Abstract
Background: Due mostly to the growth of multidrug resistant infections, antimicrobial resistance (AMR) has limited the arsenal of medical professionals against infectious diseases on a global scale. Thus, the purpose of this systematic review and meta-analysis is to present the pooled prevalence, bacterial profile, and current trend of antibiotic resistance in pregnant women who have significant bacteriuria in East Africa.
Methods: The current review covers every full-text publication in English language that is available (without date constraints) and is indexed in main scientific databases: Google Scholar, HINARI, PubMed, Science citation, and the Cochrane library. Studies of populations residing in East African countries reporting the prevalence of significant bacteriuria, bacterial profile and Drug resistance patterns were included. The data were extracted using the extraction form prepared in Microsoft Excel and exported to STATA 14.0 software for the outcome measure analyses and subgrouping.
Results: 8 articles were met the inclusion criteria selected for this systematic review and meta-analysis with 2,611 total enrolled participants and 405 Significant bacteriuria. The pooled prevalence of Significant bacteriuria among pregnant women in East Africa is found to be 16.79% (95% CI; 11,11–22.47; I2=94,98%; p<0.001) with substantial heterogeneity. The first most common bacterial isolate was Escherichia coli. The pooled incidence of Escherichia coli recovered from isolates of 405 urine samples was 43% (95% CI 37–48%). Multidrug resistance proportions of all isolates was 91% (95% CI 74–100%).
Conclusion: This meta analysis's findings indicated that pregnant women in the East Africa had substantial bacteriuria. The overall observed MDR were High. Due to the high prevalence of drug resistance, it is necessary to conduct frequent epidemiological surveillance for antibiotic resistance and to put in place an effective program for infection control and stewardship.
Keywords
Antimicrobial resistance, East Africa, Pregnant women, Meta-analysis, Significant bacteriuria, Urinary tract infection
Introduction
Urinary tract infections, which can afflict people of all ages, are the second most prevalent infectious disease in the community [1]. Urinary tract infections (UTIs) account for up to 35% of nosocomial infections; as such, UTIs are the most prevalent nosocomial infection and the second most common cause of bacteremia in hospitalized patients [2]. According to Okonko et al. [3], bacteria that enter the urethra through the opening and begin to grow there are typically the cause of the infection. Pregnancy, urethral length gender difference and the proximity of the female urethral meatus to the vaginal microbiome are the primary reasons why women are more prone to UTIs than males [4,5–7]. It was reported that up to 15% of women would have one episode of UTI at some time during their life [8].
Pregnant women are more likely to experience a UTI due to ureteral dilatation, increased bladder volume, and decreased bladder tone. Additionally, decreased ureteral tone can lead to ureterovesical reflux and increased urine stasis. Furthermore, women who are pregnant experience glycosuria, which promotes the proliferation of bacteria in the urine [4].
Pregnant women and their fetuses are negatively impacted by both the asymptomatic and symptomatic clinical presentations of UTI; additionally, recurrence of the illness even after effective therapy complicates care. Globally symptomatic UTIs are thought to cause up to seven million yearly visits to outpatient clinics, one million yearly trips to emergency rooms, and 100,000 yearly hospitalizations [9]. Asymptomatic bacteriuria is the most frequent problem in pregnant women as opposed to non-pregnant women; it affects 4–7% of healthy pregnancies. Preterm labor, the onset of acute and chronic pyelonephritis, preeclampsia, low birth weight, chronic renal illness, and perinatal death are only a few of the adverse obstetric outcomes and medical disorders that may arise from it [10]. Early detection and treatment of bacteriuria can prevent up to 80% of cases of pyelonephritis [11]. Treatment for asymptomatic bacteriuria might not be necessary. However, due to the probability of problems, pregnant women and their unborn fetuses must receive treatment [12].
Numerous problems associated with bacteriuria during pregnancy can be avoided with early detection and treatment. According to Pitout et al., [13], β-lactam agents such Cephalosporin, Penicillin, Monobactams, and Carbapenems are the most commonly administered antibiotics. Due to their broad range activity, good safety profile, and shown efficacy, cephalosporins are the class of antibiotics that are most frequently prescribed [14].
Urine culture and sensitivity testing is not done regularly for pregnant patients with urinary tract infections in Ethiopia. Antibiotics are typically provided without considering the drug-resistant patterns of the underlying cause or identifying the causative agent. This improper prescription practice ultimately leads to treatment failure and pregnancy complications.
Consequently, it's critical to ascertain the frequency of uropathogens in both asymptomatic and symptomatic pregnant women as well as their drug susceptibility patterns to certain antibacterial agents.
The bacteriological profile and drug susceptibility of UTI in pregnant women in East Africa have been the subject of numerous research to date [15–18]. Nevertheless, there are no pooled prevalence statistics in the East African region to inform clinicians and healthcare providers about the present prevalence of UTI and antimicrobial resistance (AMR) patterns in UTI during pregnancy. Thus, the purpose of this systematic review and meta-analysis is to present the pooled prevalence, bacterial profile and current trend of antibiotic resistance in pregnant women who have significant bacteriuria in East Africa.
Method
Review question
- What are the Bacterial profile among pregnant women with bacteriuria in East Africa context?
- What are the Drug resistance patterns among pregnant women with bacteriuria in East Africa context?
- What is the pooled prevalence of UTI among pregnant women in East African context?
Reporting
This systematic review and meta-analysis is based on a recommended methodology by the Joanna Briggs Institute’s approach and followed the Preferred Reporting Items for Systematic review and Meta-Analysis for Protocols (PRISMA-P) 2020 guidelines.
Search strategy
The current review covers every full-text publication in English language that is available (without date constraints) and is indexed in main scientific databases: Google Scholar, HINARI, PubMed, Science citation and the Cochrane library. A predetermined telephone system search strategy was created using the PICO (patients, interventions, comparators, and outcomes) equivalents: patients, exposures of interest, comparison, and outcomes. This allowed for the identification of potential search terms and the retrieval of Archives of the best possible set of results. As a result, the PECO framework that we used for our search was described as follows: population (P): pregnant women with bacteriuria in East Africa; exposure (E): significant bacteriuria; comparator (C): pregnant women without significant bacteriuria in East Africa; outcome (O): bacteria profile, antibiotic resistance profile. Those search terms or phrases including were: “Drug Resistance”, “Bacterial”, “Antibacterial Drug Resistance”, “Antibiotic Resistance”, “Bacterial”, “Bacteriuria”, “Bacteriurias”, “Pregnant Women”, “Pregnant Woman”, “Woman”, “Pregnant”, “Women”, “Pregnant”, “Africa”, “Eastern”, “East Africa”, “Eastern Africa”, “British Indian Ocean Territory”. Using those key terms, the following search map was applied for all Eastern African countries: ((((Drug Resistance, Bacterial[MeSH Terms]) OR (((Drug Resistance, Bacterial[Title/Abstract]) OR (Antibacterial Drug Resistance[Title/Abstract])) OR (Antibiotic Resistance, Bacterial[Title/Abstract]))) AND ((Bacteriuria[MeSH Terms]) OR ((Bacteriuria[Title/Abstract]) OR (Bacteriurias[Title/Abstract])))) AND ((Pregnant Women[MeSH Terms]) OR ((((Pregnant Women[Title/Abstract]) OR (Pregnant Woman[Title/Abstract])) OR (Woman, Pregnant[Title/Abstract])) OR (Women, Pregnant[Title/Abstract])))) AND ((Africa, Eastern[MeSH Terms]) OR ((((Africa, Eastern[Title/Abstract]) OR (East Africa[Title/Abstract])) OR (Eastern Africa[Title/Abstract])) OR (British Indian Ocean Territory[Title/Abstract]))). On both Cochran Library, and Google scholar, a build in text search were used on the advanced search section of the sources.
