Abstract
Green nanotechnology seeks eco-friendly solutions, capable of generating efficient nanotechnological protocols with low or no environmental impact. These nanosystems are marked out by intermolecular and supramolecular interactions capable of stabilizing the nanostructures and making nanotechnology effective. Many synthesis methodologies are being developed that involve a variety of biological sources. Among them, it can highlight biosynthesis using plant extracts. They are simple, cheap syntheses and have a fast reaction and low energy consumption. The dispersive vegetable medium is very complex due to its biomolecules. Extraction protocols must be established in order to isolate molecules efficient in colloidal stabilization. However, a lot has to be understood about these systems so that the present interactions have reproducible behavior worldwide.
Keywords
Green nanotechnology; Intermolecular interactions; Supramolecular interactions; Dispersive medium; Nanostructures; Nanomaterials
introduction
In the last decade, the study of nanometer-scale particles has grown exponentially worldwide. This growth is due to the broad field of nanostructures applications, which, due to their dimensions in nanometric sizes, have new properties not found in micro and macro scale. These properties result from the increase in the ratio between the surface area and volume, and the nanostructures’ size directly influences these [1]. Tolerance to temperature, variety of colors, changes in chemical reactivity, efficiency in action against microorganisms, and electrical conductivity are differentiating factors. [2].
Different synthesis pathways can synthesize nanostructured systems. In general, producing nanostructures by chemical and physical methods are more expensive and may involve the use of toxic chemicals, which involve risks to the environment and health, which limits their applications [1]. Green synthesis has been gaining attention, as it is environmentally friendly, costeffective, and can be easily scaled. Besides, there is no need to use high temperatures, pressures, chemicals that are toxic or dangerous to the environment and human health [1,2]. Thus, the methods tend to be relatively simple and use conditions closer to the environmental ones, which reduces energy expenditure [3] and requires a low concentration of precursors [4].
In the literature, synthesis procedures using biological methods that include plant extracts, bacteria, fungi, and cell extracts have been reported [5-10]. Among these, the first stands out because, although variations in seasonality can affect the synthesis [11], they do not require conditions of high asepsis and maintenance of microbial cultures [1].
The basis of green systems is associated with the extraction of biomolecules of natural origin. Whether extracted from agribusiness waste (such as leaves in tree pruning), fruit peels, leaves, seeds, roots, among others. [5,6,12-14]. The extraction protocol of biomolecules is fundamental to establish the bioactive molecules that will synthesize nanostructures. Different biomolecules such as tannins, anthocyanins, flavonoids, polyphenols, polysaccharides, and proteins can participate in biosynthesis, reducing the metallic precursor and in stabilization [6,15-21]. Besides, it is essential to emphasize that this process is complex, and the interaction with different biomolecules can be fundamental for a successful biosynthesis.
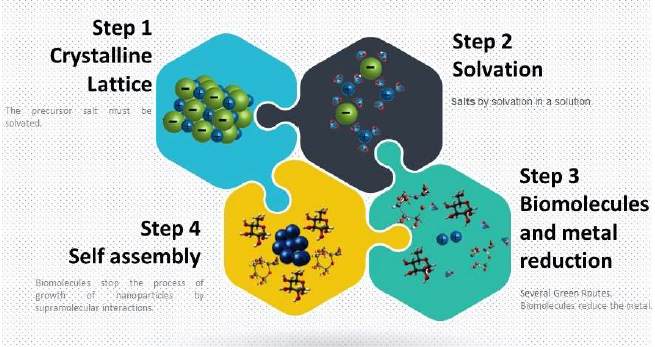
In metallic nanostructures, the synthesis routes start from a precursor, usually an inorganic salt, which already has low cytotoxicity [22]. This salt, in an aqueous medium, undergoes an ionic dissociation process. This process describes the separation of the ions that make up the salt solvated by water molecules [17]. These molecules present in the reaction medium, separate the ions, and weakens the electrostatic attraction. The ions thus separated are stable in an aqueous medium until the minimum salt concentration necessary for stabilization is established. Ionic saturated solutions do not have long-term stability. This happens because part of the salt remains in its crystalline structure, without water molecules to solvate them, due to the strong electrostatic attraction and dissociation enthalpy [11].
The minimum salt concentration is a determining factor for the establishment of stable systems. After establishing a minimum efficient salt concentration for the eco-friendly synthesis process, the dispersion of ions in a natural extractive medium is established [11,22-25]. The biomolecules, such as phenolic acids, flavonoids, anthocyanins, and other complex biomolecules, are highly reducing. These ions undergo a reduction process when they establish themselves in a dispersive medium, now in their reduced state, assuming the state of its fundamental atom [18]. At this moment, the metal atoms, originating from the precursor salt in aqueous solution, through favorable chemical kinetics, at room temperature, through a self-assembly process, or through external energies, such as controlled temperature increase, initiate a chain reaction, promote the process of nucleation and growth of nanostructures [23,24]. An efficient process related to nanotechnology must establish the nanostructures’ growth process. The parameters associated with the size of the nanostructures are in the range of 1nm to 100 nm. [25-29]. Any system that favors nanostructures’ growth up to the limit within the range of a nanotechnological product is efficient (Figure 1).
