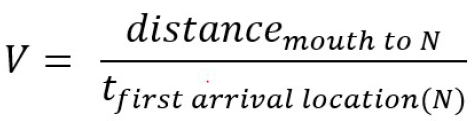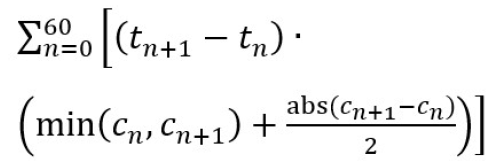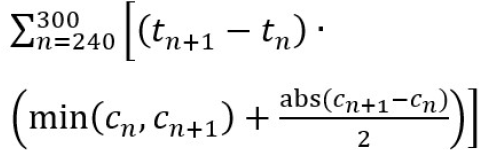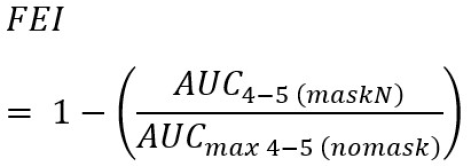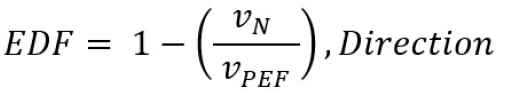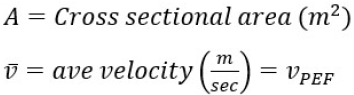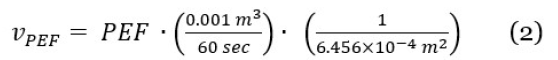Abstract
Background: For much of the SARS-CoV-2 (COVID-19) pandemic, many countries have struggled to offer definitive guidance on the wearing of masks or face coverings to reduce the highly infectious disease transmission resulting from a lack of compelling evidence on the effectiveness of communities wearing masks, and slow acceptance that aerosols are a primary SARS-CoV-2 disease transmission mechanism. Recent studies have shown that masks have been effective in several countries and populations, leaving only a lack of quantitative data on the control of airborne dispersion from human exhalation. This current study specifically has the objective to quantify the effectiveness of non-medical grade washable masks or face coverings in controlling airborne dispersion from exhalation (both droplet and aerosol) by measuring changes in direction, particle cloud velocities, and concentration.
Design: This randomized effectiveness study used a 10% NaCl nebulized polydisperse particle solution (0.3 μm up to 10 μm in size) delivered by an exhalation simulator to conduct 94 experiment runs with combinations of 8 different fabrics, 5 mask designs, and airflows for both talking and coughing. Multiple particle sensors were instrumented to measure reduction in aerosol dispersion.
Results: Three-way multivariate analysis of variance establishes that fabric, mask design, and exhalation breath level have a statistically significant effect on changing direction, reducing velocity, or concentrations of airborne particles (Fabric: P = <0.001, Wilks’ Λ = 0.000; Mask design: P = <0.001, Wilks’ Λ = 0.000; Breath level: P = <0.001, Wilks’ Λ = 0.004). There were also statistically significant interaction effects between combinations of all primary factors.
Conclusions and Relevance: The application of facial coverings or masks can significantly reduce the airborne dispersion of aerosolized particles from exhalation by diffusing the particle cloud direction and slow down its travel speed. Consequently, the results indicate that wearing masks when coupled with social distance can decrease the potentially inhaled dose of SARS-CoV-2 aerosols or droplets especially where infectious contaminants may exist in shared air spaces. The conclusion is well aligned with the concept of “time-distance-shielding” from hazardous materials emergency response. However, the effectiveness varies greatly between the specific fabrics and mask designs used.
Keywords
Masks, COVID-19, Source control, Airborne particle dispersion, Exhalation particles
Background
In light of the current pandemic from rapid transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or COVID-19) and significant morbidity, there has been inconsistent medical guidance given to the public regarding the wearing of non-medical improvised fabric masks or face coverings to reduce the transmission of COVID-19. If the SARS-CoV-2 aerosol is considered with an ability to infect for more than 3 hours with TCID50 of greater than 102 as noted in a laboratory study [1], then it is of significant importance for broad public health infectious disease strategy to understand the effectiveness of non-medical masks and face coverings to control human exhalation aerosol dispersion, especially with asymptomatic or pre-symptomatic populations.
Of concern, recent studies show that bio-droplets of all sizes are generated from normal exhalation [2-5] with 80- 90% of droplets from human exhalation in the size range of 0.1–1 μm [6,7], those from a cough can travel 23 to 27 feet (7-8 m) which is well beyond the recommended social distances of six feet or two meters, and smaller aerosols (≤5 μm) stay aloft in the air and pose a greater risk for severe infection [8,9]. In addition, social distancing is difficult to accomplish since many essential locations like grocery stores have aisles that are narrow and result in the proximity of patrons being closer than 1 meter. Reduced distance correlates to increased transmission of COVID-19 [10,11].
A lack of definitive data on establishing the effectiveness of using non-medical masks or face coverings has resulted in medical practitioners giving broad public health guidance based on professional judgement only. Existing guidance includes statements that facial coverings may offer minimal protection from small infectious particles, may only reduce large particulate matter, or only remind users to not touch their face considering infectious disease transmission from hand to face. One key challenge has been the slow acceptance of aerosol transmission as the primary SARS-CoV-2 disease transmission mechanism [9,12]. Several previous studies have been conducted to understand if wearing of masks reduce community infections of common diseases such as influenza, however most are inconclusive due to the application of masks post-exposure or lack of strict wearing compliance by study participants [13-15] Only a few well-executed studies concluded the prophylactic wearing of medical grade masks reduce community transmission of influenza or RSV [16,17]. To make matters worse, the lack of definitive guidance has also led to social and political debates on the wearing of masks or face coverings [18-20] and deters the acceptance of any new public health strategy for reducing airborne transmission of infectious diseases.
