied for subgroup comparisons (vaccine types, infection), and appropriate inferential tests (independent t-test, Mann-Whitney U) were used for two-group comparisons. P<0.05 was considered statistically significant. Analyses were performed with SPSS v20.0; GraphPad and Excel were used for figures.
Results
The mean age of the patients who participated in the study was 38.17±15.50 years. There were no significant differences between the ages of the patients who received each type of vaccine or those who experienced COVID-19 infection (P-value=0.879). Furthermore, there were no statistically significant differences between the ages of the vaccine recipients and the patients with COVID-19 infection (P-value=0.972; Table 1).
Out of 36 patients, 31 received COVID-19 vaccines and 5 had confirmed COVID-19 infection. The vaccine types included AstraZeneca (n=8), Sinopharm (n=22), and Sputnik V (n=1); no patients received mRNA vaccines due to their unavailability in Iran during the study period, which is a limitation for direct vaccine-to-vaccine comparison. No significant differences were observed between the onset of symptoms among vaccine types or between vaccination and infection groups. However, the median onset of symptoms in the AstraZeneca vaccine group was 7.0 days (IQR 5.25, 12.25), and for the COVID-19 infection group, 7.0 days (IQR 2.50, 10.50), which were higher than in other groups.
Residence (large city vs small city) did not significantly differ between groups (P=0.490), although CSU was more frequently observed in patients residing in large cities. Regarding vaccine dose, a significant difference was observed between the groups of vaccine types (P-value≤0.001; Table 1), with chronic spontaneous urticaria observed most commonly following the first dose. In terms of daily symptom pattern, no significant difference was found between groups (P=0.520); however, CSU was most commonly reported at night.
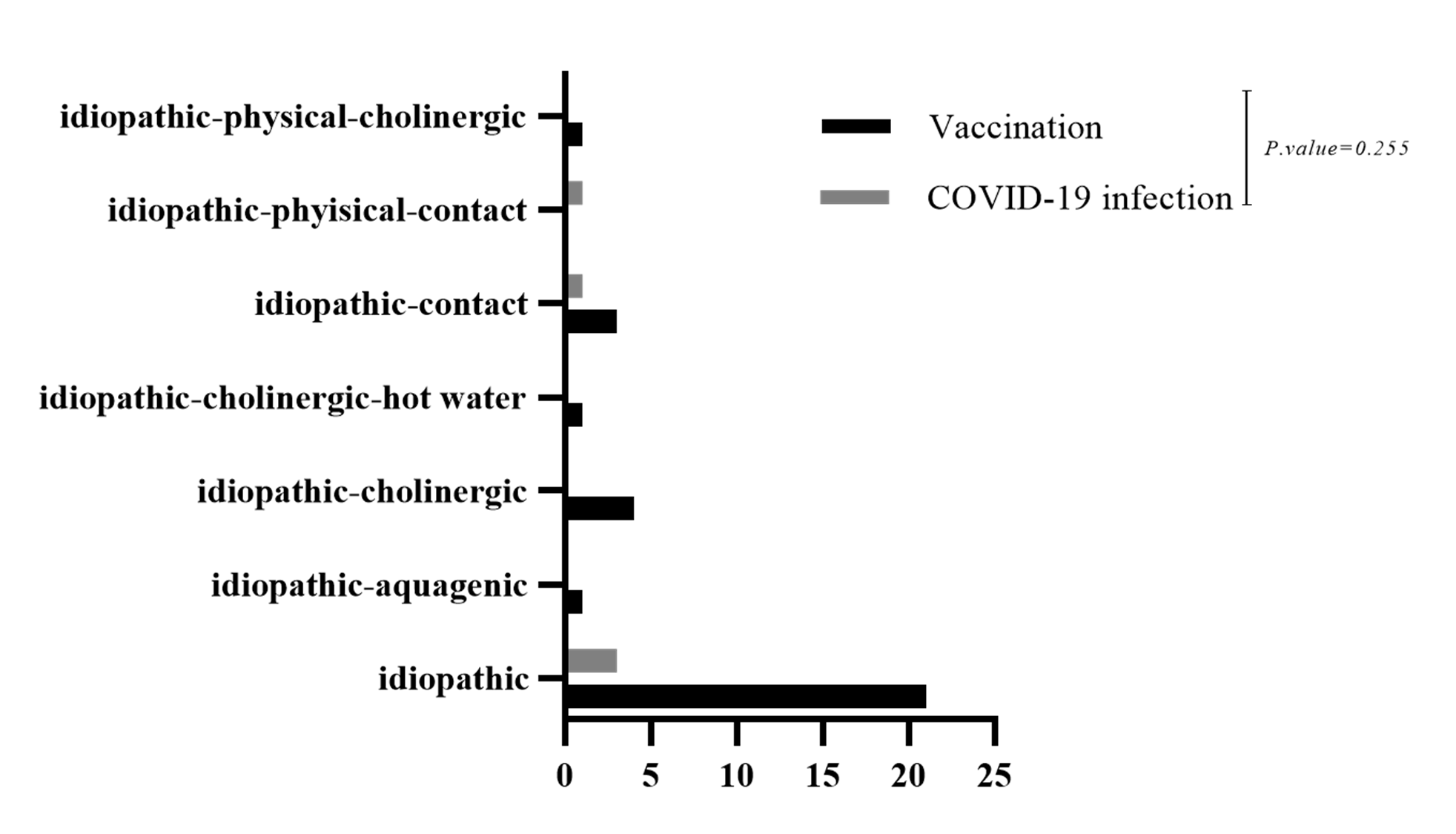
