Abstract
The local atomic structure of metallic glasses can be modified, and their physical properties can be improved by high-pressure torsion (HPT). In this article, some recent investigations are presented that reveal the HPT treatment effect on nanoglass (Zr55.7Ni10Al7Cu19Co8.3) based upon the appearance of shear bands in the HPT treated nanoglass. The residual stress rises, leading to an increase in the nanoindentation hardness. With the evolution of the free volume, the plasticity of the nanoglass is improved by HPT, exhibiting a tensile plastic strain of 0.9%. Multiple shear bands can be observed on the surface during the tensile test, and crack propagation is suppressed in HPT-treated nanoglass. Many shear bands branch and interact with each other at the tip of the cracks thus avoiding brittle behavior.
Keywords
Nanoglass, HPT, Tensile plasticity, Free volume, Shear bands.
Introduction
Metallic glasses are amorphous solids with metallic atomic bonds. Different from traditional crystalline metals, they have no long-range atomic order. Only short-range order on the scale of 0.5–1 nm can be detected [1-4]. Because of the lack of a lattice dislocation slip system, as in crystalline alloys, metallic glasses exhibit some outstanding properties, such as high elastic strain, high hardness and high strength [5-7]. The yield strengths of Zr-based bulk metallic glasses (BMGs) are approximately 2 GPa in compression. Unfortunately, this special structure also creates problems; that is, plastic deformation in BMGs is always concentrated in localized regions, namely, shear bands, resulting in catastrophic failure [8]. This brittleness restricts their industrial applications. Zr64Ni10Al7Cu19 is a BMG system [9,10] with good glass forming ability and high strength but no plastic strain under tensile testing.
Considerable studies have been performed to find a way to improve the ductility of BMGs. Limiting the propagation of shear bands is a key issue [9-12]. Recently, nanoglasses, which are amorphous solids consisting of nanometer-sized glassy regions connected by glass/glass interfaces, were reported [12-15]. Such nanoglasses could have new features; e.g., (1) denser glassy particles could hinder the propagation of shear bands that act as second phase strengthening; and (2) an enhanced free volume due to the misfit between the atoms at the boundaries between glassy particles could lead to the formation of more shear bands.
High-pressure torsion (HPT) is a severe plastic deformation technique for refining grains in polycrystalline materials [15]. Recent studies have shown that in BMGs, free volume can be enhanced by HPT, which is known as rejuvenation [16]. As a result, the velocity of shear band sliding is reduced [17], and the ductility can be improved [1]. These effects are also accompanied by the development of residual stress because of inhomogeneous plastic flow (deformation with a strain gradient) [18]. Therefore, it is of interest to perform a systematic study of severe plastic deformation (HPT) on nanoglass.
Synthesis and Structure of Nanoglass
As introduced above, compared with BMGs, nanoglasses could have improved properties. Several methods have been developed for nanoglass synthesis. Inert gas condensation was first investigated [26,27]. Nanoscale glassy clusters are evaporated from the target material into an inert gas atmosphere (helium) and then consolidated under vacuum. Magnetron sputtering is another method for nanoglass preparation. A negative potential is applied to a target, acting as a cathode. Atoms in the sputtering target are ejected by the collision of Ar+ ions and are deposited on a substrate, forming a nanoglass thin film [28]. Phase separation is a newly developed method by adding elements (such as Co) with a positive enthalpy of mixing [1]. With a particular chemical composition, nanoscale phase separation can take place in glassy structures by water-cooled copper-mold casting. Compared with inert gas condensation and magnetron sputtering, nanoglasses synthesized by the phase separation method have a large size (on the millimeter scale), making HPT treatment possible.
