Abstract
Hair follicle stem cells (HFSCs) are recognized as multipotential stem cells with exceptional proliferative capacity. Their regulatory effect on skin homeostasis is orchestrated through intricate signaling pathways, including Wnt/β-catenin, transforming growth factor-β/bone morphogenetic protein (TGFβ/BMP), Notch, and Hedgehog. Concurrently, HFSCs inhabit the bulge region of the outer root sheath of the hair follicle (HF), serving as the epicenter for skin-organizing signaling during homeostasis, engaging in dynamic interactions with other functionally specialized niche cells. This comprehensive review delineates the nuanced interplay of both cell-intrinsic and cell-extrinsic mechanisms governing HFSCs within the skin. The aspiration is that this synthesis of knowledge will contribute meaningfully to the theoretical foundations underpinning further investigations into skin disorders.
Keywords
HFSCs, Wnt/β-catenin, Skin homeostasis, LLLT
Introduction
HFSCs represent a distinct category of stem cells localized within HF, characterized by unique biological attributes and functionalities primarily implicated in the growth, development, and regeneration of hair. Notably, HFSCs are not only pivotal for fostering hair growth but also play a crucial role in the reparative processes of skin wounds [1-3]. Substantial evidence supports the assertion that HFSCs assume a central position within multiple skin components surrounding tissue HF thereby functioning as key tissue centers during adult skin homeostasis. Diverse signaling pathways, including Wnt/β-catenin, TGF-β/BMP, Notch, among others, intricately participate in the regulatory milieu governing the activities of HFSCs. These signaling pathways exert a profound impact on the self-renewal, differentiation, and cellular fate determinations of HFSCs.
HFSCs are strategically positioned within specific zones of the HF, notably within the coat sheath (outer root sheath) and the hair papilla (hair bulb) [4]. These anatomical regions are deemed critical for the viability and functionality of HFSCs. Possessing the remarkable capability for self-renewal and differentiation into diverse cell lineages, HFSCs emerge as pivotal contributors to the intricate orchestration of hair growth, instigating the generation of new hair cells that propel the continuous cycle of hair growth and renewal. Within the hair growth cycle, HFSCs actively partake in the regulation of distinct phases, encompassing the growth period (Anagen), regression period (Catagen), and rest period (Telogen) [5]. Notably, during the growth phase, HFSCs undergo active differentiation, instigating the robust growth of hair.
The skin, being the largest organ in the human body, undergoes meticulous regulation of homeostasis orchestrated by its diverse niches. Among these, HFSCs emerge as a crucial subset of stem cells, playing a pivotal role in sustaining skin homeostasis through intricate interactions with various ecological niches, including vasculature, nerves, and the extracellular matrix (ECM). In our discussion today, we delve into the signaling pathways associated with HFSCs and explore the dynamic crosstalk between HFSCs and other niches, aiming to deepen our understanding of the multifaceted functions of HFSCs within the intricate landscape of the skin.
Signaling Pathways
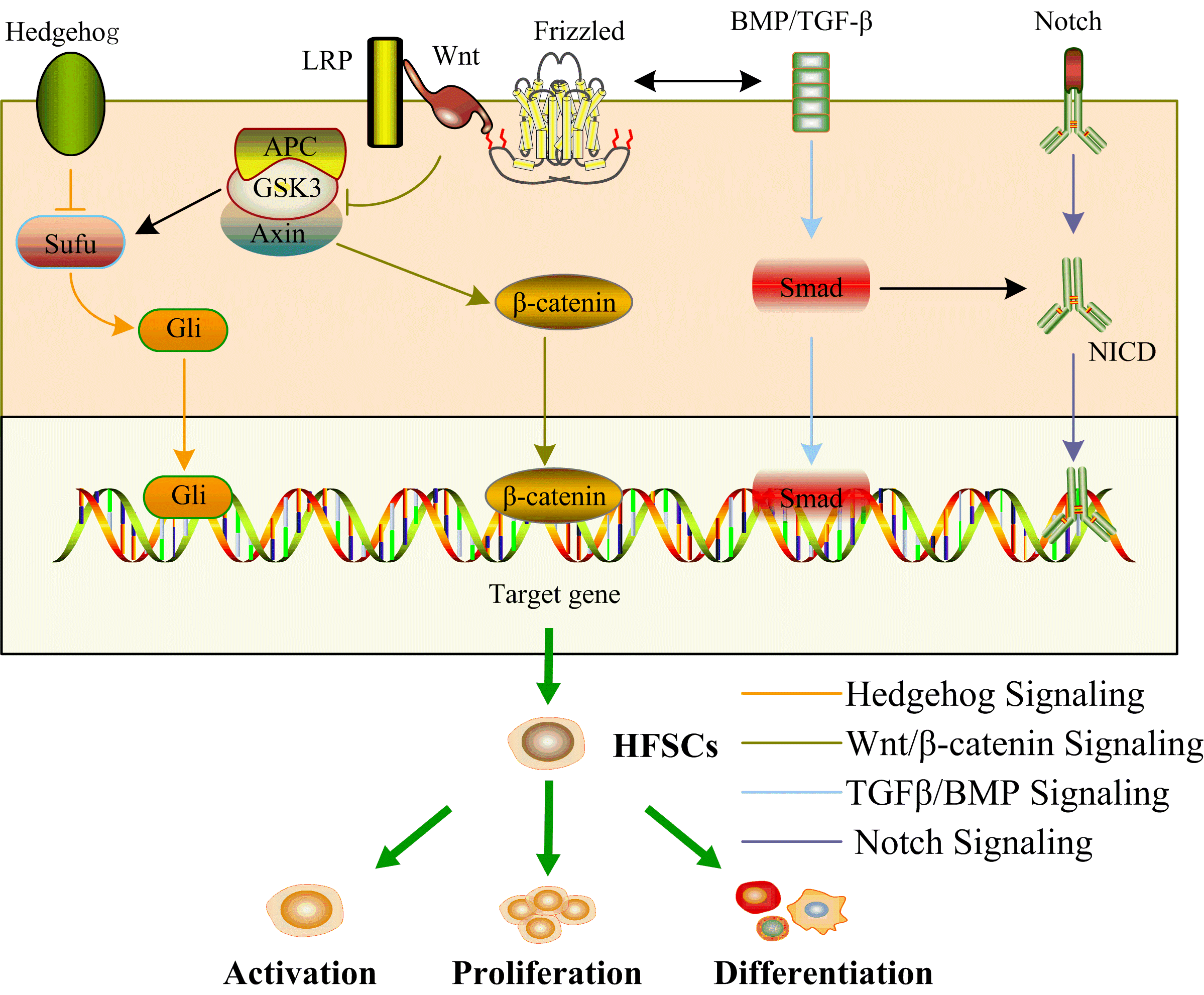