A family history of urticaria and allergy did not show a statistically significant difference between the types of vaccines (P-value=0.065). However, chronic spontaneous urticaria was more prevalent in the AstraZeneca group, and Sinopharm was more prevalent in the group with a family history of urticaria and allergy (Table 1). A positive family history of urticaria or allergy did not significantly differ between vaccine types (P=0.065), although CSU was more prevalent in the AstraZeneca group, and Sinopharm was more frequent in patients with a family history of urticaria/allergy. Most CSU cases in both groups were classified as idiopathic (Figure 1).
|
Variable |
Total (n=36) |
AstraZeneca (n=8) |
Sinopharm (n=22) |
Sputnik (n=1) |
COVID-19 Infection (n=5) |
P-value† |
P-value* |
|
Age, Mean±SD |
38.17±15.50 |
38.13±11.82 |
37.55±15.44 |
51.00 |
38.40±23.73 |
0.879 |
0.972 |
|
BMI, Mean±SD |
23.59±3.56 |
23.67±4.30 |
23.49±3.46 |
22.00 |
24.26±3.81 |
0.946 |
0.659 |
|
Onset of symptoms after COVID infection or vaccine (days), Median (IQR) |
6.5 (2.0, 7.0) |
7.0 (5.25, 12.25) |
4.0 (2.0, 7.0) |
3.0 (3.0, 3.0) |
7.0 (2.50, 10.50) |
0.449 |
0.675 |
|
Length of symptoms (months), Mean±SD |
4.25±2.19 |
5.00±3.65 |
4.07±1.45 |
3.00 |
4.10±2.46 |
0.716 |
0.872 |
|
Frequency (days/week), Mean±SD |
5.72±2.91 |
5.50±5.95 |
5.81±1.25 |
7.00 |
5.40±1.81 |
0.960 |
0.794 |
|
Sex, n (%) Female |
29 (80.6%) |
6 (20.7%) |
18 (62.1%) |
1 (3.4%) |
4 (13.8%) |
0.936 |
0.973 |
|
Sex, n (%) Male |
7 (19.4%) |
2 (28.6%) |
4 (57.1%) |
0 |
1 (14.3%) |
||
|
Family history of urticaria/allergy, n (%) |
21 (58.3%) |
7 (33.3%) |
13 (61.9%) |
0 (0.0%) |
1 (4.8%) |
0.065 |
0.061 |
|
SPT positive (any allergen), n (%) |
23 (63.9%) |
5 (21.7%) |
15 (65.2%) |
0 |
3 (13.0%) |
0.576 |
0.845 |
|
Wheal score 2 (most prevalent), n (%) |
17 (47.2%) |
3 (17.6%) |
11 (64.7%) |
0 |
3 (17.6%) |
0.305 |
0.311 |
|
Pruritus score 3 (most prevalent), n (%) |
24 (66.7%) |
5 (20.8%) |
15 (62.5%) |
1 (4.2%) |
3 (12.5%) |