A manual search for more pertinent studies in order to identify original papers that we would have lost, we simultaneously carried out exploitation references from retrieved articles and related systematic reviews. Additionally, several analysis centers and the digital library of higher establishments located in African nations were jointly investigated in order to find unpublished publications relevant to the current systematic review and meta-analysis. The searching method within the advanced looking databases was designed to leverage the "Medical Subject Headings (MeSH)" and "All fields" by connecting the Boolean operator terms "AND" and "OR" as needed. The pre-planned MEDLINE search approach was used to find pertinent papers in other databases.
Study selection and screening
The retrieved studies were exported to Endnote version 8 reference managers to remove duplicate studies. Three investigators (MG, AM, and ET) independently screened the selected studies using article's title and abstracts before retrieval of full-text papers. We used pre-specified inclusion criteria to further screen the full-text articles. Disagreements were discussed during a consensus meeting with other reviewer for the final selection of studies to be included in the systematic review and meta-analysis.
Inclusion and exclusion criteria
In this systematic review and meta-analysis, we have included studies of populations residing in East African countries reporting the prevalence of significant bacteriuria, bacterial profile, and drug resistance patterns. Those studies had published in English language without date restriction were included in this systematic review and meta-analysis. Citations without abstract and/or full-text, anonymous reports, editorials, letters, commentaries, reviews, and qualitative studies were excluded from the analysis. In addition, studies conducted among populations of world origin residing outside East Africa, Non-accessible articles because of un-published, un-retrievable from the internet were excluded. In addition, studies, which did not report our outcome of interest, were excluded after reviewing their full texts.
Study area
Only articles conducted in East African countries. East Africa is one part of Africa which is located at the horn of this continent, and it includes Burundi, Djibouti, Eritrea, Ethiopia continent, Somalia, South Sudan, Sudan, Tanzania, and Uganda.
Study design
All observational studies (cross-sectional, case controls and cohort) that contain original data reporting the prevalence of significant bacteriuria, bacterial profile and Drug resistance patterns in East African countries were considered.
Language
Literatures published in English language were incorporated.
Population
Studies conducted among pregnant women attending antenatal care were considered.
Publication condition
Both published and unpublished articles which reported the prevalence of significant bacteriuria, bacterial profile and Drug resistance patterns among pregnant women in East African countries were considered.
Quality assessment
To evaluate the quality of the studies included in this review, the researcher applied the JBI tool modified for cross-sectional studies’ quality assessment [19]. The tool consists of three major parts; the first part has five stars and assesses the methodological quality of each study. The second part of the tool assesses the comparability of the studies. The last part determines the quality of the original articles with respect to their statistical analysis. Using the tool as a checklist, the qualities of each of the original articles were evaluated. Articles with medium (fulfilling 50% of quality assessment criteria) and high quality (≥6 out of 10 scales) were considered to be included for the analysis [19].
Data Extraction
The authors developed data extraction form on the excel sheet in considering country, year of publication, study design and prevalence of significant bacteriuria reported. The data extraction sheet was piloted using 4 papers randomly, and it was adjusted after piloted the template. Two authors (MG and AM) extracted the data using the extraction form in collaboration and any discrepancy resolved through discussions with the third authors (ET) when required. These authors checked the correctness of the data independently. The mistyping of data was resolved through crosschecking with the included papers.
Synthesis of results
The authors exported the data to STATA 14 for analysis after it was extracted in excel sheet. We pooled the overall prevalence of significant bacteriuria by a random effect meta-analysis model. We examined the heterogeneity of effect size using Q statistic and the I2 statistics. In this study, the I2 statistic value of zero indicates true homogeneity, whereas the value 25, 50, and 75% represented low, moderate and high heterogeneity, respectively [19]. Subgroup analysis for congenital significant bacteriuria was done by the study country, and year of publication. Sensitivity analysis was employed to examine the effect of a single study on the overall estimation. Publication bias was checked by funnel plot and more objectively through Egger’s regression test.
Outcome measurement
This review has 3 major outcomes. The primary outcome was to see the pooled estimates of significant bacteriuria among pregnant women in East Africa. The second outcome was to assess bacterial profile and finally pattern of multidrug resistance of common uropathogens among pregnant women.