Epidermal homeostasis is intricately governed by the regulatory influence of HFSCs within the skin. This regulatory role is contingent upon a sophisticated network characterized by the interplay of multiple signaling pathways, including but not limited to the Wnt/β-catenin, TGFβ/BMP, Notch, and Hedgehog pathways, as illustrated in Figure 1.
Figure 1. Signaling pathways network of HFSCs. Wnt/β-catenin, TGFβ/BMP, Notch, and Hedgehog signaling pathways form a signaling network to jointly activate HFSCs, promoting their proliferation and differentiation. The black arrowheads show the interaction of signaling pathways.
The Wnt/β-catenin signaling pathway constitutes a pivotal cell signaling cascade, assuming a critical role in diverse biological processes encompassing embryonic development, the preservation of tissue homeostasis, and the determination of cellular fate [6]. Wnt proteins, a category of secreted signaling molecules, engage Frizzled (Fz) receptors on the cellular membrane, instigating downstream signaling events. In the absence of Wnt ligands, a destruction complex comprising Axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3β (GSK-3β) phosphorylates β-catenin, marking it for subsequent degradation by the proteasome. This mechanism maintains a basal intracellular β-catenin level. Upon Wnt ligand binding to its receptor, inhibition of the destruction complex occurs, preventing β-catenin phosphorylation and degradation. Consequently, β-catenin accumulates in the cytoplasm, translocating to the nucleus. Within the nucleus, β-catenin associates with T cell factor/lymphocyte enhancer, thereby activating target genes. The target genes modulated by β-catenin, subsequent to its nuclear translocation, participate in the orchestration of various cellular processes, encompassing but not limited to cell proliferation, differentiation, and survival [7]. A significant interrelationship is evident between the Wnt/β-catenin signaling pathway and HFSCs, exercising a crucial regulatory impact on the initiation, proliferation, and differentiation of HFSCs. The activation of the Wnt/β-catenin pathway emerges as a compelling force propelling HFSCs into the Anagen phase. Throughout this phase, the Wnt signaling pathway intricately directs the stabilization of β-catenin, enabling its translocation into the nucleus, where it orchestrates the activation of a repertoire of genes intricately linked to the process of HF growth [7,8]. Noteworthy in the Wnt/β-catenin pathway are Wnt proteins that assume a critical role in the activation of HFSCs. For instance, Wnt7b, whose protein expression initiates during the initial growth period, has been demonstrated to be indispensable for the activation of HFSCs, as its postnatal knockout halts this activation process [9,10]. Conversely, experimental overexpression of Wnt10b, a distinct member of the Wnt protein family, has been demonstrated to induce the transition of HF from the quiescent phase to the growth phase [11]. Contrarily, the targeted knockdown of Wnt10b impedes the initiation of HF into the Anagen phase [12]. HFSCs, as multipotent stem cells, manifest the capability for differentiation into a myriad of cell types, encompassing adipocytes, chondrocytes, neurons, or smooth muscle cells [13-18]. Shen et al. documented the central involvement of β-catenin in the initiation of HFSCs differentiation through the activation of the nuclear gene c-myc [19]. In summary, the intricate regulation of HFSCs by the Wnt/β-catenin signaling pathway constitutes a complex and pivotal process, bearing significant implications for various facets of HF dynamics.