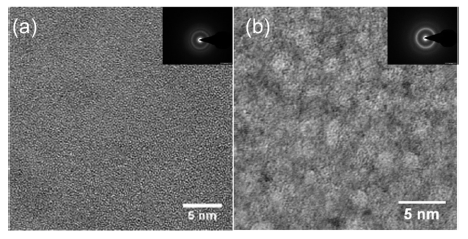
In this work, the Zr64Ni10Al7Cu19 system was selected because of its good glass forming ability [19]. Cobalt was then added to partially replace zirconium. The positive enthalpy of mixing between cobalt and copper (6 kJ/mol) constitutes the driving force for nanoscale phase separation. Transmission electron microscopy (TEM) analysis of the as-cast Zr64Ni10Al7Cu19 and Zr55.7Ni10Al7Cu19Co8.3 samples is shown in Figure 1. Electron diffraction halos confirm the amorphous structure of both samples. Nanometer-scale glassy clusters are clearly detected in the as-cast Zr55.7Ni10Al7Cu19Co8.3 sample. HPT treatments have been applied on this sample.
Figure 1. TEM images of (a) Zr64Ni10Al7Cu19 and (b) Zr55.7Ni10Al7Cu19Co8.3 metallic glass samples
High-Pressure Torsion on Nanoglass
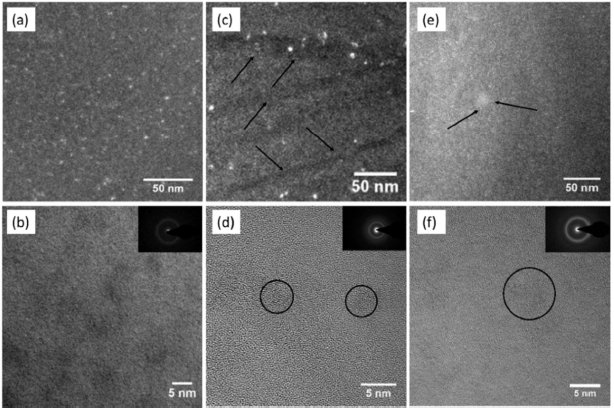
The as-cast Zr55.7Ni10Al7Cu19Co8.3 nanoglass samples show good HPT deformation capabilities. No cracks were detected in the severely plastic deformed HPT-treated samples. The shear strain γ can be calculated through where h is the initial thickness of the sample (0.4 mm), R is the distance from the sample center, and N is the number of rotations (2, 5, 10, 20). X-ray diffraction (XRD) patterns of all HPT-treated samples are shown in Figure 2. In addition to the broad diffraction peak, no sharp peaks from crystalline phases are observed for all samples. However, the TEM images in Figure 3 reveal that two rotations of HPT changed the structure of the Zr55.7Ni10Al7Cu19Co8.3 sample. Nanometer-scale crystalline particles appear as bright points in the dark-field image in Figure 3c. The content of nanocrystalline particles is small, most likely less than 1%, so that they cannot be detected by XRD. Shear bands induced during HPT treatment can also be observed in the TEM images (dark lines in the dark-field image, marked by arrows, Figure 3c).
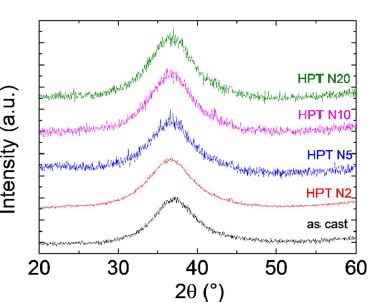
Figure 2. XRD patterns of the as-cast and HPT-treated (rotation number N=2, 5, 10, 20) Zr55.7Ni10Al7Cu19Co8.3 samples. Reprinted from [2], with the license “CC-BY 4.0” (https://creativecommons.org/licenses/by/4.0/)
Figure 3. TEM images of Zr55.7Ni10Al7Cu19Co8.3 (a) Dark-field image of the as-cast sample. (b) Bright-field HRTEM image of as-cast sample. (c)Dark-field image of the deformed sample by HPT for two rotations. (d) Bright-field HRTEM image of the deformed sample by HPT for two rotations. (e) Dark-field HRTEM image of the deformed sample by HPT for 20 rotations. (f) Bright-field HRTEM image of the deformed sample by HPT for 20 rotations. Modified from [2], with the license “CC-BY 4.0” (https://creativecommons.org/licenses/by/4.0/).