Results
Literature identification and characteristics of included studies
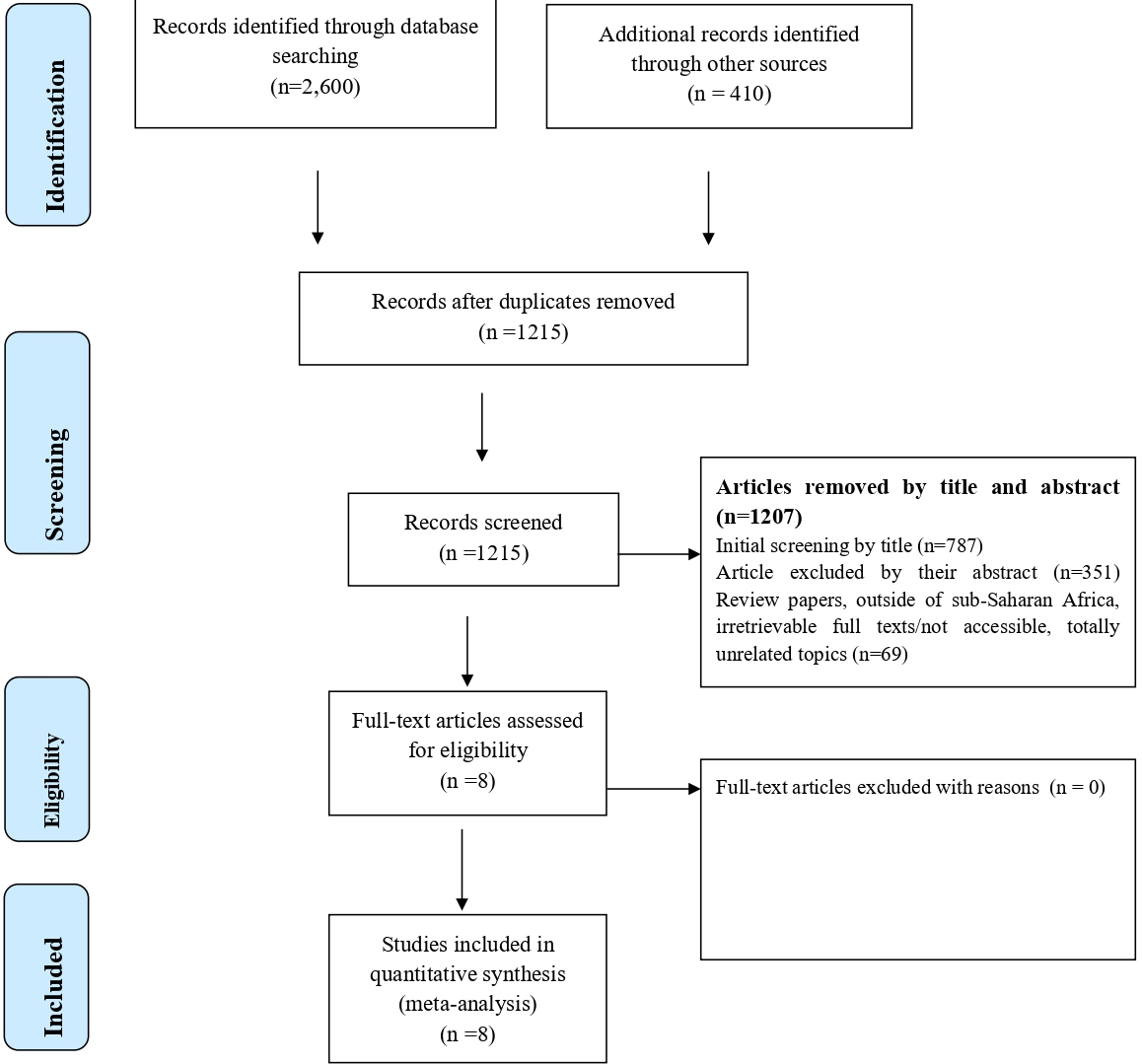
A total of 3,100 studies were identified; 2,600 Records identified through different database searching and 410 from other sources. After duplication removed, a total of 1,215 articles remained (1,885 removed by duplication). We further excluded 787 studies because they were not relevant up-on reviewing the titles. Then the full-text of the remaining 428 articles was downloaded and fully assessed for fulfilling the required criteria. We again excluded 351 articles by their abstract and Review papers, outside of sub-Saharan Africa, irretrievable full texts/not accessible, totally unrelated topics (n=69). Finally, 8 full-text studies were reviewed, and all of the articles met the inclusion criteria selected for this systematic review and meta-analysis with total 2,611enrolled participants and 405 Significant bacteriuria were detected (Figure 1). Figure 1 summarizes the process of identification, screening, checking for eligibility and inclusion into the final analysis.
Figure 1. PRISMA adapted flow diagram showed the results of the search and reasons for exclusion.
Characteristics of included studies
A total of 8 studies were included in this systematic review and meta-analyses. Significant bacteriuria was detected in 405 urine samples out of a total 2,611 samples taken from pregnant women during their antenatal care visit to the health care system. Of them 5 studies were done in Ethiopia [16,18,20–22] while 1 was in Sudan [23], 1 in Tanzania [24], 1 in Uganda [25]. Based on the study design used, 7 studies were done by cross-sectional study, 1 study conducted by case control. All of these studies were conducted from February 2010 to March 2019. Majority 6/8 (75%) of the studies were published in the year 2015–2021 and the remaining, 2/8(25%) were published in the year 2011 and 2012. The total numbers of enrolled participants included under this review ranged from 169 to 587 and the total number of Significant bacteriuria detected were ranged from 28 to 110 (Table 1). Table 1 summarizes the baseline characteristics and prevalence of significant bacteriuria among pregnant women.
|
Author |
Publication year
|
Country |
Study period
|
Sample size |
Number of isolates |
Prevalence |
Gram positive |
Gram Negative |
Symptomatic |
Asymptomatic |
JBI score |
|
Hamdan et al. [23] |
2011
|
Sudan |
February 2010 to June 2010
|
235 |
33 |
14.70% |
45.40%
|
54.50% |
12.10% |
14.70% |
8 |
|
Alemu et al. [16]
|
2012 |
Ethiopia |
March 22, 2011 to April 30, 2011
|
385 |
40 |
10.40% |
32.50% |
67.50% |
15.3% |
9% |
7 |
|
Abu et al. [20]
|
2021 |
Ethiopia |
January 2019 to February 2019
|
283 |
39 |
13.78% |
28.20% |
71.80% |
--- |
14% |
5 |
|
Tadesse et al. [18]
|
2018 |
Ethiopia |
January 1, 2018 to April 30, 2018
|
259 |
55 |
21.20% |
35.90% |
64.10% |
--- |
21% |
6 |
|
Taye et al. [21]
|
2018 |
Ethiopia |
January 2014 to April 2014
|
169 |
44 |
26.00% |
0.00% |
100.00% |
35.30% |
22% |
6 |
|
Gessese et al. [22]
|
2017 |
Ethiopia |
March 2016 to December, 2016
|
300 |
56 |
18.70% |
30.40% |
69.60% |
20.40% |
18% |
8 |
|
Kaduma et al. [24]
|
2019 |
Tanzania |
October 2019 to December, 2019
|
393 |
110 |
28% |
0.00% |
100.00% |
46.70% |
27.30% |
7 |
|
Nteziyaremye et al. [25]
|
2020 |
Uganda |
January 2019 to March 2019
|
587 |
28 |
3.75% |
46.50% |
53.50% |
--- |
4% |
6 |
Meta Analyses
Prevalence of significant bacteriuria among pregnant women
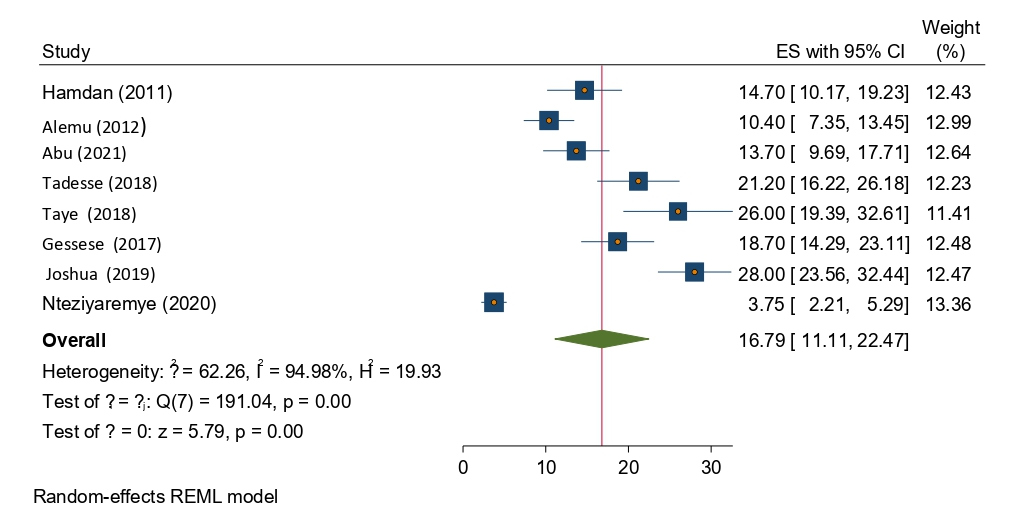
From the total of (n=8) studies reported, the prevalence of significant bacteriuria among pregnant women in Eastern Africa. The prevalence of significant bacteriuria was ranged from 3.75% to 28%. The random-effects model analysis from those studies revealed that, the pooled prevalence of significant bacteriuria among pregnant women attending antenatal care in Eastern Africa was found to be 16.79% (95% CI; 11.11–22.47; I2=94.98%; p<0.001) (Figure 2).
