Abstract
As an extension to our recently published research work in Asian Journal of Pharmaceutical and Clinical Research, entitled “Β-Sitosterol: Isolation from Muntingia Calabura Linn. Bark Extract, Structural Elucidation, and Molecular Docking Studies as Potential Inhibitor of SARS-CoV-2 Mpro (COVID-19)”, we have investigated the role of β-sitosterol as immunostimulant, antioxidant and inhibitory potential against Receptor Binding Domain (RBD) of SARS-CoV-2 Spike Glycoprotein with the aid of molecular docking. There are many studies which reveals the antioxidant and immune boosting role of β-sitosterol especially in viral infection including pneumoniae. This commentary emphasis on further potential of β-sitosterol in treatment of COVID-19 through molecular docking studies. We have targeted RBD of spike glycoprotein and performed molecular docking studies of β-sitosterol to find out its inhibitory potential of SARS-CoV-2. β-sitosterol have showed binding affinity - 7.8 kcal/mol with 0 RMSD lower and upper bound. It formed one hydrogen bond with Ala-B:419 with bond length of 2.16A0. β-sitosterol has formed five alkyl bonds with Pro-C:384 (5.0A0, 4.66A0, 5.23A0, 4.27A0) and with Lys-C:378 (4.66A0). From present commentary, we have concluded that β-sitosterol can be used to enhance immunity against the SARS-CoV-2 infection as well as to restrict the viral invasion into the host cell through angiotensin converting enzyme-2 (ACE-2) by inhibiting spike glycoprotein. If we can increase the dietary intake of β-sitosterol and other phytosterols it can modulate the immunity which is todays need to face COVID-19.
Keywords
β-sitosterol, SARS-CoV-2 spike glycoprotein, Molecular docking, 6VSB
Introduction
This article is an extension to our recently published article in Asian Journal of Pharmaceutical and Clinical Research, entitled “Β-Sitosterol: Isolation from Muntingia Calabura Linn. Bark Extract, Structural Elucidation, and Molecular Docking Studies as Potential Inhibitor of SARSCoV- 2 Mpro (COVID-19)”[1]. The article describes detailed procedure for the isolation (by Column Chromatography) and structural characterization (by FTIR, UV-Visible Spectroscopy and HPTLC) of β-sitosterol from Muntingia Calabura bark. The β-sitosterol was docked on SARSCoV- 2 Mpro to study the binding affinity (kcal/mol) in comparison with favipiravir. It has been found that favipiravir has a less binding affinity, i.e. 5.7 kcal/mol than β-sitosterol which has 6.9 kcal/mol. The number of hydrogen bonds formed by the favipiravir is much more, i.e., 4 than β-sitosterol which formed only 01 hydrogen bond with SARS-CoV-2 Mpro.
As an extension to this published research work, we have investigated the role of β-sitosterol as immunostimulant, antioxidant and inhibitory potential against receptor binding domain (RBD) of SARS-CoV-2 Spike Glycoprotein with the aid of molecular docking. There are many studies which reveals the antioxidant and immune boosting role of β-sitosterol especially in viral infection including pneumoniae. This commentary emphasized on further potential of β-sitosterol in treatment of COVID-19 through molecular docking studies.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel, zoonotic, positive-sense [2], single-stranded RNA beta-coronavirus [3] (subgenus Sarbecovirus, sub-family Orthocoronaviridae) [4]. The disease caused due to SARS-CoV-2 is termed as COVID-19 [5]. Almost every country of the world is now affected by SARS-CoV-2 infection. The World Health Organization (WHO) declared it a Public Health Emergency of International Concern on January 30, 2020, and on February 28, it upraised the worldwide threat of COVID-19 to the utmost level [6]. A global pandemic was declared on March 11, 2020 [7]. The N, E, M, and S proteins are the four structural proteins encoded by SARSCoV- 2 [8]. This S protein of SARS-CoV-2 i.e. SARS-CoV-2 spike glycoprotein causes invasion to the host cell after binding with angiotensin converting enzyme-2 (ACE-2) [9]. The SARS-CoV-2 spike glycoprotein is cleaved into two subunits during entry [10]. The S1 subunit contains a receptor binding domain (RBD) and attaches to ACE-2 [11]. The S2 subunit then facilitates membrane fusion [12-14]. Therefore, we have targeted RBD of spike glycoprotein and performed molecular docking studies of β-sitosterol to find out its inhibitory potential of SARS-CoV-2.