Only recently, several definitive case studies and broad systematic or meta-analysis shown that masks and face coverings have been effective in reducing community transmission of COVID-19 within several countries and populations [21-25], which leaves only a lack of quantitative data on the control of airborne dispersion from human exhalation. Prior studies have established the filtration efficiency of a variety of fabrics for personal protection but do not consider reducing transmission by controlling airborne dispersion of human exhalation [26-33]. Other research investigates only forward dispersion of particles from coughing or sneezing by measuring from a single optical plane [8,34]. Additional research considers the aerodynamics of exhalation particles inside various rooms or vehicles using high fidelity computational fluid dynamic simulation under ideal laminar flow conditions and possible effectiveness of masks [35-39], but do not model real world turbulent airflows nor validate the models with physical experimentation data. Several clinical studies show reductions in virus shedding when wearing face masks [2,3,5,40-42], although neither the clinical nor experimental studies have fully characterized the effectiveness of non-medical grade reusable masks in controlling aerosol dispersion of human exhalation particles.
Objective, Design, and Methods
The goal of this research is to determine the statistically significant factors and quantify the effectiveness of nonmedical grade washable masks or face coverings in the control of aerosol dispersion from human exhalation in terms of affecting direction, velocity, and concentration of various particle diameters. When considering the primary contribution of this work, there are three complimentary types of studies regarding the wearing of masks and face coverings in the context of respiratory transmission of infectious disease: mask filtration studies, clinical studies, and particle dispersion studies. Existing mask filtration studies help answer the question if they protect a user during inhalation. Clinical studies on masks help answer the question if they work for broad community reductions in transmission. Particle dispersion studies offer significant insights to the aerodynamics and biophysics of infectious particles, although there is not as much research in this category. All three types of studies are necessary to inform and construct the most holistic solutions for public health [43]. This original research offers the experimental results in the latter category of aerodynamics and biophysics of infectious airborne particles. Conclusively this study can aid in establishing public health strategies or policies that encourage the wearing of masks or face coverings to increase the effectiveness of non-pharmaceutical interventions (NPI) especially where infectious contaminants may exist in shared air spaces.
Design
We conducted the effectiveness study using a randomized full factorial design of experiments with 10% NaCl nebulized solution and an exhalation simulator to conduct 94 experiment runs with combinations of 8 different fabrics, 5 mask designs, no-mask as a control, and exhalation airflows (PEF and FEV1) that represent both talking and coughing. Randomization was created using a mathematical function in MATLAB with blocking on the two airflow settings for talking and coughing. The experiment also included randomized runs of no-mask applied as the control and a preliminary comparison with the performance of a MERV13 air filter media which has similar electrostatic filtration properties to the NIOSH N95 standard (95% filtration efficiency of 0.3μm particles).
The exhalation simulator was constructed similar to previous research [44,45], but with some differences. The exhalation simulator was driven by a dry compressed air expansion chamber and timing-controlled relay, with a port for the small volume jet nebulizer, in-line spirometer, and a corrugated tube to emulate a trachea before exiting the mouth of the CPR manikin. Details on the simulator equipment are in Supplementary Figure 1. The exhalation airflows were calibrated to simulate peak expiratory flow (PEF) of coughing with a range 507L/min to 650L/ min, and PEF for talking of approximately 120L/min as established by previous research [44–47]. The typical air flows for talking are similar to that of singing [47].
Four laser scattering particle concentration sensors (Plantower PMS5003) that measured particles from 0.3μm to 10μm in size were placed at specific locations inside a non-airtight fume hood (Supplementary Figure 2) to detect aerosol dispersion directly downward, laterally from the mid-line, and 1 meter forward of the mouth. Preliminary testing of the configuration identified that the optimal position when used with various masks in this fume hood was 43 cm below the level of the mouth for all sensors. The frontal sensor represents an approximate halfway point of a 2 meter or 6 feet social distance.
Interventions and Exposures
A NaCl aqueous solution was selected as a polydisperse test aerosol which is also used as the exposure for NIOSH N95 respirator test methods. 10% NaCl was used to generate a sufficient quantity of particles for the open-air fume hood environment and also to stay beneath the PMS sensor maximum (65,535 particle count per 0.1L for any given size). The aerosol was produced by nebulizing the solution at 103 kPa (15 psi) for 5 seconds into the aerosol chamber of the exhalation simulator followed by a 3 ms delay before exhalation from the manikin through the applied masks. The simulated exhalations were driven by timing controlled compressed air at 827 kPa (120 psi) for “coughing” and 206 kPa (30 psi) for “talking”.
The intervention was provided by non-medical grade fabric masks sourced from local materials that were available during the COVID-19 supply chain interruptions. The washable fabrics were selected are shown in (Supplementary Table 1) (fabric bolts were unavailable) which included natural fibers, polyesters, and other materials. The mask designs were selected from variety of community-based designs which included a bandana style, surgical mask style, folded no-sew, a simple mask with earloops, and a stylistic mask that had more coverage of the nose. Microscopic images of the fabric weave and fibers were taken (Keyence VHX-S660E) to further understand and explain the results. More details regarding the fabrics and masks designs and the basic test procedure are also included in the (Supplementary Appendix).