Figure 1. The influence of intermolecular and supramolecular interactions in green nanotechnological systems.
For the nanostructure formation process’s efficiency, the biomolecules present in the dispersive medium are critical. The molecular volume present in the established system is called colloidal. The size and complexity of biomolecules promote the separation by intermolecular and supramolecular interactions. Moreover, it is possible to remove nanostructures favoring isolation and controlling growth [15]. A protocol for an efficient dispersive medium is essential for isolating biomolecules and establishing a supramolecular system.
The isolated biomolecules of dispersive medium interact and protect the nanoparticles. It is fundamental to an efficient colloidal system. The biomolecules from the extract establish the nanostructures by steric isolation. Nanostructures are stable by separating by size, structure, or/and resonance effect by molecules and solvation. Biomolecules interact by Van der Waals forces. It occurs because of the aromatic rings and large chains. The effect of the steric impediment establishes a stable system. The nanostructures are protected and maintain their size. The system’s stability foresees that more significant interaction between the biomolecules keeps the nanostructured system. More stable since it keeps the nanostructures well dispersed and with a regular size. [15].
The inter-relation between biomolecules promotes the establishment of a nanosystem by supramolecular interactions. The intermolecular and supramolecular interactions in the system provide efficiency in green nanostructured systems [30]. However, it is necessary to provide parameters, often not established in the literature, regarding nanostructured systems’ stability. In the scientific literature associated with these systems, little is yet discussed about the different aspects of specific biomolecules’ synthesis and isolation routes. In this way, it can predict an efficient and, at the same time, reproducible system anywhere on the planet. The efficient green nanostructures synthesis considers variables such as seasonality, pollution, and local biome, which influence the biomolecules available [31,32].
Another factor that must be considered is that these systems can be variable according to seasonality and other parameters such as temperature, humidity, and exposure to sunlight. The system’s kinetic energy can increase molecular shocks and cause an end to the stability of the colloidal system. These parameters must be evaluated for nanotechnological products [33].
Another critical parameter in the biosynthesis of nanostructures is pH. More alkaline pHs accelerate the formation of nanostructures that are more stable and monodispersed. In contrast, at more acidic pHs, aggregation is observed, consequently forming larger structures. [6,19,34]. This tendency to aggregate at more acidic pH may result from a lower availability of functional groups of the molecules present in the extracts to act in the formation of new nuclei, which give rise to new nanostructures. Thus, with less formed nuclei, there will be more ions of the metallic precursor available in solution to interact and lead to the growth of the nanostructure [35]. As the extracts’ pH may vary according to the plant, extraction protocol, or even the plant organ used, protocol optimizations may be necessary [35]. However, from a green synthesis perspective, it is expected that it will occur at the natural pH of the extract produced [36].
Many important factors establish parameters that seek to be reproducible anywhere on the planet. Synthesis conditions are defined on systems based on intermolecular and supramolecular interactions. The comprehension of these nanosystems is essential to the maintenance of the nanostructures’ colloidal system. In this way, it is established nanotechnological products efficient anywhere in the world.
References
2. Hasan S. A review on nanoparticles: their synthesis and types. Research Journal of Recent Sciences. 2015; 2277:2502.
3. Keat CL, Aziz A, Eid AM, Elmarzugi NA. Biosynthesis of nanoparticles and silver nanoparticles. Bioresources and Bioprocessing. 2015 Dec;2(1):1-1.
4. Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA. Bacteriogenic silver nanoparticles: synthesis, mechanism, and applications. Applied Microbiology and Biotechnology. 2015 Jun 1;99(11):4579-93.
5. Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitology Research. 2015 Sep 1;114(9):3315-25.
6. Parameshwaran R, Kalaiselvam S, Jayavel R. Green synthesis of silver nanoparticles using Beta vulgaris: Role of process conditions on size distribution and surface structure. Materials Chemistry and Physics. 2013 Jun 15;140(1):135-47.
7. Jaganathan A, Murugan K, Panneerselvam C, Madhiyazhagan P, Dinesh D, Vadivalagan C, et al. Earthworm-mediated synthesis of silver nanoparticles: A potent tool against hepatocellular carcinoma, Plasmodium falciparum parasites and malaria mosquitoes. Parasitology International. 2016 Jun 1;65(3):276-84.
8. Fayaz AM, Girilal M, Rahman M, Venkatesan R, Kalaichelvan PT. Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochemistry. 2011 Oct 1;46(10):1958-62.
9. Barabadi H, Tajani B, Moradi M, Kamali KD, Meena R, Honary S, et al. Penicillium family as emerging nanofactory for biosynthesis of green nanomaterials: a journey into the world of microorganisms. Journal of Cluster Science. 2019 Jul 1:1-4.