Figure 2. Forest plot showing the overall prevalence of significant bacteriuria among pregnant women in East Africa, 2024.
Subgroup analysis for the pooled prevalence of significant bacteriuria among pregnant women in Eastern Africa
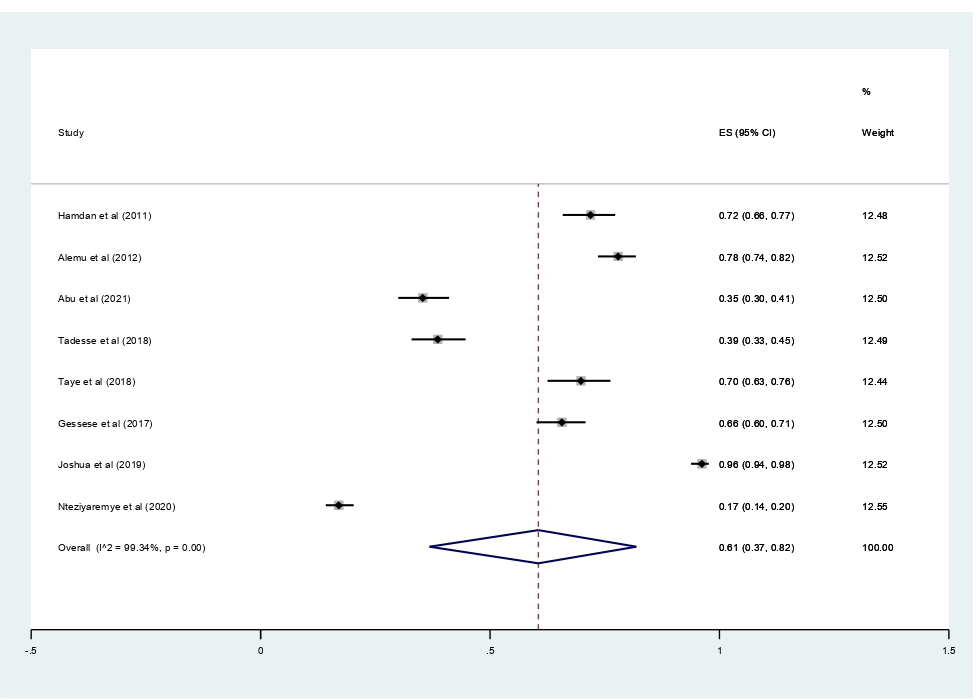
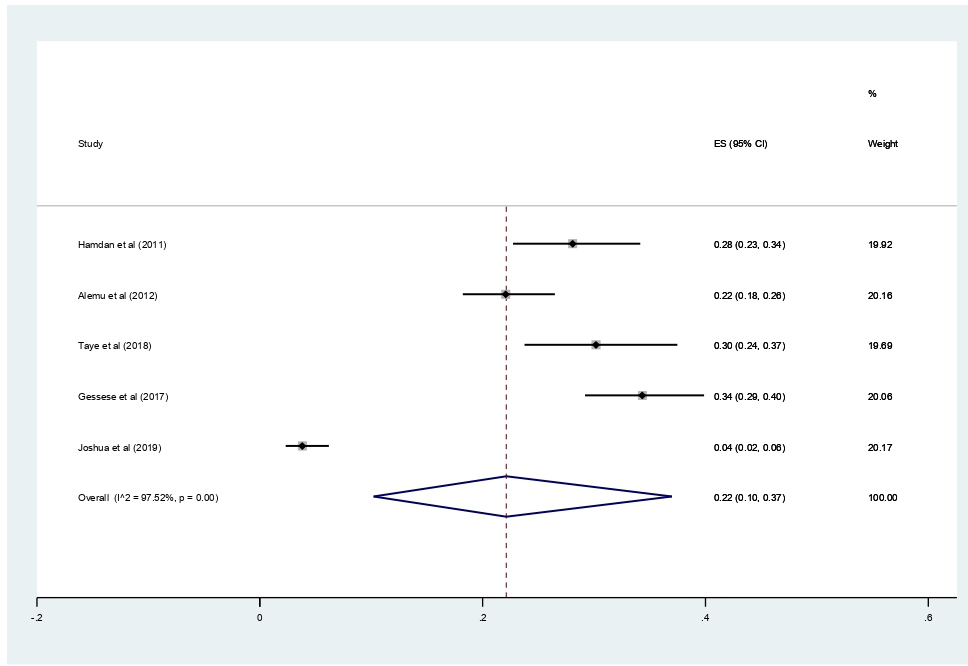
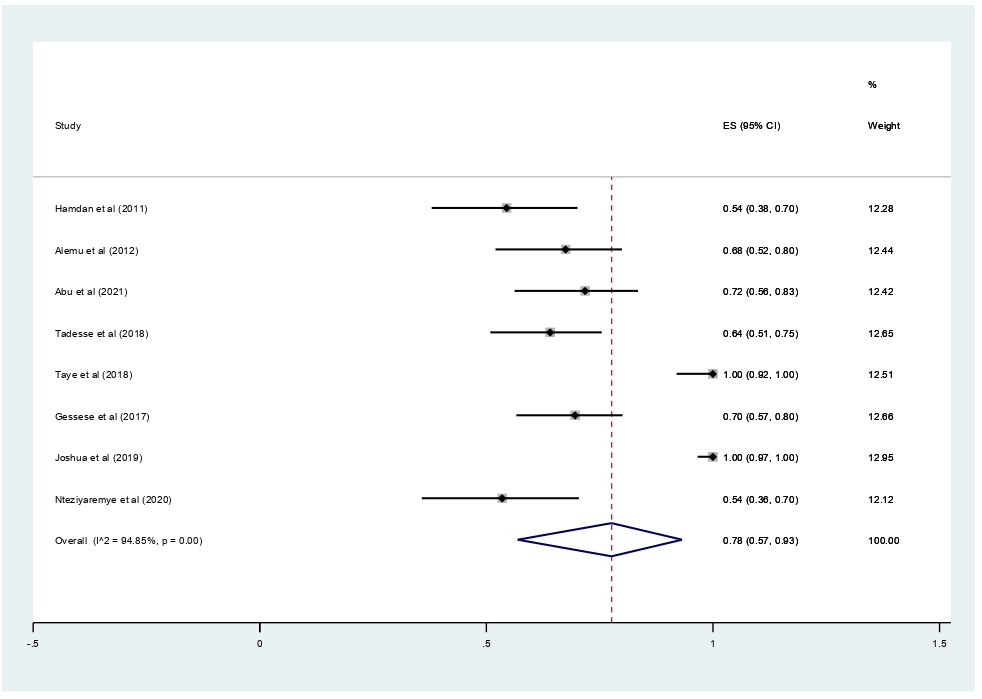
Subgroup analyses revealed that the proportion of pregnant women developed asymptomatic bacteriuria was 61% (95% CI 37%–42%, I2 = 99.34%, p<0.001) as shown in Figure 3, whereas only 22% (95% CI 10–37%, I2 = 97.52%, p<0.001, Figure 4) developed symptomatic bacteriuria. Gram-negative bacteria accounted for 78% (95% CI 57–93%, I2=94.85%, p<0.001, Figure 5) of bacteriuria in participants included in this study.
Figure 3. Forest plot showing the prevalence of asymptomatic bacteriuria among pregnant women in East Africa, 2024.