The activation of TGF-β/BMP signaling pathway can elicit a spectrum of cellular responses, encompassing cell proliferation, differentiation, apoptosis, as well as the synthesis of the ECM [20,21]. TGF-β and BMP represent two closely related classes of cytokines functioning as signaling molecules that mediate cellular communication. These cytokines initiate signaling by binding to their respective receptors, culminating in the phosphorylation of Smad proteins and the formation of an activated Smad protein complex. This complex translocates into the nucleus, where it engages with other transcription factors to intricately regulate the transcription of specific genes. Analogously, the TGF-β/BMP signaling pathway assumes a pivotal role in the development, growth, and regulation of HFSCs. Within the context of this signaling pathway, the nuclear interaction between TGF-β/BMP and Smad proteins serves as a facilitator for the induction of transcription of pertinent target genes [22-26]. This transcriptional activity functions as a regulatory mechanism, delicately modulating the proliferation and differentiation of HFSCs. The collective evidence underscores the pivotal role of the TGF-β/BMP signaling pathway in shaping the dynamics of HFSCs in a consistent manner. Notably, the BMP antagonist, Noggin, emerges as an instrumental factor in the regulation of HFSCs. BMP-4 intricately interacts with Noggin to finely modulate the differentiation of HFSCs, guiding them towards the development of sebaceous glands, sweat glands, and epidermal cells through the overexpression of lymphoid enhancer-binding factor (LEF) molecules [27]. Concurrently, the TGF-β/BMP signaling pathway demonstrates regulatory control over the Wnt/β-catenin signaling pathway by promoting the upregulation of Dickkopf3 (DKK3) molecules [28,29]. This intricate interplay underscores the nuanced regulatory mechanisms involved in orchestrating the activities of HFSCs.
The Notch signaling pathway represents a highly conserved mechanism of cellular signaling [30]. The interaction between the Notch receptor and its ligand serves as a regulatory mechanism governing cell proliferation, differentiation, and fate decisions [31,32]. The Notch signaling pathway has the capacity to promote the differentiation of HFSCs into HF cells while concurrently inhibiting their differentiation into epidermal cells through the Notch/RBP-J mechanism [33,34]. The Notch signaling pathway additionally functions as a downstream pathway of Wnt/β-catenin signaling, activating the transcription of target genes such as hair and split enhancers (Hes), runt-associated transcription factors (Runx), and Notch inhibitory membrane proteins (Numb) [35]. Yet another conserved mechanism of cellular signaling, the Hedgehog signaling pathway, is imperative for the activation of β-catenin activity [36]. All these signaling pathways intricately interact, forming a finely tuned regulatory network to govern the activities of HFSCs.