Outcomes
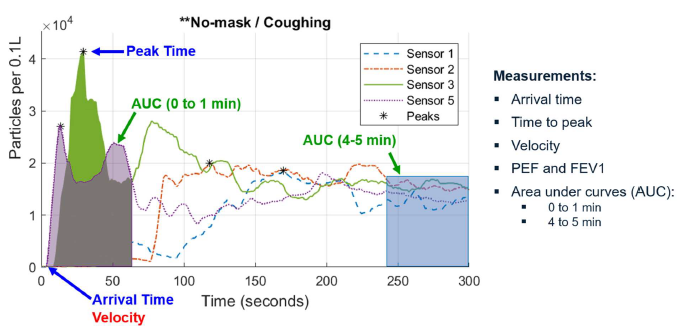
The primary outcome was to measure any significant reduction in aerosol dispersion velocity, quantity of particles, and change in dispersion direction. Measurements used in this study included peak expiratory flow (PEF), forced expiratory volume (FEV1), as well as aerosol arrival time, time to peak concentration, aerosol velocity, area under curve (AUC) for first minute and last minute as shown in Figure 1. A change in direction from sensor 5, reduction in velocity, or AUC are considered a positive effect. Two novel metrics of Filtration Efficiency Indicator (FEI) and Expiratory Flow Dispersion Factor (EDF) are established in this study to present quantitative values that give relative indicators to the dispersion control performance of non-medical masks using simple and repeatable measurement techniques of the research. A description of all measurements and outcomes are presented in Table 1.
Figure 1. Examples of the measurements performed on the time-series data from all experiment runs for each of the four sensors and particle sizes of 0.3µm, 0.5µm, 1µm, 2.5µm, 5µm, and 10µm.
AUC0 to 1 min as described in Table 1 characterizes the leading wave of the particle cloud. A higher value indicates a more particle concentration in the airspace over time. Lower values result from abrupt changes to particle concentration due to an overall lower amount of particles from exhalation or a rapid movement of the particle cloud movement. In general, a lower value is more desirable which represents less time and concentration that a person might be exposed to a particle cloud before it disperses.
FEI is a ratio of the particle concentration remaining after exhalation through a mask compared to no-mask and provides a quantitative indicator that aids in the filtration performance characterization. It is a ratio of remaining particle concentration (AUC4 to 5 min) with a mask applied compared to the worst-case AUC of no-mask applied; a higher value indicates better filtration. Since this experiment does not lend itself to directly measure the particle concentration in the aerosol chamber prior to exhalation nor does it use the same measurement equipment, test orifice and tube size, airflow dynamics, and other equipment from the NIOSH N95 test standard [48], the filtration efficiency indicator values are relative to each experiment. Likewise, many other recent studies seeking to establish the filtration efficiencies of non-medical grade masks or fabrics have the same constraint on relativity of results without NIOSH calibrated test orifice, tube size, and airflow characteristics. However, while the actual values are relative to this study the FEI measurement technique is broadly applicable to all exhalation dispersion studies.
We also present EDF as a measurement of the reduction in particle velocity and change in direction when a mask is applied. As shown in Table 1, it is the ratio of particle cloud velocity at the first arriving sensor and that of the exhalation airflow velocity derived from the PEF measurement. A higher value indicates better performance with more reduction in particle velocities. The theory of EDF is based on the airflow from exhalation simulator (bounded volume) and aerosol velocity in fume hood (unbounded turbulent airflow) which are correlated by Bernoulli’s ideal-gas law and further described in the field of kinetic theory of gases. Full derivation of the volumetric flow formula is provided in literature [49]; in this study the airflow of PEF equals the cross-sectional area of the spirometer multiplied by the average velocity of the air stream shown in Equation (1)

The inside diameter of the MIR SmartOne spirometer was measured to be 28.67 mm which allows for an area calculation. Using algebraic relationships, the formula for calculating the velocity of the exhalation using PEF measurement is shown in Equation (2) along with the unit conversion to meters per second.

Sample Size
The overall sample size from the full factorial combination of 40 distinct masks, several randomly inserted test runs of no-mask as the control, and 2 exhalation levels resulted in 94 experiment runs. The overall experiment with four sensors and average sampling rate of 1 second, generated over 1.694 million time-series sensor data measurements for this study. The sample size of n=94 resulted in 2,496 measurements for each dependent response variable across all particle diameters.
Statistical Analysis
Multivariable and multivariate analysis was conducted from the perspective of a null hypothesis that non-medical improvised masks do not affect the dispersion or offer any source control. This study determines if the three independent variables (mask designs, fabrics and breathing levels) have a statistically significant effect on any of the dependent responses (direction, AUC0 to 1 min, FEI, and EDF) at various sensor locations. Three-way multivariate analysis of variance (MANOVA) is used to simultaneously understand the significance of multiple effects from the independent variables and their correlations while minimizing type I statistical errors (false positives). Details on the use of MANOVA and validation against the assumptions of the data, including homogeneity of covariance, normality, independence of observations and multicollinearity [50-54] are provided in (Description of Statistical Methods in the Supplementary Appendix).
MATLAB version R2019a was used to import the raw data files, compute the response variable values, and calculate summary statistics. SPSS version 1.0.0.1327 was used to perform MANOVA. To additionally validate the statistical results and measured outcomes, graphical analysis of the data was also performed to identify any anomalies that were not expected in the response variables.
Results
The mean (SD) PEF for simulated coughing was 532.08 (75.65) L/min and FEV1 of 5.92 (0.1) L. Likewise, the mean (SD) PEF for simulated talking was 148.35 (43.29) L/min and FEV1 of 1.79 (0.07) L. Both simulated exhalation levels are within range of previous studies [44–47] The aerosol particle concentration was measured at the one-meter frontal sensor during the last minute of all no-mask (control) runs and resulted in concentration levels and distribution that indicates good polydisperse particle generation (Supplementary Figure 3). The mean (SD) concentrations for simulated talking generated peak concentrations of 32,199 (3,683) for 0.3μm particle diameters representing aerosols, and 201(42) for 10μm particle diameters representing droplets. Likewise, simulated coughing generated peak concentrations of 27,731 (9,837) for 0.3μm particle diameters, and 131(27) for 10μm particle diameters.