Figure 4. Forest plot showing the prevalence of symptomatic bacteriuria among pregnant women in East Africa, 2024.
Figure 5. Forest plot showing the prevalence of Gram-negative bacteriuria among pregnant women in East Africa, 2024.
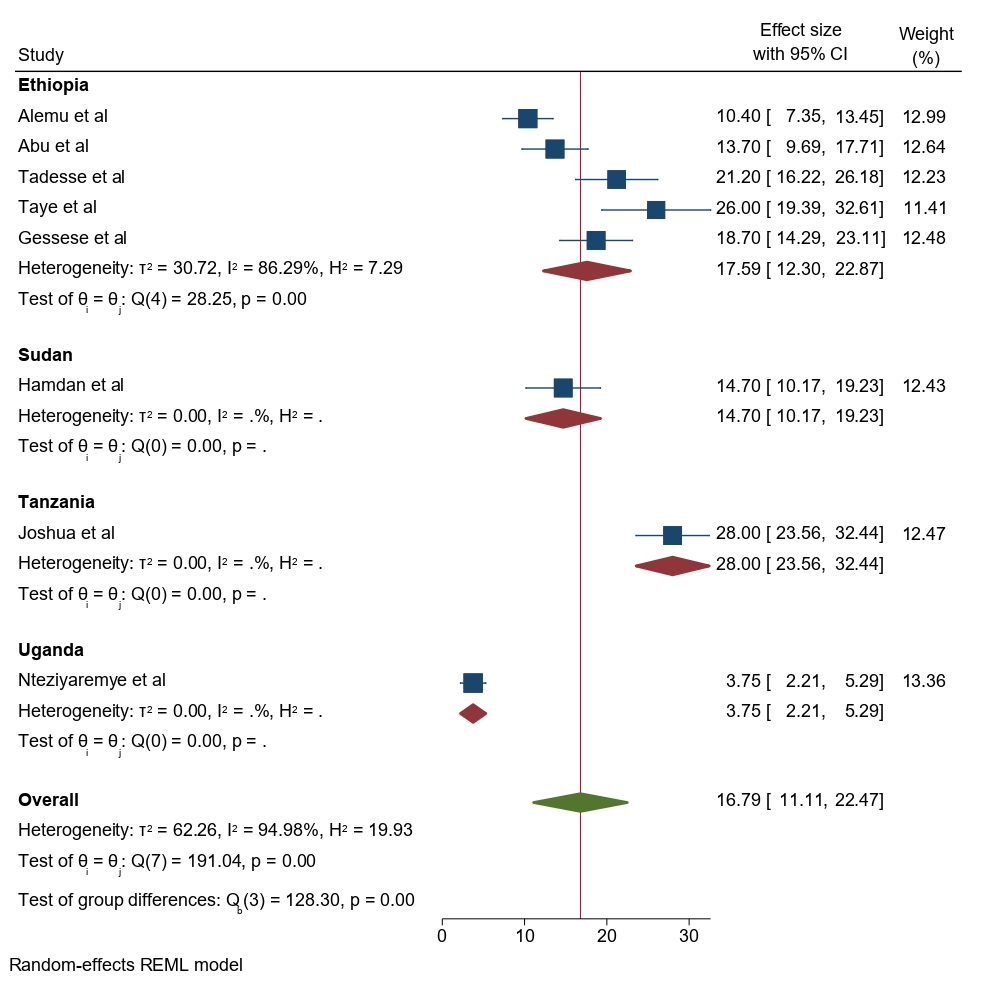
The subgroup analysis was done through stratified by country and year of publication. Based on the country study, the pooled prevalence of significant bacteriuria among pregnant women was found to be 17.59% (95% CI: 12.30–22.87) in Ethiopia, 14.70% (95% CI: 10.17, 19.23) in Sudan, 28.00% (95% CI: 23.56, 32.44) in Tanzania, and 3.75% (95%CI: 2.21, 5.59) in Uganda (Figure 6).
Figure 6. Forest plot showing the prevalence of bacteriuria among pregnant women by country study in East Africa, 2024.