Cell-extrinsic Mechanisms
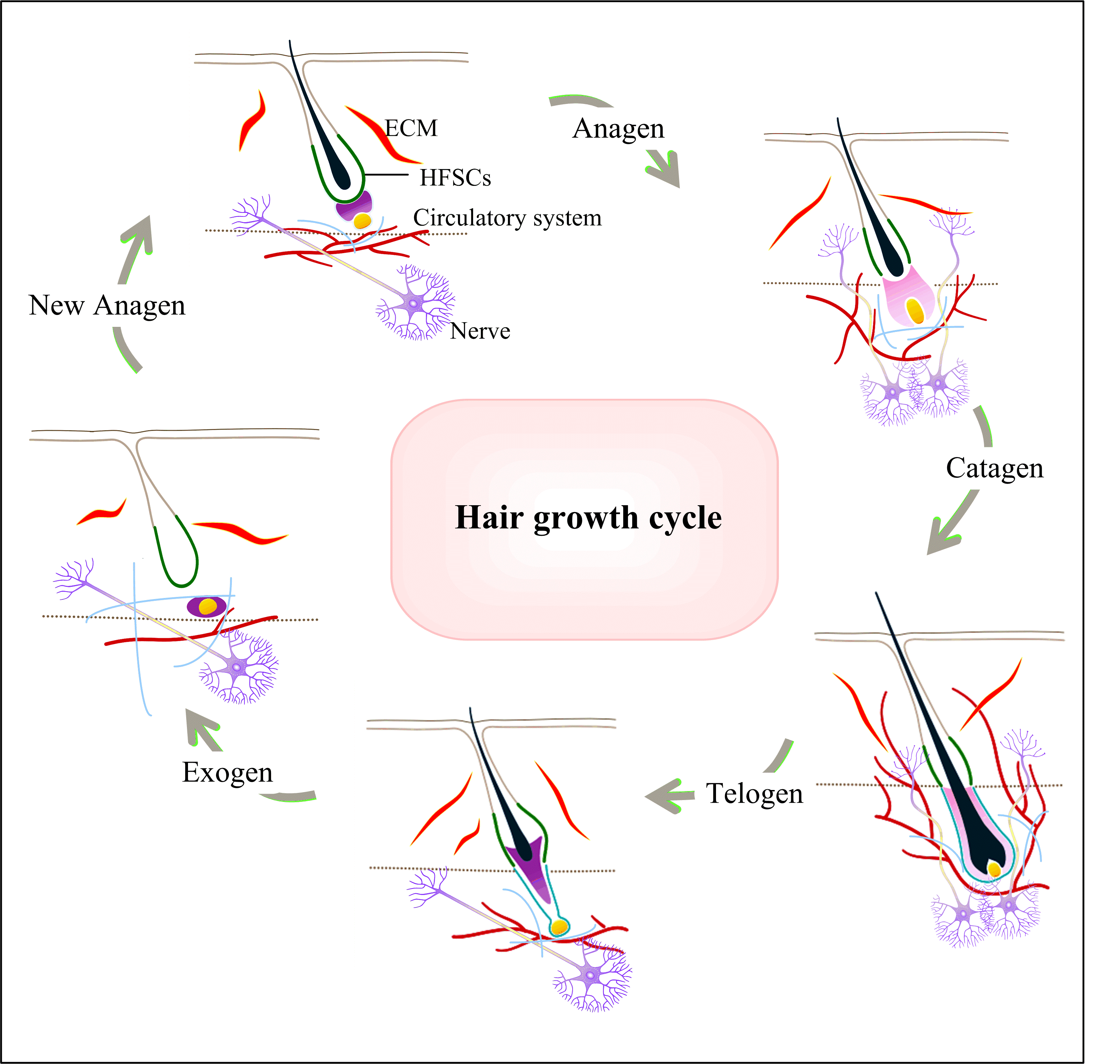
As a crucial ecological niche within the skin, HFSCs possess the capacity to interact with other niches, contributing to the maintenance of skin homeostasis and regulate hair growth, as illustrated in Figure 2. The normal functioning of HFSCs necessitates an adequate vascular supply. The circulatory system plays a crucial role in supporting the growth and differentiation of HFSCs by furnishing them with essential elements such as oxygen, nutrients, and various growth factors [37]. The lymphatic system plays a pivotal role in removing tissue waste and supporting immune surveillance for HFSCs [38]. Simultaneously, upon activation of HFSCs, there is a transient increase in lymphatic vessel caliber, accompanied by the dissociation of lymphatic capillaries in close proximity to HFSCs [39,40]. However, during quiescence, lymphatic capillaries closely associate with HFSCs [39]. Hence, there exist dynamic changes in the association between lymphatic capillaries and HFSCs. Collectively, the vasculature assumes a pivotal role in the health and functionality of HFSCs. This interaction encompasses not only the provision of nutrients and oxygen but also involves cell signaling, niche maintenance, and participation in the healing process. Therefore, ensuring an adequate blood supply may prove crucial for the maintenance of HF health and the prevention of hair loss.