0.816 |
0.793 |
|
Treatment outcome: Not cured |
9 (25.0%) |
3 (33.3%) |
4 (44.4%) |
1 (11.1%) |
1 (11.1%) |
0.478 |
0.825 |
|
Treatment outcome: Symptom-free |
10 (27.8%) |
1 (10.0%) |
8 (80.0%) |
0 |
1 (10.0%) |
||
|
Treatment outcome: Controlled |
17 (47.2%) |
4 (23.5%) |
10 (58.8%) |
0 |
3 (17.6%) |
||
|
ESR, Median (IQR) mm/hr |
12 (8–18) |
— |
— |
— |
— |
— |
— |
|
CRP, Median (IQR) mg/L |
2.1 (1.1–3.8) |
— |
— |
— |
— |
— |
— |
|
Abbreviations: BMI: Body Mass Index; SPT: Skin Prick Test; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; IQR: Interquartile Range |
|||||||
Figure 1. Distribution of chronic spontaneous urticaria subtypes (idiopathic vs. other causes) among patients who developed CSU after COVID-19 vaccination or infection. Idiopathic CSU was most common in both groups.
Wheal score did not significantly differ between vaccine or infection groups (P=0.305); a wheal score of 2 was most prevalent, particularly in the Sinopharm and infection groups. Pruritus score 3 was the most frequent among all groups, without significant difference between vaccination and infection (P=0.816), but CSU was more frequently seen in patients with a pruritus score of 3 (Table 1).
Nine patients out of 36 (25%) did not get cured with daily doses of second-generation H1 antihistamines, whereas 10 (27.8%) became symptom-free and 17 (47.2%) had controlled symptoms with the same therapy (Fexofenadine 120 mg twice daily; Table 1).
Regarding the history of COVID-19 infection, a significant difference was seen between any vaccination and COVID-19 infection in patients with chronic spontaneous urticaria (P-value=0.042; Table 1).
There was a significant difference regarding prior COVID-19 infection between vaccinated and infected groups (P=0.042; Table 1). The severity of urticaria post-infection also differed significantly between groups (P=0.022), with most CSU cases not worsening after infection. Smoking history differed significantly between groups (P=0.027).