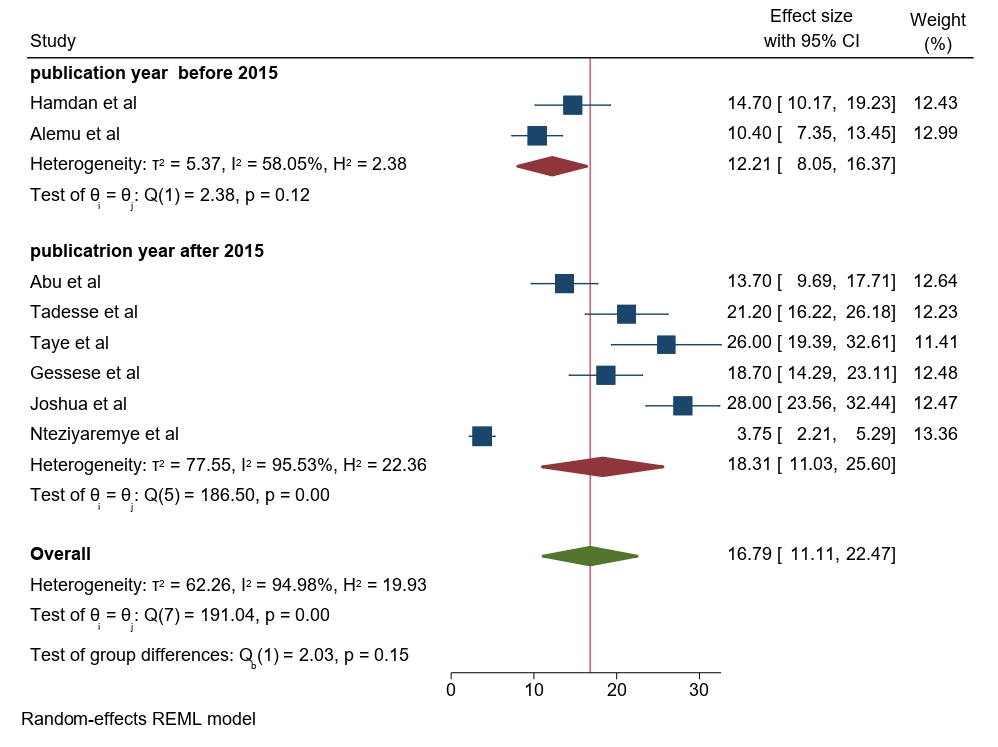
Based on the year of publication, the prevalence of significant bacteriuria among pregnant women was 12.21% (95% CI: 8.05–16.37) in studies published before 2015 and 18.31% (95% CI: 11.03–25.60) in studies published after 2015 (Figure 7).
Figure 7. Forest plot showing the prevalence of bacteriuria among pregnant women by publication year in East Africa, 2024.
Types of bacterial isolates
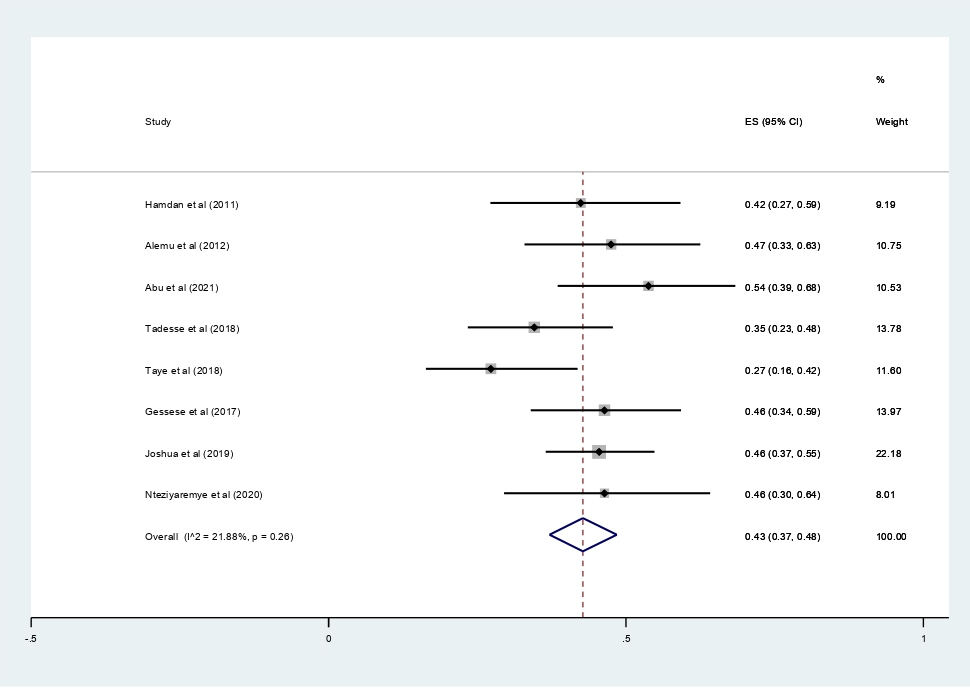
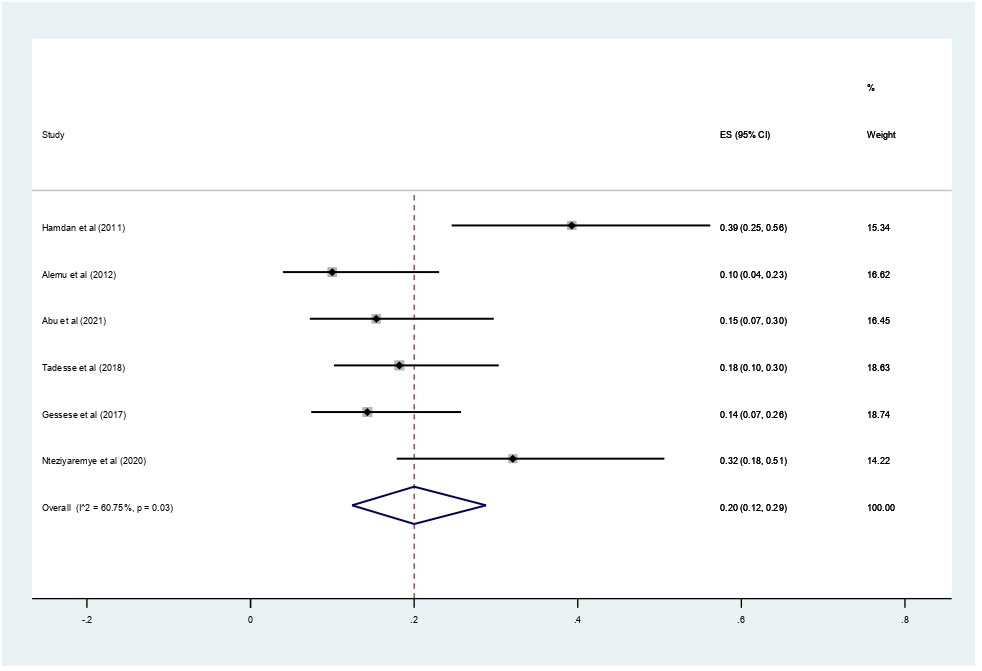
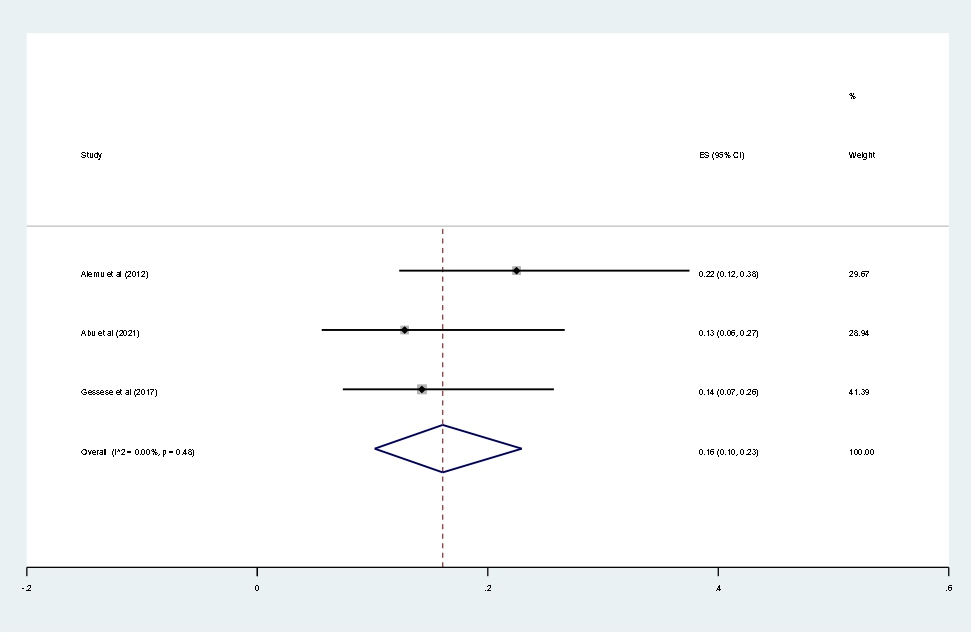
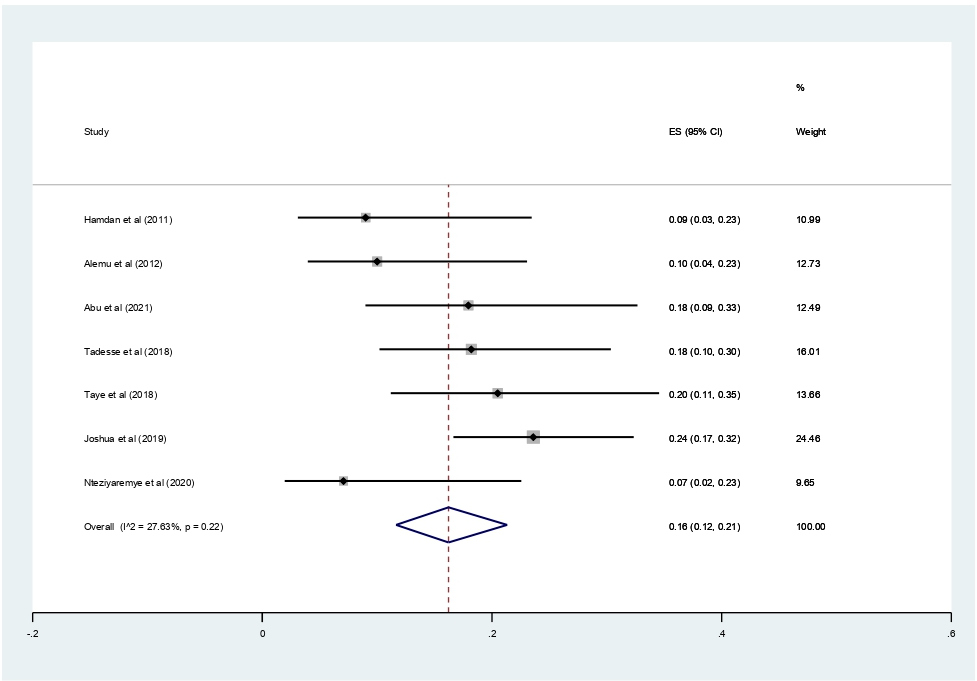