β-sitosterol as Immunostimulant, as Antiviral and as Antioxidant
Cheng et al. investigated the effects of dietary β-sitosterol at different levels on serum lipid levels, immune function, oxidative status, and intestinal morphology in broilers. They have concluded that dietary β-sitosterol supplementation could regulate serum cholesterol level, promote immune function, and improve intestinal oxidative status and morphology in broilers [15]. Fraile et al. reported that β-sitosterol can be considered an immunomodulator in pigs [16]. Bouic and Lamprecht reported that this phytosterol complex seems to target specific T-helper lymphocytes, the Th1 and Th2 cells, helping normalize their functioning and resulting in improved T-lymphocyte and natural killer cell activity. The re-establishment of these immune parameters may be of help in numerous disease processes relating to chronic immune-mediated abnormalities, including chronic viral infections, tuberculosis, rheumatoid arthritis, allergies, cancer, and autoimmune diseases [17]. Bouic et al. concluded that phytosterols could be used to prevent the subtle immunosuppression associated with excessive physical stress [18]. There are many studies which have reported the immunostimulant activity of the phytosterols [19-24].
Li et al. reported that β-sitosterol is a candidate for the development of anti-virulence agents against pathogens that rely on cholesterol-dependent toxins for successful infections [25]. Zhou et al. reported that β-sitosterol blocks the immune response mediated by RIG-I signaling and deleterious IFN production, providing a potential benefit for the treatment of influenza [26]. Parvez et. al. reported the antioxidative and hepatoprotective efficacy of of G. senegalensis leaves extract. HPTLC analysis of β-amyrin, β-sitosterol, lupeol and ursolic acid strongly supported the anti-HBV efficacy of GSLE via abating the cellular oxidative stress molecules [27]. There are many studies that have reported the antioxidant activity of β-sitosterol [27-34].
Molecular Docking Studies of β-sitosterol with RBD of SARS-CoV-2 Spike Glycoprotein
Autodock vina 1.1.2 in PyRx-Virtual Screening Tool 0.8 were used to perform the docking studies [35]. The active amino acid residues in the protein were identified and noted using BIOVIA Discovery Studio Visualizer (version-19.1.0.18287) [36]. The complete docking procedure along with ligand preparation and target preparation have been performed as described in the reference article [1]. The recently elucidated structure pre-fusion 2019-nCoV (SARS-CoV-2) spike glycoprotein with a single receptor-binding domain up was obtained from the RCSB Protein Data Bank (PDB ID: 6VSB) which was released on 26 February 2020 (https://www.rcsb. org/structure/6VSB) [37]. RBD from SARS-CoV-2 spike glycoprotein was identified from the official website of Protein Data Bank in Europe (EMBL-EBI) (https:// www.ebi.ac.uk/pdbe/entry/pdb/6vsb). There were three sequence domains in the 6VSB crystal structure; Spike receptor binding domain; Spike glycoprotein N-terminal domain; Coronavirus spike glycoprotein S1, C-terminal, along with three chains in the structure (Chain A, B, C). For molecular docking simulation, the three-dimensional grid box (size_x = 76.1623A°; size_y = 84.3011A°; size_z = 62.4413A°) was designed (to define area for interactions) around the RBD (to occupy) using Autodock tool 1.5.6 with exhaustiveness value of 8 [35,36].
Results and Discussion
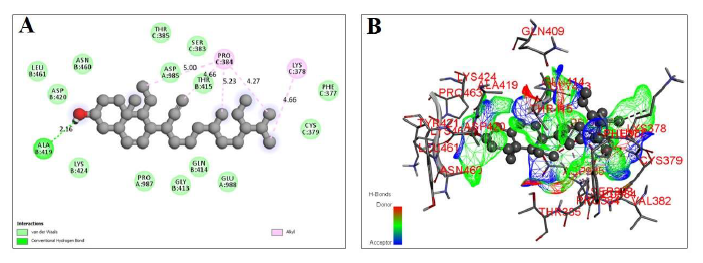
RBD comprises of amino acid residues from chain A, chain B, and chain C as well, therefore, the most potent inhibitor will be the one which interacts with amino acid residues from all the chains. β-sitosterol has showed a binding affinity of 7.8 kcal/mol with 0 RMSD lower and upper bound. The 2D- and 3D-Docking poses of the β-sitosterol represented in figure 1A & 1B respectively. It formed one hydrogen bond with Ala-B:419 with bond length of 2.16A0. β-sitosterol has formed five alkyl bonds with Pro-C:384 (5.0A°, 4.66A°, 5.23A°, 4.27A°) and with Lys-C:378 (4.66A°). It also shows van der Waals attraction with Thr-C:385, Ser-C:383, Asp-A:985, Thr-B:415, Phe-C:377, Cys-C:379, Glu-A:988, Gln-B:414, Gly-B:413, Pro-A:987, Lys-B:424, Asp-B:420, Leu-B:461, Asn-B:460. As β-sitosterol is interacting with amino acids from every chain (A, B, C), it indicates that it is a potent inhibitor of RBD of SARS-CoV-2 spike glycoprotein. The interacting residues, bond length, and binding affinity are represented in table 1.