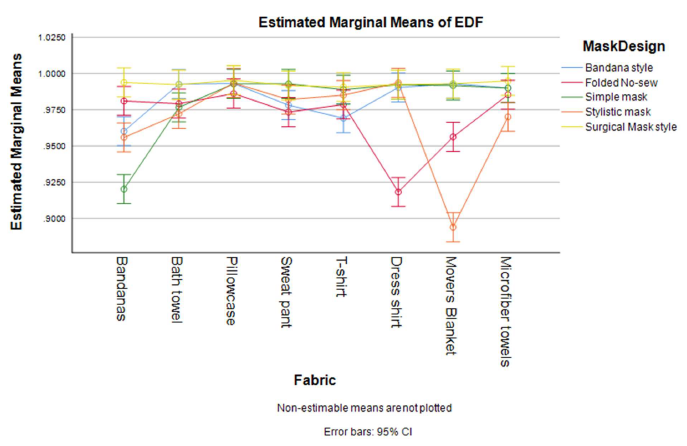
Table 2 shows descriptive summary statistics of variables with respect to the primary dispersion direction and gives some insight into the generalized responses. The best overall performing mask is the surgical style with internal non-woven layers [AUC0 to 1 min=7.721x105 (6.606x105), FEI=0.468(0.158), EDF=0.993(0.005)]. The best overall fabric depends on a desired characteristic of reduced velocity and direction or increased filtration performance; a general comparison of EDF across mask designs is shown in Figure 2. The velocity-ratio related performance, EDF, indicates the overall slowdown of particles due to turbulence and aerodynamics in an open-air system. The large standard deviations represent the divergence between the responses for each exhalation breath level (Supplementary Figures 6–13) and also indicate that the interactions between multiple factors and the multivariate responses.
Figure 2. Estimated means of EDF resulting from MANOVA, showing the combined effects of fabrics and mask design. The test condition for “no-mask” resulted in particle cloud velocities that exceeded the sampling rate of the PMS sensors and were not captured. Consequently, an EDF for “no-mask” was not calculated.
| n | Direction | Velocity of Dispersion | AUC 0to1 | FEI | EDF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fabric | Mode | % | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| **No-mask | 14 | 5 | 100.0% | -- a | -- | 1.059 x106 | 2.237 x105 | 0.092 | 0.084 | -- a | -- |
| Bandana, 100% cot- ton bandana | 10 | 1 | 100.0% | 0.216 | 0.217 | 1.846 x106 | 9.194 x105 | 0.409 | 0.167 | 0.962 | 0.045 |
| Bath towel 100% combed cotton |
10 | 3 | 83.3% | 0.170 | 0.213 | 1.314 x106 | 7.995 x105 | 0.471 | 0.166 | 0.983 | 0.013 |
| Pillowcase, 60% cot- ton, 40% polyester |
10 | 5 | 66.7% | 0.059 | 0.058 | 1.516 x106 | 8.360 x105 | 0.352 | 0.200 | 0.992 | 0.005 |
| Sweat pant, 60% cot- ton, 40% polyester | 10 | 2 | 83.3% | 0.155 | 0.178 | 1.591 x106 | 8.081 x105 | 0.469 | 0.173 | 0.984 | 0.012 |
| T-shirt, 60% cotton, 40% polyester |
10 | 1 | 100.0% | 0.122 | 0.087 | 1.839 x106 | 8.884 x105 | 0.444 | 0.109 | 0.982 | 0.012 |
| Dress shirt, 100% polyester |
10 | 5 | 100.0% | 0.100 | 0.166 | 1.298 x106 | 9.573 x105 | 0.304 | 0.151 | 0.977 | 0.049 |
| Mover blanket, 85% polyester, 15% recy- cled cotton |
10 | 5 | 100.0% | 0.138 | 0.228 | 1.601 x106 | 1.060 x106 | 0.331 | 0.099 | 0.966 | 0.066 |
| Microfiber towels, 80% polyester, 20% polyamide | 10 | 1 | 100.0% | 0.090 | 0.079 | 1.382 x106 | 8.543 x105 | 0.405 | 0.165 | 0.986 | 0.011 |
| Mask Design | |||||||||||
| **No-mask | 14 | 5 | 100.0% | -- a | -- | 1.059 x106 | 2.237 x105 | 0.092 | 0.084 | -- a | -- |
| Bandana style | 16 | 1 | 100.0% | 0.113 | 0.123 | 1.709 x106 | 9.205 x105 | 0.435 | 0.142 | 0.983 | 0.020 |
| Folded No-sew | 16 | 1 | 83.3% | 0.176 | 0.166 | 1.772 x106 | 7.754 x105 | 0.484 | 0.085 | 0.970 | 0.040 |
| Simple mask (short with earloops) | 16 | 1 | 100.0% | 0.119 | 0.159 | 1.651 x106 | 9.351 x105 | 0.263 | 0.175 | 0.981 | 0.035 |
| Stylistic mask | 16 | 1 | 100.0% | 0.208 | 0.240 | 1.839 x106 | 6.844 x105 | 0.341 | 0.127 | 0.968 | 0.050 |
| Surgical Mask style (internal non-woven) | 16 | 5 | 100.0% | 0.040 | 0.017 | 7.721 x105 | 6.606 x105 | 0.468 | 0.158 | 0.993 | 0.005 |
| Exhalation Level | |||||||||||
| Coughing | 48 | 1 | 100.0% | 0.154 | 0.158 | 1.388 x106 | 8.291 x105 | 0.353 | 0.191 | 0.988 | 0.013 |
| Talking | 46 | 5 | 66.7% | 0.104 | 0.151 | 1.567 x106 | 8.333 x105 | 0.352 | 0.186 | 0.971 | 0.042 |
The results from MANOVA on the complete data set are reported in Table 3 with all of the primary factors of fabric, mask design, and exhalation breath level resulting in an overall statistically significant effect on the dependent variables of direction, velocity, AUC0 to 1 min, FEI and EDF (Fabric: F(35, 36) = 8.526, P = <0.001, Wilks’ Λ = 0.000, Partial η2 =0.846; Mask design: F(20, 27) = 15.691, P = <0.001, Wilks’ Λ = 0.000, Partial η2 =0.876; Breath level: F(5, 8) = 371.6, P = <0.001, Wilks’ Λ = 0.004, Partial η2 =0.996). Similarly, Table 3 shows that the results using Pillai’s Trace are also significant (in case the MANOVA assumptions of homogeneity of variance-covariance were violated). Therefore, the null hypothesis that masks or face coverings have no effect on exhalation dispersion or source control is rejected.