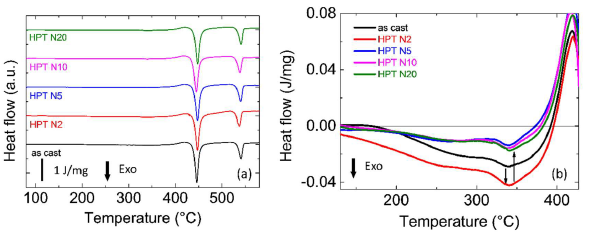
Differential scanning calorimentry (DSC) measurements were also performed for as-cast and HPT-treated nanoglass samples. Zr55.7Ni10Al7Cu19Co8.3 nanoglass exhibits a two-step crystallization at 701 K and 794 K, which most likely results from the phase separation in the samples. HPT treatments do not affect the crystallization processes with no detectable change in Tg, Tx or crystallization enthalpy, as shown in Figure 4a. However, interesting changes take place in the relaxation processes prior to Tg (Figure 4b). After two rotations of HPT, more energy release during relaxation is observed, indicating a release of enhanced free volume. By further increasing the rotation number (N=5, 10, 20), the free volume decreases further. This decrease could be due to heating and/or mechanical annealing effects during plastic deformation [20].
Figure 4. (a) Di fferential scan calorimetry curves of as-cast and HPT-treated (rotation number N=2, 5, 10, 20) Zr55.7Ni10Al7Cu19Co8.3 samples.(b) Relaxation energy release prior to Tg of as-cast and HPT-treated Zr55.7Ni10Al7Cu19Co8.3 samples. Reprinted from [2], with the license “CC-BY 4.0” (https://creativecommons.org/licenses/by/4.0/).
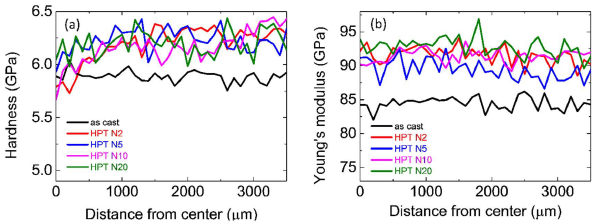
Figure 5 (a) shows the radial distributions of the nanoindentation hardness values of the as-cast state and HPTtreated (N=2, 5, 10, 20) Zr55.7Ni10Al7Cu19Co8.3 samples. In the central region (0-500 μm from the center), there is a relatively small increase of the hardness after HPT treatments of about 3%. In the outer region (500-3500 μm from the center), the hardness of the treated sample increases from 5.9 ± 0.1 to 6.3 ± 0.1 GPa. As reported in [18], the residual compressive stress is aligned parallel to the radial direction introduced by the disk material flow during HPT deformation [21]. The Young’s modulus radial distributions of the as-cast and HPT-treated Zr55.7Ni10Al7Cu19Co8.3 samples are shown in Figure 5b. After HPT treatment, the Young’s modulus of the nanoglass increases from 84 ± 2 to 92 ± 2 GPa, i.e. about 10% [22].
Figure 5. (a) Nano indentation hardness and (b) Young ’s modulus distribution of as-cast and HPT-treated (rotation number N = 2, 5, 10, 20) Zr55.7Ni10Al7Cu19Co8.3 samples [22].
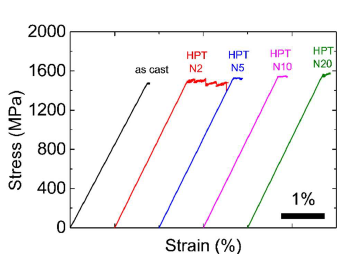
Unlike compression, metallic glasses have zero ductility under tensile loading. A load-displacement curve and scanning electron microscopy (SEM) images can be obtained at the same time during the in situ tensile testing performed within the SEM chamber test. The tensile stress–strain curves of the as-cast and HPT-treated Zr55.7Ni10Al7Cu19Co8.3 nanoglasses are shown in Figure 6. Similar to the results reported in other publications [23-25], the as-cast metallic glasses show almost no plastic strain during the tensile test. However, after HPT for two rotations, the ductility of the Zr55.7Ni10Al7Cu19Co8.3 nanoglass sample is improved significantly. Its tensile plastic strain reaches almost 1.0%, which is significantly improved in comparison with untreated monolithic metallic glasses. From the serrated plastic deformation plateau on the stress–strain curve, it might be deduced that more than one major shear band initiated during the tensile test. However, the plastic strain of the treated samples decreases again when the HPT increases to 5-20 rotations. As discussed above, the reason is the variation in free volume. An excessively large deformation degree might facilitate a temperature rise in the sample, leading to free volume annihilation in the structure.