| Name of Molecule |
Binding Affinity (kcal/mol) |
Types of Bond | Active Amino Residues with Bond Length (A°) |
|---|---|---|---|
| β-sitosterol | -7.8 | Hydrogen Bond | Ala-B:419 (2.16A°) |
| Alkyl Bond | Pro-C:384 (5.0A°, 4.66A°, 5.23A0, 4.27A°), Lys-C:378 (4.66A°) | ||
| Van Der Waals | Thr-C:385, Ser-C:383, Asp-A:985, Thr-B:415, Phe-C:377, Cys-C:379, Glu-A:988, Gln-B:414, Gly-B:413, Pro-A:987, Lys-B:424, Asp-B:420, Leu-B:461, Asn-B:460 |
Conclusion
Currently, there is no specific treatment available for SARS-CoV-2 infection. Use of immunostimulants, antivirals, and antioxidants can help to reduce the risk of COVID-19. Literature supports the immunostimulant, antiviral, and antioxidant activity of the β-sitosterol.
Also, molecular docking studies have found very good binding affinity of β-sitosterol with RBD of SARS-CoV-2 spike glycoprotein which can restrict the viral invasion into the host cell. It has formed one hydrogen bond with Ala-B:419, which is good for better inhibition. From present commentary, we have concluded that β-sitosterol can be used to enhance immunity against the SARS-CoV-2 infection as well as to restrict the viral invasion into the host cell through angiotensin converting enzyme-2 (ACE- 2) by inhibiting spike glycoprotein. If we can increase the dietary intake of β-sitosterol and other phytosterols it can modulate the immunity which is todays need to face COVID-19.
Conflict of Interest
The authors have no conflicts of interest.
References
1. Chaudhari RN, Khan SL, Chaudhary RS, Jain SP, Sidduqui FA. ?-SITOSTEROL: ISOLATION FROM MUNTINGIA CALABURA LINN BARK EXTRACT, STRUCTURAL ELUCIDATION AND MOLECULAR DOCKING STUDIES AS POTENTIAL INHIBITOR OF SARS-CoV-2 Mpro (COVID-19). Asian Journal of Pharmaceutical and Clinical Research. 2020 May;13(5):204-9.
2. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 May;581(7807):215-20.
3. Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020 Apr 9;181(4):894–904.e9.
4. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of covid-19. Viruses. 2020 Apr;12(4):372.
5. Singhal T. A review of coronavirus disease-2019 (COVID-19). The Indian Journal of Pediatrics. 2020 Apr;87(4):281-286.
6. Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research. 2020 Mar 13;7(1):11.
7. Kumar D, Malviya R, Sharma PK. Corona virus: a review of COVID-19. Eurasian Journal of Medicine and Oncology. 2020;4:8-25.
8. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). Vol. 251, Journal of Pathology. 2020 May 17;251(3):228-48.
9. Musarrat F, Chouljenko V, Dahal A, Nabi R, Chouljenko T, Jois SD, et al. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. Journal of Medical Virology. 2020 May 6.
10. Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell research. 2020 Apr;30(4):343-55.
11. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020 Mar 9;181(2):281–292.e6.
12. Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020 May 04;369(6501):330-3.
13. Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proceedings of the National Academy of Sciences. 2004 Mar 23;101(12):4240- 5.
14. Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020 May;583(7815):290–5.
15. Cheng Y, Chen Y, Li J, Qu H, Zhao Y, Wen C, et al. Dietary ?-sitosterol regulates serum lipid level and improves immune function, antioxidant status, and intestinal morphology in broilers. Poultry Science. 2020 Mar 1;99(3):1400-8.
16. Fraile L, Crisci E, Córdoba L, Navarro MA, Osada J, Montoya M. Immunomodulatory properties of betasitosterol in pig immune responses. International Immunopharmacology. 2012 Jul 1;13(3):316-21.