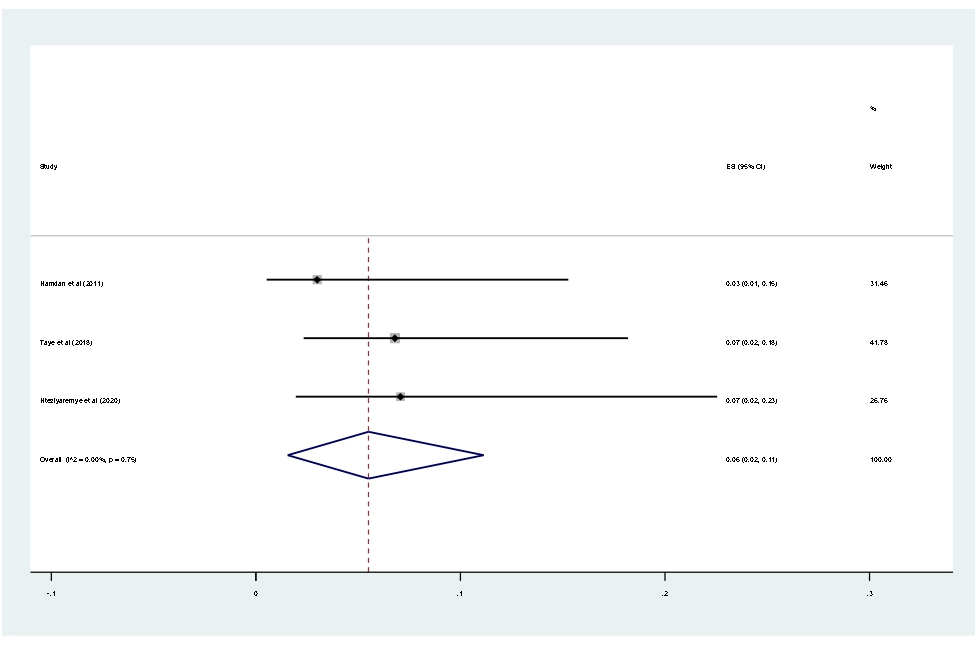
Data of 2,611 urine samples from 8 studies conducted in 4 countries were pooled. Different types of bacterial isolates were extracted from studies included in this review. The most common bacterial isolate of both asymptomatic and symptomatic bacteriuria was E. coli with an overall prevalence of 43% (95% CI 37–48%, Figure 8) followed by Staphylococcus aureus 20% (12–29%, Figure 9), Coagulase negative Staphylococci 16% (10–23% Figure 10), Klebsiella species 16% (12–21%, Figure 11), and P. aeruginosa 0.1% (2–11%, Figure 12).