Figure 6. Tensile stress–strain curves of Zr55.7Ni10Al7Cu19Co8.3 samples before and after the HPT process (rotation number N = 2, 5, 10, 20).Modified from [2], with the license “CC-BY 4.0” (https://creativecommons.org/licenses/by/4.0/).
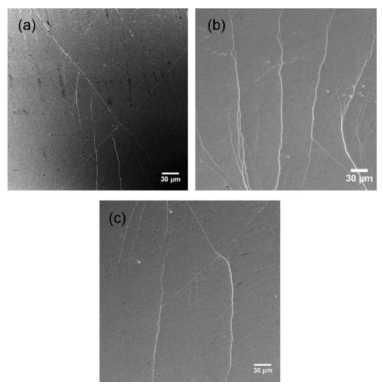
The initiation and evolution of shear bands on the sample’s surfaces during tensile deformation were also observed by in situ SEM measurement. The images in Figure 7 exhibit the surfaces of the as-cast and HPT-treated nanoglass samples under yield stress loading conditions. Figure 7a shows that two major shear bands cut through the surface of the as-cast Zr55.7Ni10Al7Cu19Co8.3 sample, with a few shear bands on the side. The deformation is concentrated within the major shear bands. Unlike the compression process with restricted load, this shear band easily extends to a crack under tension, which leads to very rapid catastrophic fracture after yielding. The two-rotation HPT-treated Zr55.7Ni10Al7Cu19Co8.3 sample shows the best ductility. Multiple shear bands can be observed on the surface with an orientation perpendicular to the tension direction (Figure 7b), resulting in large plastic strain. Further increasing the HPT rotation number (5-20) reduced the number of shear bands (Figure 7c) because of the annihilation of free volume in the structure and the plasticity of the samples is reduced.
Figure 7. Shear bands on the surface of the Zr55.7Ni10Al7Cu19Co8.3 sample (a) as-cast, (b) N = 2, and (c) N = 20, under yielding load. Modified from [2], with the license “CC-BY 4.0” (https://creativecommons.org/licenses/by/4.0/).
Finite element analysis of the tensile tests on nanoglass has been done by Mo Li et al. [30]. It shows that, in the heterogeneous structure, initial deformation started at the soft regions, continuing to be localized around their original location. When localized regions have finally developed, the deformation bands do not look smooth and straight, rather rugged and zigzag with many side bands. The localized deformation zones are spread more widely, resulting in improved plasticity [30]. This simulation is coincident with the experimental results as shown in Figure 7.
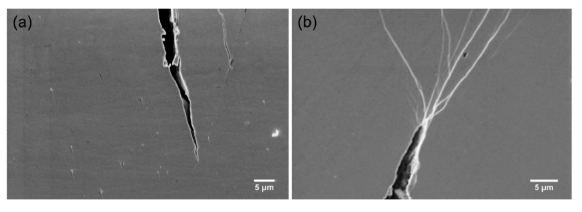
Figure 8 illustrates the SEM images for crack tips on two rotations of HPT-treated Zr64Ni10Al7Cu19 BMG and Zr55.7Ni10Al7Cu19Co8.3 nanoglass samples just before fracture. It is clear that on the treated Zr55.7Ni10Al7Cu19Co8.3 nanoglass, many shear bands branch and interact with each other at the crack tip (Figure 8b), which cannot be detected on the treated Zr64Ni10Al7Cu19 BMG (Figure 8a). In this treated nanoglass, the crack tips reached the HPT–introduced pre-existing shear bands during propagation, resulting in the formation of newly formed shear bands. The propagation of the newly formed shear bands is inhibited again by branching and intersection [29,31]. Thus, the localized plasticity is improved significantly [2].