17. Bouic PJD, Lamprecht JH. Plant sterols and sterolins: A review of their immune-modulating properties. Altern Med Rev. 1999 Jun 1;4(3):170-7.
18. Bouic PJD, Clark A, Lamprecht J, Freestone M, Pool EJ, Liebenberg RW, et al. The effects of B-sitosterol (BSS) and B-sitosterol glucoside (BSSG) mixture on selected immune parameters of marathon runners: Inhibition of post marathon immune suppression and inflammation. International Journal of Sports Medicine. 1999 May;20(04):258-62.
19. Park YJ, Bang IJ, Jeong MH, Kim HR, Lee DE, Kwak JH, et al. Effects of ?-Sitosterol from Corn Silk on TGF- ?1-Induced Epithelial-Mesenchymal Transition in Lung Alveolar Epithelial Cells. Journal of agricultural and food chemistry. 2019 Aug 2;67(35):9789-95.
20. Boukes GJ, Van de Venter M. In vitro modulation of the innate immune response and phagocytosis by three Hypoxis spp. and their phytosterols. South African Journal of Botany. 2016 Jan 1;102:120-6.
21. Liu R, Hao D, Xu W, Li J, Li X, Shen D, et al. ?-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharmaceutical Biology. 2019 Jan 1;57(1):161-8.
22. Ju YH, Clausen LM, Allred KF, Almada AL, Helferich WG. ?-Sitosterol, ?-Sitosterol Glucoside, and a Mixture of ?-Sitosterol and ?-Sitosterol Glucoside Modulate the Growth of Estrogen-Responsive Breast Cancer Cells in Vitro and in Ovariectomized Athymic Mice. The Journal of Nutrition. 2004 May;134(5):1145-51.
23. Bouic PJ. Sterols and sterolins: new drugs for the immune system?. Drug Discovery Today. 2002 Jul 1;7(14):775-8.
24. Bouic PJD. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Current Opinion in Clinical Nutrition & Metabolic Care. 2001 Nov 1;4(6):471-5.
25. Li H, Zhao X, Wang J, Dong Y, Meng S, Li R, et al. ?-sitosterol interacts with pneumolysin to prevent Streptococcus pneumoniae infection. Scientific Reports. 2015 Dec 3;5(1):1-9.
26. Zhou BX, Li J, Liang XL, Pan XP, Hao YB, Xie PF, et al. ?-sitosterol ameliorates influenza A virus-induced proinflammatory response and acute lung injury in mice by disrupting the cross-talk between RIG-I and IFN/STAT signaling. Acta Pharmacologica Sinica. 2020 Jun 5:1-9.
27. Parvez MK, Alam P, Arbab AH, Al-Dosari MS, Alhowiriny TA, Alqasoumi SI. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm J. 2018;26(5):685–93.
28. Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS. Antidiabetic and antioxidant potential of ?-sitosterol in streptozotocin-induced experimental hyperglycemia. Journal of Diabetes. 2011 Mar;3(1):29-37.
29. Vivancos M, Moreno JJ. ?-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Biology and Medicine. 2005 Jul 1;39(1):91-7.
30. Baskar AA, Al Numair KS, Gabriel Paulraj M, Alsaif MA, Muamar MA, Ignacimuthu S. ?-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1, 2-dimethylhydrazineinduced colon cancer. Journal of Medicinal Food. 2012 Apr 1;15(4):335-43.
31. Ambavade SD, Misar AV, Ambavade PD. Pharmacological, nutritional, and analytical aspects of ?-sitosterol: A review. Oriental Pharmacy and Experimental Medicine. 2014 Sep 1;14(3):193-211.
32. Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. ?-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Molecular Nutrition & Food Research. 2010 Apr;54(4):551-8.
33. López-Cervantes J, Sánchez-Machado DI, Cruz-Flores P, Mariscal-Domínguez MF, Servín de la Mora-López G, Campas-Baypoli ON. Antioxidant capacity, proximate composition, and lipid constituents of Aloe vera flowers. Journal of Applied Research on Medicinal and Aromatic Plants. 2018 Sep 1;10:93-8.
34. Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. Journal of Agricultural and food Chemistry. 2001 Jun 18;49(6):2774-9.
35. Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods in Molecular Biology. 2015;1263(1263):243-50.
36. Dassault Systèmes. Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment. 2017; Available at: https://www.3dsbiovia.com/about/citations-references/
37. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 Mar 13;367(6483):1260-3.