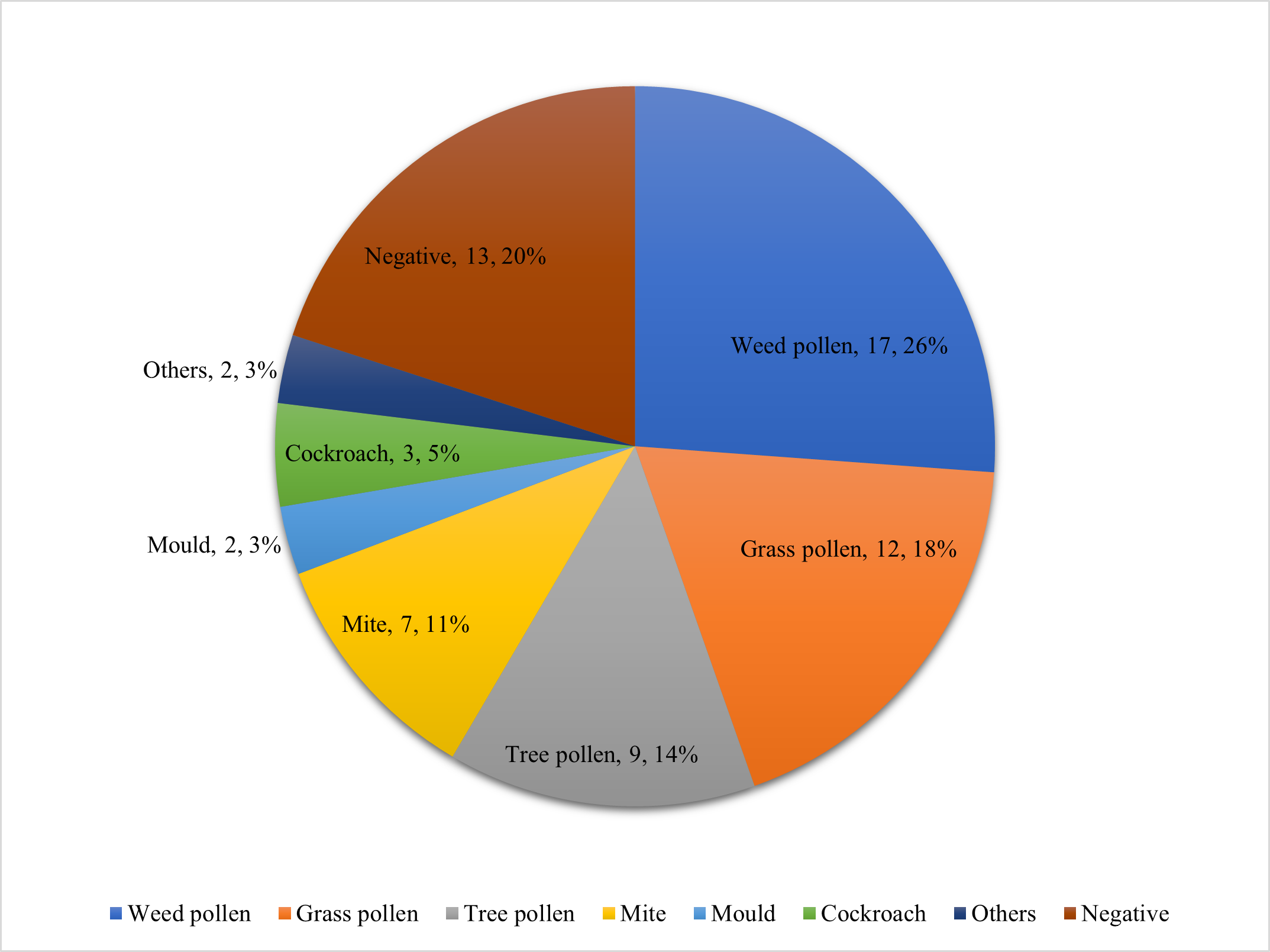
Table 2 compares the SPT results of the patients with CSU after vaccination and COVID-19 infection. Most of the positive SPT results come from vaccinated patients. Additionally, 13 out of 36 patients had negative SPT results, and 23 had positive ones. Of the 23 patients with positive SPT, some had sensitization to only one allergen; however, most had multiple sensitizations to indoor and outdoor allergens, as shown in Table 2 and Figure 2.