Figure 2. HFSCs with other niches during the hair growth cycle. The hair growth cycle includes Anagen, Catagen, Telogen, Exogen, and then it can begin a new Anagen. In different periods, the interaction of HFSCs with nerve and circulatory system is different. When the HF undergoes hair growth cycle, the nerve and circulatory system also make periodic changes. As the HFSC is activated, the HF enters Anagen. The subcutaneous vasculature structures that normally oriented horizontally and fill the vessels below the hair ula disperse and become more vertical. Meanwhile, angiogenesis occurs in the cutaneous vasculature, which can provide both oxygen and nutrients. In Telogen, the present vasculature becomes horizontal again, forming a dense vascular structure and maintaining this structure until the onset of the next Anagen. HF innervation and the density of cutaneous peripheral nerves increase during the growth phase and decrease during the degenerative phase and maintaining to next Anagen. The ECM itself is the true ecological niche of the HFSCs, and ECM from hair epithelium, the ECM proteins are mediators of various components in the HFSC microenvironment.
Nerves and neurons communicating with various tissue stem cells, including hematopoietic stem cells [41], intestinal stem cells [42-44] and muscle stem cells [35,45,46], have been extensively documented. HFSCs are no exception to this phenomenon. HFSCs are not only regulated by the niche in wound healing through sensory nerves but are also activated by sympathetic nerves [35,48-50]. Similarly, HFSCs express neurotrophic factors to modulate their neural niche [51]. The regulation of HFSCs by the nervous system constitutes a complex network, encompassing various aspects such as neuroendocrine regulation, neurovascular regulation, and neural-immune interactions. A more profound understanding of these interactions is crucial for comprehending mechanisms underlying hair loss, advancing treatment strategies, and preserving overall hair health.
The ECM, comprising components such as collagen, integrins, proteoglycans, and other structural macromolecules, serves as a tissue scaffold that offers crucial structural support. It plays a pivotal role in cell adhesion, migration, and cell signaling [52]. The mutual crosstalk between stem cells and the ECM represents a prominent and current research focus. The ECM stands as a pivotal component within the HF niche, furnishing essential support for the growth and differentiation requisite for HFSCs [14,52-54]. The ECM encompasses a diverse array of proteins, polysaccharides, and other biomolecules. These components collectively construct a microenvironment essential for the survival and optimal functioning of HFSCs [14,53,57].
All in all, the interaction between HFSCs and other skin niches forms an integrated regulatory network. This interaction occurs through various means, including the exchange of nutrients, exudates, signaling molecules, etc.
Treatment of Hair Loss
The periodic growth of HF is orchestrated by HFSCs, which assume a pivotal role in the treatment of hair loss. Research data indicates that HFSCs can drive HF regeneration through interventions such as drugs, laser therapy, and cell transplantation.
Minoxidil initially emerged as a medication for hypertension in the 1970s; however, its use revealed instances of hair regeneration and generalized hirsutism in bald patients [58]. Over time, 2% and 5% formulations of minoxidil have been employed for the treatment of hair loss. Mori and Uno observed a significant reduction in the Telogen phase in rats treated with minoxidil compared to the untreated group [59]. Simultaneously, it was discovered that minoxidil could markedly upregulate vascular endothelial growth factor in dermal papillary cells, a phenomenon typically robustly expressed during the Anagen phase [60]. The effects of minoxidil can vary from person to person, with some individuals experiencing a significant slowdown in the hair loss process and promotion of new hair growth. However, not everyone responds in the same way, and common side effects, such as irritant contact dermatitis with typical symptoms of itching and desquamation, may occur in some individuals.
Low-level laser therapy (LLLT) is a biological intervention relying on low-intensity laser irradiation, employing a single type of non-thermal radiation with wavelengths in the red to near-infrared range of the electromagnetic spectrum [61]. LLLT is most commonly employed in clinical settings for various purposes, including irradiation of injured sites to promote wound healing, remodeling, or reduce inflammation. It is utilized for inducing nerve cells to relieve analgesia, reducing lymph node edema and inflammation, as well as promoting muscle relaxation and alleviating pain [62-64]. In the 1960s, Endre Mester discovered that a low-power ruby laser (694 nm) enhanced hair growth in the shaved area of the back [65]. The introduction of LLLT marked the first demonstration of its beneficial effects on hair growth, opening up new avenues for the treatment of hair loss [66]. Subsequently, an increasing number of studies have revealed the efficacy of LLLT in the context of hair loss. In 2007 and 2011, the U.S. Food and Drug Administration (FDA) granted approval for LLLT as a safe treatment for male and female-pattern alopecia, respectively [67]. In 2021, Jin et al. discovered that LLLT mitigates hair loss through the activation of HFSCs [68]. They found that LLLT can stimulate the activation of quiescent HFSCs and alleviate HF atrophy. This is achieved through the induction of reactive oxygen species (ROS), which activate the PI3K/AKT/GSK-3β signaling pathway, thereby inhibiting the proteasomal degradation of β-catenin in the HFSC. On the other hand, LLLT accelerates microvascular blood flow, leading to increased blood oxygen content, thereby promoting HF regeneration [69]. LLLT not only has the capacity to regulate the signaling pathways of HFSCs but also exerts an effect on the ecological niche of HFSCs.