Figure 8. SEM images of crack tips during tensile tests of the deformed samples (a) Zr64Ni10Al7Cu19 and (b) Zr55.7Ni10Al7Cu19Co8.3 by HPT for two rotations. Modified from [2], with the license “CC-BY 4.0” (https://creativecommons.org/licenses/by/4.0/).
Conclusions
Nanoscale crystalline particles (about 1 vol%) and shear bands form in the nanoglass (Zr55.7Ni10Al7Cu19Co8.3) during HPT. The free volume in the structure is increased by two rotations of HPT. Nanoindentation analysis shows that the hardness of the outer region (500-2500 μm from the center) is increased about 10% by HPT, indicating an increase in residual compressive stress. The localized tensile plasticity of the nanoglass is improved by two rotations of HPT treatments, and its tensile plastic strain reaches about 1%. The reason is the increased free volume and pre-existing shear bands introduced by the two rotations of HPT. During tensile deformation, multiple shear bands form on the tip of cracks; they branch and interact with each other, enhancing the ductility. With this improved property, the HPT treated nanoglass could have large application potential.
Acknowledgement
The financial support of DLR (50WM2143) and fruitful discussions with Prof. H. Gleiter and Dr. A. Haghighiradare in KIT are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
2. Dong Y, Liu SY, Biskupek J, Cao QP, Wang XD, Jiang JZ, et al. Improved tensile ductility by severe plastic deformation for a nanostructured metallic glass. Materials. 2019 May 16;12(10):1611.
3. Liu CT, Heatherly L, Horton JA, Easton DS, Carmicheal CA, Wright JL, et al. Test environment and mechanical properties of Zr-base bulk amorphous alloys. Metallurgical and Materials Transactions A. 1988 Jul;29(7):1811-1820.
4. Inoue A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Materialia. 2000 Jan;48(1):279-306.
5. Johnson WL, Samwer K. A universal criterion for plastic yielding of metallic glasses with a (T/Tg)2/3 temperature dependence. Physical Review Letters. 2006 Nov 4;95:195501.
6. Gu XJ, McDermoot AG, Poon SJ, Shiflet GJ. Critical Poisson’s ratio for plasticity in Fe-Mo-C-B-Ln bulk amorphous steel. Applied Physics Letters. 2006 May 24;88:211905.
7. Shen B, A Inoue. Enhancement of fracture strength and glassforming ability of CoFeTaB bulk glassy alloy. Journal of Physics: Condensed Matter. 2005 Sep 2;17(37):5647.
8. Greer AL, Cheng YQ, Ma E. Shear bands in merallic glasses. Materials Science and Engineering R. 2013 Apr;74(4):71-132.
9. Chen LY, Fu ZD, Zhang GQ, Hao XP, Jiang QK, Wang XD, et al. New class of plastic bulk metallic glass. Physical Review Letters. 2008 Feb 20;100:075501.
10. Du XH, Huang JC, Hsieh KC, Jang JSC, Liaw PK, Chen HM, et al. Designing ductile Zr-based bulk metallic glasses with phase separated microstructure. Advanced Engineering Materials. 2009 May 27;11(5):387-391.
11. Cao QP, Liu JW, Yang KJ, Xu F, Yao ZQ, Minkow A, et al. Effect of pre-existing shear bands on the tensile mechanical properties of a bulk metallic glass. Acta Materialia. 2010 Feb;58(4):1276-1292.
12. Gleiter H. Our thoughts are ours, their ends none of our own: Are there ways to synthesize materials beyond the limitations of today? Acta Materialia. 2008 Nov;56(19):5875-5893.
13. Wang XD, Cao QP, Jiang JZ, Franz H, Schroers J, Valiev RZ, et al. Atomic-level structural modifications induced by severe plastic shear deformation in bulk metallic glasses. Scripta Materialia. 2011 Jan;64(1):81-84.