|
Skin Test Result |
Vaccination n (%) |
Infection n (%) |
P-value |
|
Grass pollen |
2 (100.0%) |
0 (0.0%) |
0.554 |
|
Mite–weed pollen |
1 (100.0%) |
0 (0.0%) |
|
|
Negative |
11 (84.6%) |
2 (15.4%) |
|
|
Tree pollen |
0 (0.0%) |
1 (100.0%) |
|
|
Tree pollen–grass pollen |
2 (100.0%) |
0 (0.0%) |
|
|
Tree pollen–weed pollen |
1 (100.0%) |
0 (0.0%) |
|
|
Tree pollen–weed pollen–grass pollen–mite |
0 (0.0%) |
1 (100.0%) |
|
|
Tree pollen–weed pollen–grass pollen–mite–moulds–cockroach |
1 (100.0%) |
0 (0.0%) |
|
|
Tree pollen–weed pollen–mite–cockroach |
1 (100.0%) |
0 (0.0%) |
|
|
Triclosan–turpentine oil |
1 (100.0%) |
0 (0.0%) |
|
|
Weed pollen |
3 (75.0%) |
1 (25.0%) |
|
|
Weed pollen–cockroach |
1 (100.0%) |
0 (0.0%) |
|
|
Weed pollen–grass pollen |
2 (100.0%) |
0 (0.0%) |
|
|
Weed pollen–grass pollen–tree pollen–mite |
1 (100.0%) |
0 (0.0%) |
|
|
Weed pollen–grass pollen–mite |
1 (100.0%) |
0 (0.0%) |
|
|
Weed pollen–grass pollen–mold Alternaria |
1 (100.0%) |
0 (0.0%) |
|
|
Weed pollen–mite |
1 (100.0%) |
0 (0.0%) |
|
|
Weed pollen–tree pollen–grass pollen |
1 (100.0%) |
0 (0.0%) |
|
|
Abbreviations: SPT: Skin Prick Test |
|||
Figure 2. Number and type of indoor (mite, mold, cockroach) and outdoor (grass, weed, tree pollens) aeroallergen sensitizations among CSU patients following COVID-19 vaccination or infection, as determined by skin prick test. Most positive patients were polysensitized.
Discussion
This study is among the first in Iran to evaluate the risk factors and associations of CSU with COVID-19 vaccination. While several international studies have examined post-vaccine urticaria, data on the regional population and vaccine types commonly used in Iran remain limited. Previous reports indicate that both whole-virus and mRNA COVID-19 vaccines can cause urticaria, particularly following the first dose [12,13]. Exacerbation rates after viral vector and mRNA vaccines have been reported at 13–14%, mainly among patients with comorbidities such as hypertension, allergic rhinitis, diabetes mellitus, or preexisting CSU [8]. In a systematic review of 80 studies involving 1,415 reactions, urticaria accounted for 9% of all cases after mRNA and AstraZeneca vaccines, with most reactions classified as delayed onset [14]. There are currently no approved live attenuated COVID-19 vaccines [15]. Live vaccines for other diseases, such as monkeypox, are not specifically associated with urticaria, but may carry other risks in immunocompromised patients [16].
Our findings demonstrate that CSU most frequently developed following the first dose of COVID-19 vaccination, consistent with other studies [17]. Notably, most patients in this study had received inactivated virus vaccines (Sinopharm), reflecting national vaccine availability, which limited direct comparison between vaccine type (a study limitation). No mRNA vaccines were administered during our study period.
While some studies report fewer or milder reactions after the second dose [18], others, such as Johnston et al., describe similar or recurrent cutaneous reactions with subsequent doses. This apparent discrepancy may be related to differences in patient characteristics, vaccine types, or genetic and environmental factors [6].
The higher frequency of CSU among vaccinated patients compared to those with COVID-19 infection in our cohort may reflect immunological responses specific to vaccination, including type IIb autoimmunity and sensitization to vaccine components [10,19]. A recent study also demonstrated that a history of atopy was associated with urticaria following inactivated virus vaccines, while comorbidities such as hypertension or hyperlipidemia increased risk among non-atopic individuals [20]. Sinopharm was the most common vaccine in our study population, which may partially account for the distribution of CSU cases.