Cell transplantation emerges as a therapeutic approach for hair loss, involving the implantation of HF or other cell sources into the scalp to stimulate the growth of new hair. Hair loss can be addressed through either the direct transplantation of HF or by fostering their regeneration with transplanted stem cells. Research findings suggest that neural stem cells directly regulate the HF niche, inducing core growth factors to promote hair regeneration through the TGF-β and BMP signaling pathway [70]. Despite the numerous available treatments for hair loss, the underlying mechanisms warrant further investigation.
Conclusion
The term "niche" denotes the specific position or role occupied by an organism for its survival and lifestyle. This encompasses biological functions, behaviors, adaptation strategies, and interactions with the surrounding environment and other organisms. Within the context of skin biology, HFSCs function as a pivotal signaling center. They are not only subject to regulation by a diverse array of signaling pathways but also engage in intricate interactions with other elements, forming an extensive network mediation center crucial for maintaining skin homeostasis.
In summary, the investigation of signaling pathways and the niche environment surrounding HFSCs holds promise for identifying effective strategies in addressing skin diseases, potentially serving as a target for conditions such as alopecia in the near future. This scholarly discourse underscores the potential translational impact of understanding HFSCs and their niche in the realm of skin health.
Conflicts of Interest
None.
Funding Statement
This work was supported by the Science and Technology Program of Guangzhou (202201010291), the Guangdong Provincial Department of Education Key Areas Special Project for Regular Higher Education Institutions (2023ZDZX2022), and the Guangdong Provincial Department of Education Special Innovative Projects for Regular Higher Education Institutions (2022KTSCX032).
References
2. Li B, Hu W, Ma K, Zhang C, Fu X. Are hair follicle stem cells promising candidates for wound healing?. Expert Opinion on Biological Therapy. 2019 Feb 1;19(2):119-28.
3. Li G, Wang Q, Liu H, Yang Z, Wu Y, He L, et al. Fabricating Composite Cell Sheets for Wound Healing: Cell Sheets Based on the Communication Between BMSCs and HFSCs Facilitate Full-Thickness Cutaneous Wound Healing. Tissue Engineering and Regenerative Medicine. 2023 Nov 23:1-15.
4. Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. Journal of Investigative Dermatology. 2008 May 1;128(5):1071-80.
5. Alonso L, Fuchs E. The hair cycle. Journal of Cell Science. 2006 Feb 1;119(3):391-3.
6. Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circulation Research. 2010 Jun 25;106(12):1798-806.
7. Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annual review of cell and developmental biology. 1998 Nov;14(1):59-88.
8. Bejsovec A. Wnt signaling: an embarrassment of receptors. Current Biology. 2000 Dec 14;10(24):R919-22.
9. Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proceedings of the National Academy of Sciences. 2013 Jan 22;110(4):1351-6.
10. Kandyba E, Kobielak K. Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem cells. 2014 Apr 1;32(4):886-901.
11. Lei M, Lai X, Bai X, Qiu W, Yang T, Liao X, et al. Prolonged overexpression of Wnt10b induces epidermal keratinocyte transformation through activating EGF pathway. Histochemistry and Cell Biology. 2015 Sep;144:209-21.
12. Li YH, Zhang K, Yang K, Ye JX, Xing YZ, Guo HY, et al. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. Journal of Investigative Dermatology. 2013 Jan 1;133(1):42-8.
13. Nath M, Offers M, Hummel M, Seissler J. Isolation and in vitro expansion of Lgr6-positive multipotent hair follicle stem cells. Cell and Tissue Research. 2011 Jun;344(3):435-44.
14. Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004 Jan 16;303(5656):359-63.
15. Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proceedings of the National Academy of Sciences. 2005 Apr 12;102(15):5530-4.
16. Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, et al. From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle. 2015 Jul 18;14(14):2362-6.