| Effect | Value | F | df | Error df | P value |
Partial η2 |
Noncentrality Parameter | |
|---|---|---|---|---|---|---|---|---|
| Intercept | Pillai's Trace | 1.000 | 4.12x106b | 5.000 | 8.000 | <0.001 | 1.000 | 20.618x106 |
| Wilks' Lambda | 0.000 | 4.12x106b | 5.000 | 8.000 | <0.001 | 1.000 | 20.618x106 | |
| Fabric | Pillai's Trace | 3.243 | 3.164 | 35.000 | 60.000 | <0.001 | 0.649 | 110.731 |
| Wilks' Lambda | 0.000 | 8.526 | 35.000 | 36.083 | <0.001 | 0.846 | 198.845 | |
| MaskDesign | Pillai's Trace | 2.879 | 5.650 | 20.000 | 44.000 | <0.001 | 0.720 | 113.009 |
| Wilks' Lambda | 0.000 | 15.691 | 20.000 | 27.483 | <0.001 | 0.876 | 194.446 | |
| BreathLevel | Pillai's Trace | 0.996 | 371.599b | 5.000 | 8.000 | <0.001 | 0.996 | 1857.993 |
| Wilks' Lambda | 0.004 | 371.599b | 5.000 | 8.000 | <0.001 | 0.996 | 1857.993 | |
| Fabric * MaskDesign | Pillai's Trace | 4.420 | 3.269 | 140.000 | 60.000 | <0.001 | 0.884 | 457.631 |
| Wilks' Lambda | 0.000 | 6.925 | 140.000 | 44.549 | <0.001 | 0.954 | 930.323 | |
| Fabric * BreathLevel | Pillai's Trace | 3.355 | 3.497 | 35.000 | 60.000 | <0.001 | 0.671 | 122.397 |
| Wilks' Lambda | 0.000 | 7.820 | 35.000 | 36.083 | <0.001 | 0.836 | 184.155 | |
| MaskDesign * BreathLevel | Pillai's Trace | 2.857 | 5.496 | 20.000 | 44.000 | <0.001 | 0.714 | 109.923 |
| Wilks' Lambda | 0.001 | 10.703 | 20.000 | 27.483 | <0.001 | 0.835 | 139.136 | |
| Fabric* MaskDesign * BreathLevel | Pillai's Trace | 4.390 | 3.083 | 140.000 | 60.000 | <0.001 | 0.878 | 431.560 |
| Wilks' Lambda | 0.000 | 5.729 | 140.000 | 44.549 | <0.001 | 0.945 | 771.142 | |
| aDesign: Intercept + Fabric + MaskDesign + BreathLevel + Fabric * MaskDesign + Fabric * BreathLevel + MaskDesign * BreathLevel + Fabric * MaskDesign * BreathLevel; bExact statistic |
||||||||
The statistics of Wilks Lambda and Pillai’s trace (Table 3) converge at η2 = .996 and indicate that 99.6% of the variance of dependent variables are associated with exhalation breath levels of talking or coughing. It should also be noted that there were statistically significant interaction effects between fabric, mask design, breath levels and the combination of all three independent variables also reported in Table 3 (Fabric*Mask Design, Fabric*Breath level, Mask design*Breath level, Fabric*Mask design*Breath level). In some cases, the between-subject effects were marginally significant (P-value closer to .05), however the vast majority of individual between-subject effects 30 of 35 (85.7%) are significant. A full multivariate analysis of variance and multivariate tests of betweensubject effects and interactions are provided in (online Supplementary Table 2 and 3).
Discussion
Conclusively, this quantitative effectiveness study establishes that improvised non-medical grade mask designs or fabric combinations are statistically significant in reducing airborne dispersion of particles from exhalation as defined by direction, velocity, AUC0 to 1 min, FEI and EDF. The statistically significant interaction effects between combinations of all primary factors and partial η2 values further establish the strong correlation of outcomes to fabrics used, mask design, and exhalation breath levels. This foundational research offers an orthogonal but complimentary result to previous research on respiratory protection and personal protective equipment which exclusively looks at inhalation filtration and airflow pressure gradients that support respiration.