Symptom onset in our patients occurred a median of 6.5 days after vaccination, comparable to prior studies, though minor variations may relate to vaccine formulation or ethnic background [21,22]. Some studies report delayed localized hypersensitivity reactions—primarily in females—which typically resolve within three weeks, whereas in our study, CSU persisted for at least two months [18]. This distinction may be explained by differing underlying pathogenesis between localized delayed reactions and chronic generalized urticaria.
Assessment of allergy background revealed that 63.9% of patients had positive SPT results for indoor or outdoor aeroallergens, and a substantial proportion reported a history of atopy or allergic diseases. This aligns with emerging evidence that allergy history and comorbidities increase susceptibility to CSU after vaccination. In contrast, several case series report predominantly negative SPT results for suspected vaccine allergens, suggesting that vaccine excipients are not the sole contributors [23–25]. Our data supports the need for comprehensive allergy evaluation in this context.
In addition, we evaluated basic inflammatory markers (ESR, CRP) in our patients, and no significant elevations were observed, consistent with non-systemic inflammatory presentation of CSU (See Table 1).
Importantly, no patients in this cohort experienced life-threatening reactions; most were managed successfully with second-generation H1 antihistamines. Most patients had moderate wheal and severe pruritus scores, which, while not dangerous, significantly affected quality of life.
Limitations
The present study has several limitations. First, the sample size is small, reflecting the relative rarity of CSU after vaccination and limiting generalizability. Second, due to the constraints of national vaccine policy, mRNA vaccines were not administered, restricting direct vaccine comparisons. Third, no true control group was available, as non-exposed or pre-pandemic controls could not be reliably selected. Therefore, causality between vaccination and CSU cannot be established. Finally, although we collected data on allergy and basic inflammatory markers, detailed immunologic profiling was not feasible.
Strengths
To our knowledge, this is one of the first studies in the region to evaluate risk factors, allergy history, and SPT patterns in CSU following COVID-19 vaccination. The comprehensive assessment of allergy background and documentation of clinical course adds to the understanding of this underrecognized complication.
Future research should include larger, multicenter studies with well-matched controls and broader immunological workup to clarify the pathogenesis and risk profile for CSU after COVID-19 vaccination.
Conclusion
Our results indicate that CSU can develop after COVID-19 vaccination or infection, most frequently after the first vaccine dose and within a week of exposure. A history of allergy or atopy and a positive skin prick test were common among affected patients, suggesting that an allergic predisposition may increase risk. The majority of cases were mild to moderate and were effectively managed with second-generation H1 antihistamines. Ongoing surveillance and further research are needed to better delineate risk factors and immunologic mechanisms.
List of Abbreviations
CSU: Chronic Spontaneous Urticaria; SPT: Skin Prick Test
Declarations
Conflicts of interests
Authors declare that there is no conflict of interest regarding the publication of this article.
Funding
Authors have no funding source for this research.
Acknowledgments
This manuscript is based on the thesis for the MD degree of the first corresponding author.
References
2. Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: A worldwide review. JAAD Int. 2021;2:119–33.
3. Zhou Z, Zhu Y, Chu M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front Immunol. 2022;13:898192.
4. Abufares HI, Oyoun Alsoud L, Alqudah MAY, Shara M, Soares NC, Alzoubi KH, et al. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int J Mol Sci. 2022;23(23):15415.
5. Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open. 2021;4(12):e2140364.
6. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64(10):1417–26.
7. Miller M, Tracey M, Simpson M, Mikita C. Pearls and pitfalls: Adverse cutaneous reactions after COVID-19 vaccination. Allergy Asthma Proc. 2022;43(6):555–8.
8. Lee JH, Shin E, Kim HK, Song WJ, Kwon HS, Kim TB, et al. Exacerbation of Chronic Spontaneous Urticaria Following Coronavirus Disease 2019 (COVID-19) Vaccination in Omalizumab-Treated Patients. J Allergy Clin Immunol Pract. 2023;11(8):2403–10.