17. Yamazaki A, Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, et al. Isoproterenol directs hair follicle-associated pluripotent (HAP) stem cells to differentiate in vitro to cardiac muscle cells which can be induced to form beating heart-muscle tissue sheets. Cell Cycle. 2016 Mar 3;15(5):760-5.
18. Liu JY, Peng HF, Gopinath S, Tian J, Andreadis ST. Derivation of functional smooth muscle cells from multipotent human hair follicle mesenchymal stem cells. Tissue engineering Part A. 2010 Aug 1;16(8):2553-64.
19. Shen Q, Yu W, Fang Y, Yao M, Yang P. Beta-catenin can induce hair follicle stem cell differentiation into transit-amplifying cells through c-myc activation. Tissue and Cell. 2017 Feb 1;49(1):28-34.
20. Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone research. 2015 Apr 14;3(1):1-20.
21. Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone research. 2016 Apr 26;4(1):1-21.
22. Owens P, Bazzi H, Engelking E, Han G, Christiano AM, Wang XJ. Smad4-dependent desmoglein-4 expression contributes to hair follicle integrity. Developmental biology. 2008 Oct 1;322(1):156-66.
23. Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes & development. 1996 Jul 1;10(13):1580-94.
24. Botchkarev VA, Kishimoto J. Molecular control of epithelial–mesenchymal interactions during hair follicle cycling. Journal of Investigative Dermatology Symposium Proceedings. 2003 Jun 1;8(1):46-55.
25. Rishikaysh P, Dev K, Diaz D, Qureshi WM, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. International Journal of Molecular Sciences. 2014 Jan 22;15(1):1647-70.
26. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004 Sep 3;118(5):635-48.
27. Zhang W, Wang N, Zhang T, Wang M, Ge W, Wang X. Roles of melatonin in goat hair follicle stem cell proliferation and pluripotency through regulating the Wnt signaling pathway. Frontiers in Cell and Developmental Biology. 2021 Jun 4;9:686805.
28. Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003 Mar 20;422(6929):317-22.
29. Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem cells. 2006 Jun 1;24(6):1476-86.
30. Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D'antonio M, Parkinson DB, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nature neuroscience. 2009 Jul;12(7):839-47.
31. Aubin-Houzelstein G. Notch signaling and the developing hair follicle. Notch Signaling in Embryology and Cancer. 2012 Jan 1:142-60.
32. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017 Jun 1;169(6):985-99.
33. Yamamoto N, Tanigaki K, Han H, Hiai H, Honjo T. Notch/RBP-J signaling regulates epidermis/hair fate determination of hair follicular stem cells. Current Biology. 2003 Feb 18;13(4):333-8.
34. Huang C, Du Y, Nabzdyk CS, Ogawa R, Koyama T, Orgill DP, et al. Regeneration of hair and other skin appendages: A microenvironment-centric view. Wound Repair and Regeneration. 2016 Sep;24(5):759-66.
35. Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell stem cell. 2011 May 6;8(5):552-65.
36. Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998 Nov 25;95(5):605-14.
37. Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. Journal of Investigative Dermatology Symposium Proceedings. 2000 Dec 1;5(1):14-19.
38. Hampton HR, Chtanova T. Lymphatic migration of immune cells. Frontiers in immunology. 2019 May 28;10:1168.
39. Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, et al. Stem cell–driven lymphatic remodeling coordinates tissue regeneration. Science. 2019 Dec 6;366(6470):1218-25.
40. Peña‐Jimenez D, Fontenete S, Megias D, Fustero‐Torre C, Graña‐Castro O, Castellana D, et al. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. The EMBO Journal. 2019 Oct 1;38(19):e101688.
41. Agarwala S, Tamplin OJ. Neural crossroads in the hematopoietic stem cell niche. Trends in Cell Biology. 2018 Dec 1;28(12):987-98.
42. Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PloS One. 2011 Feb 9;6(2):e16295.
43. Davis EA, Zhou W, Dailey MJ. Evidence for a direct effect of the autonomic nervous system on intestinal epithelial stem cell proliferation. Physiological Reports. 2018 Jun;6(12):e13745.
44. Puzan M, Hosic S, Ghio C, Koppes A. Enteric nervous system regulation of intestinal stem cell differentiation and epithelial monolayer function. Scientific Reports. 2018 Apr 20;8(1):6313.
45. Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. Journal of Neuroscience. 2006 May 24;26(21):5739-49.
46. Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, et al. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. American Journal of Physiology-Cell Physiology. 2009 Aug;297(2):C238-52.
47. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiological Reviews. 2013 Jan;93(1):23-67.
48. Martínez-Martínez E, Galván-Hernández CI, Toscano-Márquez B, Gutiérrez-Ospina G. Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PloS One. 2012 May 4;7(5):e36421.
49. Fan SM, Chang YT, Chen CL, Wang WH, Pan MK, Chen WP, et al. External light activates hair follicle stem cells through eyes via an ipRGC–SCN–sympathetic neural pathway. Proceedings of the National Academy of Sciences. 2018 Jul 17;115(29):E6880-9.
50. Shwartz Y, Gonzalez-Celeiro M, Chen CL, Pasolli HA, Sheu SH, Fan SM, et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell. 2020 Aug 6;182(3):578-93.
51. Cheng CC, Tsutsui K, Taguchi T, Sanzen N, Nakagawa A, Kakiguchi K, et al. Hair follicle epidermal stem cells define a niche for tactile sensation. Elife. 2018 Oct 25;7:e38883.
52. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. Journal of Cell Science. 2010 Dec 15;123(24):4195-200.
53. Chermnykh E, Kalabusheva E, Vorotelyak E. Extracellular matrix as a regulator of epidermal stem cell fate. International Journal of Molecular Sciences. 2018 Mar 27;19(4):1003.
54. Miyachi K, Yamada T, Kawagishi‐Hotta M, Hasebe Y, Date Y, Hasegawa S, et al. Extracellular proteoglycan decorin maintains human hair follicle stem cells. The Journal of Dermatology. 2018 Dec;45(12):1403-10.
55. Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011 Feb 4;8(2):177-87.
56. Rahmani W, Sinha S, Biernaskie J. Immune modulation of hair follicle regeneration. NPJ Regenerative Medicine. 2020 May 11;5(1):9.
57. Chacón‐Martínez CA, Klose M, Niemann C, Glauche I, Wickström SA. Hair follicle stem cell cultures reveal self‐organizing plasticity of stem cells and their progeny. The EMBO Journal. 2017 Jan 17;36(2):151-64.
58. Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981 Oct;22:257-78.
59. Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Patents on Inflammation & Allergy Drug Discovery. 2012 May 1;6(2):130-6.
60. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 2004 Feb 1;150(2):186-94.
61. Schindl A, Schindl M, Pernerstorfer-Schön H, Schindl L. Low-intensity laser therapy: a review. Journal of Investigative Medicine: the official publication of the American Federation for Clinical Research. 2000 Sep 1;48(5):312-26.
62. Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. InSeminars in Cutaneous Medicine and Surgery. 2013 Mar;32(1):41.
63. Gavish L, Houreld NN. Therapeutic efficacy of home-use photobiomodulation devices: a systematic literature review. Photobiomodulation, Photomedicine, and Laser Surgery. 2019 Jan 1;37(1):4-16.
64. Carroll JD, Milward MR, Cooper PR, Hadis M, Palin WM. Developments in low level light therapy (LLLT) for dentistry. Dental Materials. 2014 May 1;30(5):465-75.
65. Mester E, Ludany G, Sellyei M, Szende B, Gyenes G, Tota GJ. Studies on the inhibiting and activating effects of laser beams. Langenbecks Archiv fur Chirurgie. 1968 Jan 1;322:1022-7.
66. Barolet D. Light-emitting diodes (LEDs) in dermatology. Seminars in Cutaneous Medicine and Surgery. 2008 Dec 31;27(4):227-38.
67. Wikramanayake TC, Rodriguez R, Choudhary S, Mauro LM, Nouri K, Schachner LA, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers in Medical Science. 2012 Mar;27:431-6.
68. Jin H, Zou Z, Chang H, Shen Q, Liu L, Xing D. Photobiomodulation therapy for hair regeneration: A synergetic activation of β-CATENIN in hair follicle stem cells by ROS and paracrine WNTs. Stem Cell Reports. 2021 Jun 8;16(6):1568-83.
69. Sun SQ, Shen JJ, Wang YF, Jiang YT, Chen LF, Xin H, et al. Red organic light-emitting diodes based photobiomodulation therapy enabling prominent hair growth. Nano Research. 2023 May;16(5):7164-70.
70. Hwang I, Choi KA, Park HS, Jeong H, Kim JO, Seol KC, et al. Neural stem cells restore hair growth through activation of the hair follicle niche. Cell Transplantation. 2016 Aug;25(8):1439-51.