When considering airborne dispersion control (also known as source control in some literature) it is important to understand the primary mechanisms that affect the dispersion. The field of filtration theory offers significant understanding with the primary mechanisms for the respiratory use case: Interception, Brownian Diffusion, Inertial Impaction, and Sieving or blocking filtration [55-58]. Since the fibers and fabric meshes are typically larger than small aerosols or infectious particles (5μm diameters or smaller), the first three filtration mechanisms are most applicable to this study and other studies in the field of personal protective equipment (PPE). Special emphasis is placed on inertial impaction and Brownian diffusion to disrupt the velocity and direction of airborne dispersion. Further discussion on filtration mechanisms is provided in (Supplementary Appendix).
One observation from this study is that the effectiveness in dispersion control varies greatly between the specific fabrics and mask design combinations. For example, the factors of fabric, mask design, and the interaction of fabric and exhalation breath level that have significant effect on FEI, while other interactions are not significant (Supplementary Table 3). This suggests that a fabric’s dynamic characteristics such as pliability (i.e., conforms to the face for fit and coverage) and dynamic response to airflow force (i.e., stretch characteristics) have an effect on the overall filtration of exhaled particles. Ad hoc test data of a stretch fabric commercially available mask is consistent with this statement (online Supplementary Table 5). Further materials analysis and characterization is justified to fully understand this observation however it does emphasize that proper wearing of masks fully covering the mouth and nose [59-61] is important for PPE usage as well as dispersion control. Characterization of fabric thickness, fiber density and weave, as well as layering will also aid in establishing accurate predictor coefficients of a dispersion control linear regression model for specific fabrics and masks.
The strength of this study is that it offers quantitative evidence on the effectiveness of non-medical improvised masks for helping to establishing public health strategies or policies that encourage the wearing of masks or face coverings. Fundamentally the effectiveness of nonpharmaceutical interventions (NPI) can be increased by reducing exhalation particle dispersion and is especially important where infectious contaminants may exist in shared air spaces. Universal application of masks will reduce overall particle quantities and slow down the dispersion speed which gives people in the vicinity of the dispersion (from coughing or talking) time to react and avoid inhalation exposure to potentially infectious particle clouds. The slowing down of a particle cloud’s dispersion also means that social distances will be more effective considering the relationship of velocity, time, and distance. This concept is well aligned with typical hazardous materials and bio-chem emergency response considerations of “time-distance-shielding” [62-64] where masks provide shielding during inhalation, and also slows exhalation particles to give others time and distance to avoid the particle cloud.
An overall public health strategy must consider the additive effect of wearing masks and face coverings for inhalation filtration (PPE) and that of dispersion and source control. However, the strategy would also need to account for the non-ideal performance of various fabrics and masks, where the ideal performance would offer 95% filtration efficiencies and dispersion contained to the user’s body. Combining this research with recent community SIR modeling [65] can help provide significant insights to the public health strategy. To summarize, it would be of most benefit for all people in community settings to wear masks and get full effect of controlling exhalation particle dispersion to reduce transmission of highly infectious respiratory diseases such as COVID-19.
Limitations
One limitation of this study is that it provides approximations of human exhalation using polydisperse NaCl solution rather than human exhalation which adds additional compositions of particles that can be smaller than 0.3μm in diameters, moisture, proteins, gases, and other bio material [66]. The effectiveness of masks for source or dispersion control from long term use (such as during a 4 hour or 8 hour workdays) cannot be directly established from this data, however this study utilizes industry and NIOSH accepted proxy for testing respiratory barriers of NaCl. Another limitation was the PMS sensor performance: measurement minimum of 0.3μm particles and a slower intake fan speed at 0.1 CFM limited its ability to accurately measure all characteristics of fast-moving aerosol clouds from that of no-mask applied. Regardless, the sensor data and experiment design were sufficient to determine statistical conclusions on the effects of wearing masks and face coverings of different fabrics and designs. Future works should consider using a large test chamber and more sensors to result in more accurate measurement of airborne dispersion and turbulent airflows.
Conclusions
The results show that the application of various nonmedical grade mask designs or fabric combinations were statistically significant in reducing airborne dispersion of particles from exhalation during coughing and talking as well as singing. However, the effectiveness varies greatly between the specific fabrics and mask designs used. The best overall performing mask design is a surgical style with internal non-woven layers, while the best overall fabric depends on a desired characteristic of reduced velocity, change in direction, or increased filtration performance.
Conclusively this study can aid in establishing public health strategies or policies that encourage the wearing of masks or face coverings to increase the effectiveness of non-pharmaceutical interventions (NPI) especially where infectious contaminants may exist in shared air spaces.
Author Contributions
NE was the principal investigator and primary author, RW created the design of experiment and conducted the mathematical analysis, RP conducted background research and guided the approach to experimentation and rigor of analysis, MG assisted in the laboratory configuration and testing.
Acknowledgment
We extend our appreciation to The American Red Cross of Southeastern Colorado and El Paso County Public Health for allowing the use of CPR training equipment and manikin to rapidly conduct this study. We also acknowledge Mr. Asher Edwards for his contributions to the early code development on the data acquisition system, and Ms. Lydia Edwards for providing continuous awareness of open news research and public vetting of concepts.
Data Availability Statement
The datasets generated from this study are available to U.S. national, state and regional agencies as well as healthcare systems on request to the corresponding author.
Conflict 0f Interest Disclosures
The authors have no conflicts of interest to report.
Funding/Support
This work was supported by independent research and development funding provided by the author’s organization, The MITRE Corporation. ©2020 The MITRE Corporation. Approved for Public Release, Distribution Unlimited. Public Release Case Number 20-1583.
References
2. Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathogens. 2013 Mar 7;9(3):e1003205.