9. Chi WY, Li YD, Huang HC, Chan TEH, Chow SY, Su JH, et al. COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J Biomed Sci. 2022;29(1):82.
10. Tuzer C, Terzioglu K. Evaluation of the Autoimmunity and Preexisting Risky Conditions for Hypersensitivity Reactions to COVID-19 Vaccines. Int Arch Allergy Immunol. 2022;183(6):651–61.
11. Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77(3):734–66.
12. Im JH, Kim E, Lee E, Seo Y, Lee Y, Jang Y, et al. Adverse Events with the Pfizer-BioNTech COVID-19 Vaccine among Korean Healthcare Workers. Yonsei Med J. 2021;62(12):1162–8.
13. Katherine Yih W, Daley MF, Duffy J, Fireman B, McClure D, Nelson J, et al. Tree-based data mining for safety assessment of first COVID-19 booster doses in the Vaccine Safety Datalink. Vaccine. 2023;41(2):460–6.
14. Kroumpouzos G, Paroikaki ME, Yumeen S, Bhargava S, Mylonakis E. Cutaneous Complications of mRNA and AZD1222 COVID-19 Vaccines: A Worldwide Review. Microorganisms. 2022;10(3):624.
15. Mbaeyi S, Oliver SE, Collins JP, Godfrey M, Goswami ND, Hadler SC, et al. The Advisory Committee on Immunization Practices' Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines - United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545–52.
16. Abdelaal A, Reda A, Lashin BI, Katamesh BE, Brakat AM, Al-Manaseer BM, et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious against Monkeypox, or Is There a Need for Killed Virus and mRNA Vaccines? Vaccines (Basel). 2022;10(9):1419.
17. Tuchinda P, Kulthanan K, Chularojanamontri L, Pongkittilar B, Pochanapan O, Rujitharanawong C. Disease activity of patients with chronic urticaria receiving COVID-19 vaccines. Asian Pac J Allergy Immunol. 2022;42(2):123–31.
18. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021;157(6):716–20.
19. Pescosolido E, Muller YD, Sabaté-Brescó M, Ferrer M, Yerly D, Caubet JC, et al. Clinical and immunological data from chronic urticaria onset after mRNA SARS-CoV-2 vaccines. Clin Exp Allergy. 2022;52(11):1343–6.
20. Chiewchalermsri C, Hengkrawit K, Srinithiwat P, Kiatsermkachorn W, Luecha O. Risk of Adverse Events of Live-Attenuated COVID-19 Vaccination Among Atopic Patients. J Asthma Allergy. 2022;15:1605–21.
21. Shakoei S, Kalantari Y, Nasimi M, Tootoonchi N, Ansari MS, Razavi Z, et al. Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases. Dermatol Ther. 2022;35(8):e15651.
22. Pitlick MM, Joshi AY, Gonzalez-Estrada A, Chiarella SE. Delayed systemic urticarial reactions following mRNA COVID-19 vaccination. Allergy Asthma Proc. 2022;43(1):40–3.
23. Ben-Fredj N, Chahed F, Ben-Fadhel N, Mansour K, Ben-Romdhane H, Mabrouk RSE, et al. Case series of chronic spontaneous urticaria following COVID-19 vaccines: an unusual skin manifestation. Eur J Clin Pharmacol. 2022;78(12):1959–64.
24. Pitlick MM, Sitek AN, D'Netto ME, Dages KN, Chiarella SE, Gonzalez-Estrada A, et al. Utility and futility of skin testing to address concerns surrounding messenger RNA coronavirus disease 2019 vaccine reactions. Ann Allergy Asthma Immunol. 2022;128(2):153–60.
25. Magen E, Yakov A, Green I, Israel A, Vinker S, Merzon E. Chronic spontaneous urticaria after BNT162b2 mRNA (Pfizer-BioNTech) vaccination against SARS-CoV-2. Allergy Asthma Proc. 2022;43(1):30–6.