14. Wang XL, Jiang F, Hahn H, Li J, Gleiter H, Sun J, et al. Sample size effects on strength and deformation mechanism of Sc75Fe25 nanoglass and metallic glass. Scripta Materialia. 2016 Apr;116:95-99.
15. Haddad M, Ivanisenko Y, Courtois-Manara E, Fecht H-J. In-situ tensile test of high strength nanocrystalline bainitic steel. Materials Science and Engineering: A. 2014 Jan 3;620:30-35.
16. Dmowski W, Yokoyama Y, Chuang A, Ren Y, Umemoto M, Tsuchiya K, et al. Structural rejuvenation in a bulk metallic glass induced by severe plastic deformation. Acta Materialia 2010 Jan;58(2):429-438.
17. Denis P, Meylan CM, Ebner C, Greer AL, Zehetbauer M, Fecht H-J. Rejuvenation decreases shear band sliding velocity in Pt-based metallic glasses. Materials Science and Engineering: A. 2017 Jan 27;684:517-523.
18. Adachi N, Todaka Y, Yokoyama Y, Umemoto M. Cause of hardening and softening in the bulk glassy alloy Zr50Cu40Al10 after high-pressure torsion. Materials Science and Engineering: A. 2015 March 11;627:171-181.
19. Liu YH, Wang G, Wang RJ, Zhao DQ, Pan MX, Wang WH. Super plastic bulk metallic glasses at room temperature. Science. 2007 Mar 09;315(5817):1385-1388.
20. Yang B, Morrison ML, Liaw PK, Buchanan RA, Wang G, Liu CT, et al. Dynamic evolution of nanoscale shear bands in a bulk-metallic glass. Applied Physics Letter 2005 Mar 30;86:141904.
21. Adachi N, Todaka Y, Yokoyama Y, Umemoto M. Cause of hardening and softening in the bulk glassy alloy Zr50Cu40Al10 after high pressure torsion. Materials Science and Engineering: A. 2015 Jan 6;627:171-181.
22. Dong Y. Structure, mechanical properties and post-processing of nano-structured metallic glasses. Open Access Repository of Ulm University and Ulm University of Applied Sciences. 2021 Mar 4:Dissertation.
23. Zhang ZF, Eckert J, Schultz. Fatigue and fracture behavior of bulk metallic glass. Metallurgical and Materials Transactions A. 2004 Nov;35:3489-3498.
24. Zhang ZF, Eckert J, Schultz. Difference in compressive and tensile fracture mechanisms of Zr59Cu20Al10Ni8Ti3 bulk metallic glass. Acta Materialia 2003 Feb;51(4):1167-1179.
25. Mukai T, Nieh TG, Kawamura Y, Inoue A, Higashi K. Dynamic response of a Pd40Ni40P20 bulk metallic glass in tension. Scripta Materialia 2002 Jan;46(1):43-47.
26. Jing J, Krämer A, Birringer R, Gleiter H, Gonser U. Modified atom structure in a Pd70Fe3Si27 nanoglass: A Mössbauer study. Journal of Non-Crystalline Solids. 1989 Dec 4;113(2-3):167-170.
27. Gleiter H. Are there ways to synthesize materials beyond the limits of today? Metallurgical and Materials Transactions A. 2009 May 27;40:1499-1509.
28. Chen N, Frank R, Asao N, Louzguine-Luzgin DV, Sharma P, Wang JQ, et al. Formation and properties of Au-based nanograined metallic glasses. Acta Materialia. 2011 Sep;59(16):6433-6440.
29. Xie S, George EP. Hardness and shear band evolution in bulk metallic glasses after plastic deformation and annealing. Acta Materialia. 2008 Oct;56(18):5202-5213.
30. Wang Y, Li M, Xu J. Toughen and harden metallic glass through designing statistical heterogeneity. Scripta Materialia. 2016 Mar 1:113:10-13.
31. Liu JW, Cao QP, Chen LY, Wang XD, Jiang JZ. Shear band evolution and hardness change in cold-rolled bulk metallic glasses. Acta Materialia. 2010 Aug;58(14):4827-4840.