3. Leung NH, Chu DK, Shiu EY, Chan KH, McDevitt JJ, Hau BJ, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine. 2020 May;26(5):676-80.
4. Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Scientific Reports. 2019 Feb 20;9(1):1-0.
5. Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proceedings of the National Academy of Sciences. 2020 Jun 2;117(22):11875-7.
6. Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. Journal of Aerosol Medicine. 1997;10(2):105-16.
7. Morawska LJ, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, Chao CY, Li Y, Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science. 2009 Mar 1;40(3):256-69.
8. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. Jama. 2020 May 12;323(18):1837-8.
9. Pan M, Lednicky JA, Wu CY. Collection, particle sizing and detection of airborne viruses. Journal of Applied Microbiology. 2019 Dec;127(6):1596-611.
10. Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet. 2020 Jun 1.
11. Atkinson J. Natural Ventilation for Infection Control in Health-Care Settings, Geneva: World Health Organization.
12. Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infectious Diseases. 2019 Dec 1;19(1):101.
13. MacIntyre CR, Cauchemez S, Dwyer DE, Seale H, Cheung P, Browne G, et al. Face mask use and control of respiratory virus transmission in households. Emerging Infectious Diseases. 2009 Feb;15(2):233.
14. Mukerji S, MacIntyre CR, Newall AT. Review of economic evaluations of mask and respirator use for protection against respiratory infection transmission. BMC Infectious Diseases. 2015 Dec 1;15(1):413.
15. National Academies of Sciences Engineering and Medicine. Rapid expert consultation on the effectiveness of fabric masks for the COVID-19 pandemic. Washington, DC. April. 2020 Apr 8;8:2020.
16. Aiello AE, Murray GF, Perez V, Coulborn RM, Davis BM, Uddin M, et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. The Journal of Infectious Diseases. 2010 Feb 15;201(4):491-8.
17. Agah R, Cherry JD, Garakian AJ, Chapin M. Respiratory syncytial virus (RSV) infection rate in personnel caring for children with RSV infections: routine isolation procedure vs routine procedure supplemented by use of masks and goggles. American Journal of Diseases of Children. 1987 Jun 1;141(6):695-7.
18. Relman E. Trump shares tweet that says masks represent “slavery and social death” - Business Insider. 2020. https://www.businessinsider.com/trump-sharestweet- that-says-masks-represent-slavery-and-socialdeath- 2020-5.
19. McCann M. Mandatory Masks Aren’t About Safety, They’re About Social Control. 2020. https://thefederalist. com/2020/05/27/mandatory-masks-arent-about-safetytheyre- about-social-control/.
20. Chuck E, Einhorn E, Gamboa S, Hassan A, Kingkade T, Smith S. The mask wearing debate is dividing America. And the messaging isn’t getting any clearer. MSN. 2020. https://www.msn.com/en-us/news/us/the-maskwearing- debate-is-dividing-america-and-the-messagingisnt- getting-any-clearer/ar-BB15BxDd.
21. Brooks JT, Butler JC, Redfield RR. Universal masking to prevent SARS-CoV-2 transmission—the time is now. Jama. 2020 Jul 14.
22. Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. Jama. 2020 Aug 18;324(7):703-4.
23. Fisher KA, Barile JP, Guerin RJ, Esschert KL, Jeffers A, Tian LH, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020.
24. Mills M, Rahal C, Akimova E. Face masks and coverings for the general public: behavioural knowledge, effectiveness of cloth coverings and public messaging [Internet]. The Royal Society & The British Academy; 2020.
25. Hendrix MJ. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy—Springfield, Missouri, May 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69.
26. Mueller AV, Fernandez LA. Assessment of Fabric Masks as Alternatives to Standard Surgical Masks in Terms of Particle Filtration Efficiency. MedRxiv. 2020 Jan 1.
27. Gobi N, Evangelin S, Kasthuri R. Multilayer nonwoven fabrics for filtration of micron and submicron particles. Journal of Textile Engineering & Fashion Technology. 2019;5(2):81-4.
28. Rengasamy S, Eimer B, Shaffer RE. Simple respiratory protection—evaluation of the filtration performance of cloth masks and common fabric materials against 20– 1000 nm size particles. Annals of Occupational Hygiene. 2010 Oct 1;54(7):789-98.
29. Konda A, Prakash A, Moss GA, Schmoldt M, Grant GD, Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020 Apr 24;14(5):6339-47.
30. Shakya KM, Noyes A, Kallin R, Peltier RE. Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. Journal of Exposure Science & Environmental Epidemiology. 2017 May;27(3):352-7.
31. Michigan Mask Response. DIY Mask FAQ - Test Results. DIY Mask FAQ. https://www.maskfaq.com/testresults.
32. Zangmeister CD, Radney JG, Vicenzi EP, Weaver JL. Filtration Efficiencies of Nanoscale Aerosol by Cloth Mask Materials Used to Slow the Spread of SARS-CoV-2. ACS Nano. 2020;14:9188-200.
33. Lustig S, Biswakarma JJ, Rana D, Tilford SH, Hu W, Su M, Rosenblatt MS. Effectiveness of Common Fabrics to Block Aqueous Aerosols of Virus-like Nanoparticles. ACS nano. 2020 May 21.
34. Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Physics of Fluids. 2020 Jun 1;32(6):061708.
35. Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerging Infectious Diseases. 2020 Jul;26(7):1628.
36. Dietz L, Horve PF, Coil DA, Fretz M, Eisen JA, Van Den Wymelenberg K. novel coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems 5: e00245-20.