10. Li J, Webster TJ, Tian B. Functionalized nanomaterial assembling and biosynthesis using the extremophile Deinococcus radiodurans for multifunctional applications. Small. 2019 May;15(20):1900600.
11. Portnov IV, Potemkin II. Interpolyelectrolyte Complex Dissociation vs. Polyelectrolyte Desorption from Oppositely Charged Surface upon Salt Addition. The Journal of Physical Chemistry B. 2020 Jan 14;124(5):914- 20.
12. Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector-Borne and Zoonotic Diseases. 2012 Mar 1;12(3):262-8.
13. Vijayakumar M, Priya K, Nancy FT, Noorlidah A, Ahmed AB. Biosynthesis, characterisation and antibacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Industrial Crops and Products. 2013 Jan 1;41:235-40.
14. Puišo J, Jonkuvien D, Mačionien I, Šalomskien J, Jasutien I, Kondrotas R. Biosynthesis of silver nanoparticles using lingonberry and cranberry juices and their antimicrobial activity. Colloids and Surfaces B: Biointerfaces. 2014 Sep 1;121:214-21.
15. Backx BP. Nanobiotechnology and Supramolecular Mechanistic Interactions on Approach for Silver Nanoparticles for Healthcare Materials. In: Nanostructures for Antimicrobial and Antibiofilm Applications 2020 (pp. 185-207). Springer, Cham.
16. Santos OAL, Backx B. Ciências da Saúde. Atena Editora; 2019. Chapter 14, Estudo da eficiência da síntese de nanopartículas de prata em extrato de Beta vulgaris para aplicação em têxteis com atividade antimicrobiana; p. 143-157.
17. Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice”. Science 274: 99-102. Hsiao, K.(1998). Transgenic mice expressing Alzheimer amyloid precursor proteins”. Experimental Gerontology. 1996;33:883-9.
18. Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Scientific Reports. 2017 Nov 20;7(1):1-3.
19. Nalawade P, Mukherjee P, Kapoor S. Biosynthesis, characterization and antibacterial studies of silver nanoparticles using pods extract of Acacia auriculiformis. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014 Aug 14;129:121-4.
20. Peng H, Yang A, Xiong J. Green, microwaveassisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium. Carbohydrate Polymers. 2013 Jan 2;91(1):348-55.
21. Ali M, Kim B, Belfield KD, Norman D, Brennan M, Ali GS. Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract—a comprehensive study. Materials Science and Engineering: C. 2016 Jan 1;58:359-65.
22. Kirchhübel N, Fantke P. Getting the chemicals right: Toward characterizing toxicity and ecotoxicity impacts of inorganic substances. Journal of Cleaner Production. 2019 Aug 1;227:554-65.
23. Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. Journal of Nanobiotechnology. 2018 Dec 1;16(1):84.
24. Imran Din M, Rani A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness. International Journal of Analytical Chemistry. 2016 Jun 19;2016.
25. Aziz N, Fatma T, Varma A, Prasad R. Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. Journal of Nanoparticles. 2014;2014.
26. Aziz N, Faraz M, Pandey R, Shakir M, Fatma T, Varma A, et al. Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir. 2015 Oct 27;31(42):11605-12.
27. Aziz N, Pandey R, Barman I, Prasad R. Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Frontiers in Microbiology. 2016 Dec 15; 7:1984.
28. Aziz N, Faraz M, Sherwani MA, Fatma T, Prasad R. Illuminating the anticancerous efficacy of a new fungal chassis for silver nanoparticle synthesis. Frontiers in Chemistry. 2019 Feb 8; 7:65.
29. Prasad R, Jha AK, Prasad K, editors. Exploring the realms of nature for nanosynthesis. New York: Springer; 2018 Oct 24.
30. Reimers JR, Ford MJ, Marcuccio SM, Ulstrup J, Hush NS. Competition of van der Waals and chemical forces on gold–sulfur surfaces and nanoparticles. Nature Reviews Chemistry. 2017 Feb 8;1(2):1-3.
31. Liu YS, Chang YC, Chen HH. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. Journal of Food and Drug Analysis. 2018 Apr 1;26(2):649-56.
32. Grassian VH. When size really matters: sizedependent properties and surface chemistry of metal and metal oxide nanoparticles in gas and liquid phase environments. The Journal of Physical Chemistry C. 2008 Nov 27;112(47):18303-13.
33. Santos DS, Santos Filho DL. AS, Santana SDCJ, de Souza MF et al. Can green synthesis of nanoparticles be efficient all year long. Nanomaterial Chemistry and Technology. 2019:32-5.
34. Ramesh PS, Kokila T, Geetha D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015 May 5; 142:339-43.
35. Akhtar MS, Panwar J, Yun YS. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustainable Chemistry & Engineering. 2013 Jun 3;1(6):591-602.
36. Barua S, Konwarh R, Bhattacharya SS, Das P, Devi KS, Maiti TK, et al. Non-hazardous anticancerous and antibacterial colloidal ‘green’ silver nanoparticles. Colloids and Surfaces B: Biointerfaces. 2013 May 1; 105:37-42.