37. Blocken B, Malizia F, van Druenen T, Marchal T. Towards aerodynamically equivalent COVID19 1.5 m social distancing for walking and running. Questions and Answers. Website Bert Blocken, Eindhoven University of Technology (The Netherlands) and KU Leuven (Belgium). Disponibile su: http://www. urbanphysics. net/COVID19. html (ultimo accesso 21 aprile 2020). 2020.
38. Grush L. How Sneeze Particles Travel Inside an Airplane. Popular Science. 2014. https://www.popsci. com/article/science/how-sneeze-particles-travel-insideairplane/.
39. Gröndahl M, Goldbaum C, White J. What Happens to Viral Particles on the Subway. The New York Times. 2020 Aug 10. https://www.nytimes.com/interactive/2020/08/10/nyregion/nyc-subwaycoronavirus. html.
40. Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic?. Disaster medicine and public health preparedness. 2013 Aug;7(4):413-8.
41. Patel RB, Skaria SD, Mansour MM, Smaldone GC. Respiratory source control using a surgical mask: an in vitro study. Journal of Occupational and Environmental Hygiene. 2016 Jul 2;13(7):569-76.
42. Abd-Elsayed A, Karri J. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesthesia and Analgesia. 2020 Apr 20.
43. Eikenberry SE, Mancuso M, Iboi E, Phan T, Eikenberry K, Kuang Y, et al. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infectious Disease Modelling. 2020 Apr 21.
44. Lindsley WG, King WP, Thewlis RE, Reynolds JS, Panday K, Cao G, et al. Dispersion and exposure to a coughgenerated aerosol in a simulated medical examination room. Journal of Occupational and Environmental Hygiene. 2012 Dec 1;9(12):681-90.
45. Lindsley WG, Reynolds JS, Szalajda JV, Noti JD, Beezhold DH. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol Science and Technology. 2013 Aug 1;47(8):937- 44.
46. Hui DS, Chow BK, Chu L, Ng SS, Lee N, Gin T, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. Plos One. 2012 Dec 5;7(12):e50845.
47. Rothenberg M, Miller D, Molitor R, Leffingwell D. The control of air flow during loud soprano singing. Journal of Voice. 1987 Jan 1;1(3):262-8.
48. NIOSH. Procedure no. TEB-APR-STP-0059, revision 2.0. Determination of particulate filter efficiency level for N95 series filters against solid particulates for nonpowered, air purifying respirators standard testing procedure (STP).
49. Ower E, Pankhurst RC. The measurement of air flow. Elsevier; 2014 May 9.
50. Warne RT. A Primer on Multivariate Analysis of Variance (MANOVA) for Behavioral Scientists. Practical Assessment, Research & Evaluation. 2014 Nov 1;19.
51. Ito PK. 7 robustness of anova and manova test procedures. Handbook of Statistics. 1980 Jan 1;1:199-236.
52. Tinsley HE, Brown SD, editors. Handbook of applied multivariate statistics and mathematical modeling. Academic press; 2000 May 22.
53. Tinsley HE, Brown SD, editors. Handbook of applied multivariate statistics and mathematical modeling. Academic press; 2000 May 22.
54. Crichton N. Information point: Wilks’ lambda. Journal of clinical nursing. 2000 May;9(3):381-.
55. Schmidt EW, Gieseke JA, Gelfand P, Lugar TW, Furlong DA. Filtration theory for granular beds. Journal of the Air Pollution Control Association. 1978 Feb 1;28(2):143-6.
56. Ranz WE, Katz EJ. Determining Impaction Efficiencies of Mist Collection Equipment. Journal of the Air Pollution Control Association. 1959 Feb 1;8(4):328-32.
57. Ripperger S, Gösele W, Alt C, Loewe T. Filtration, 1. fundamentals. Ullmann’s Encyclopedia of Industrial Chemistry. 2000 Jun 15:1-38.
58. Sipes J. Basics of Air Filtration. Price Industries. https://www.priceindustries.com/content/uploads/ assets/literature/technical-papers/basics-of-air-filtration. pdf.
59. Desai AN, Aronoff DM. Masks and coronavirus disease 2019 (covid-19). Jama. 2020 Apr 17.
60. CDC. Use of Cloth Face Coverings to Help Slow the Spread of COVID-19. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/ coronavirus/2019-ncov/prevent-getting-sick/diy-clothface- coverings.html.
61. CDC. Use of Cloth Face Coverings to Help Slow the Spread of COVID-19. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/ coronavirus/2019-ncov/prevent-getting-sick/diy-clothface- coverings.html.
62. Robert A. Time, distance, shielding: Minimizing radiation exposure. FireRescue1. 2019. https://www. firerescue1.com/fire-products/hazmat-equipment/ articles/time-distance-shielding-minimizing-radiationexposure- yUjpcxrDyYFdibTa/.
63. U.S. Department of Health and Human Services. Chemical Hazards Emergency Medical Management - Quick Response Guide. 2020. https://chemm.nlm.nih.gov/quickresponseguide.htm. Accessed 12 Aug 2020.
64. U.S. Department of Health and Human Services. The Golden First Minutes — Initial Response to a Chemical Hazardous Materials Incident - CHEMM. 2020. https:// chemm.nlm.nih.gov/detailedinfo.htm.
65. Stutt RO, Retkute R, Bradley M, Gilligan CA, Colvin J. A modelling framework to assess the likely effectiveness of facemasks in combination with ‘lock-down’in managing the COVID-19 pandemic. Proceedings of the Royal Society A. 2020 Jun 24;476(2238):20200376
66. Popov TA. Human exhaled breath analysis. Annals of Allergy, Asthma & Immunology. 2011 Jun 1;106(6):451-6.