Figure 8. Forest plot showing the prevalence of E. coli among pregnant women in East Africa, 2024.
Figure 9. Forest plot showing the prevalence of S. aures among pregnant women in East Africa, 2024.
Figure 10. Forest plot showing the prevalence of CONs among pregnant women in East Africa, 2024.
Figure 11. Forest plot showing the prevalence of K. pneumonia among pregnant women in East Africa, 2024.
Figure 12. Forest plot showing the prevalence of P. arogenosa among pregnant women in East Africa, 2024.
Antibiotic resistance pattern of isolates
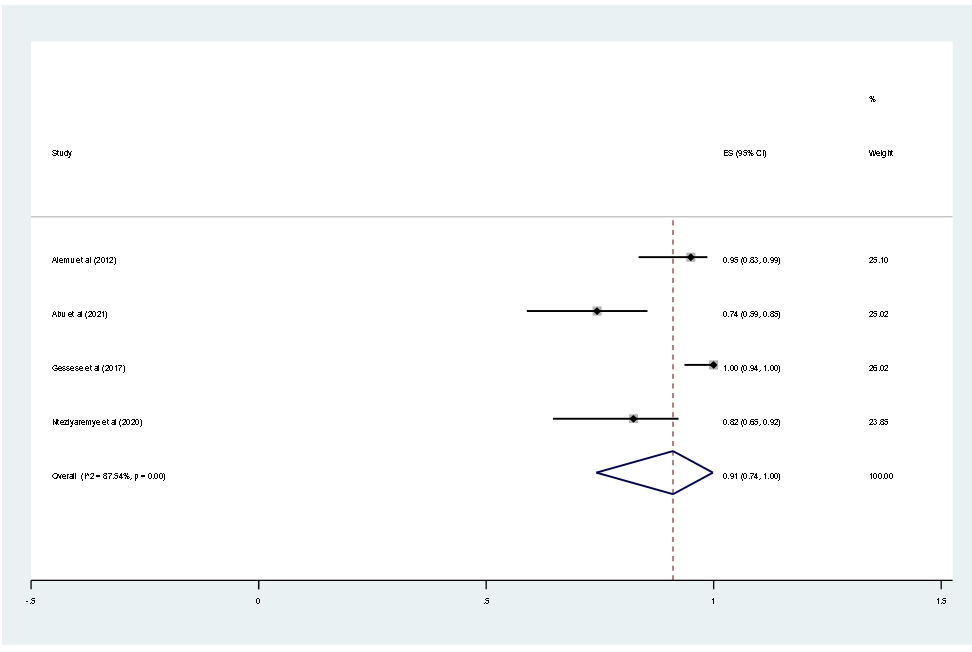
This meta-analysis result showed the prevalence of multidrug resistance isolated from urine samples of pregnant women with significant bacteriuria was 91% (95% CI; 74–100; I2=87.54%; p<0.001, Figure 13).
Figure 13. Forest plot showing the prevalence of MDR among pregnant women in East Africa, 2024.
Heterogeneity
Sensitivity analysis for significant bacteriuria: We employed a leave-one-out sensitivity analysis to identify influence of individual study on the pooled prevalence of significant bacteriuria in East Africa. The results of this sensitivity analysis showed that these findings were not dependent on a single study.
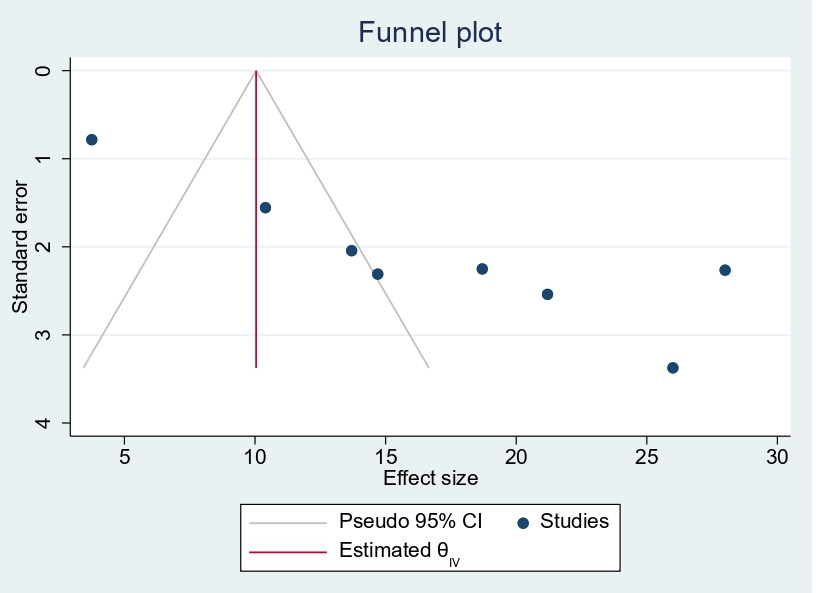
Publication bias: A funnel plot showed asymmetrical distribution. The Egger’s regression test-value was (β=9.47, 95% CI 4.30–12.30, p<0.001, Figure 14), which indicated the presence of publication bias.
Figure 14. Funnel plot showing publication bias among pregnant women in East Africa, 2024.
Discussion
Increased Enterobacteriaceae associated with UTI during pregnancy have negative impact on pregnant women and birth outcomes, such as low birth weight, intrauterine growth restriction, fetal death, premature rupture of the membranes and neonatal infections [26]. Studies are needed to determine UTI colonization and infection rates among pregnant women and their antimicrobial susceptibility patterns in East Africa. Thus, this meta-analysis was intended to determine the total pooled prevalence of bacteriuria among asymptomatic and symptomatic pregnant women, the common bacterial causative agent, and their antimicrobial susceptibility patterns.
The pooled prevalence of significant bacteriuria among pregnant women attending antenatal care in Eastern Africa was found to be 16.79%. Our result is in line with previous findings in Ethiopia (15.37%) [27]. In contrast to our findings, significantly higher pooled prevalence of significant bacteriuria has been reported in sub-Saharan Africa (32.12% versus 16.79%) [28]. Lower finding of significant bacteriuria among pregnant women attending antenatal care has been reported from another study done in Ethiopia (14.50% versus 16.79%) [29]. Also, a little bit lower prevalence of significant bacteriuria has been reported in pregnant women with bacteriuria in Ethiopia (15 % versus 16.79%) [30]. These may be connected to the various prenatal variables that are avoidable and modifiable. Anemia, lowered immunity, sexual activity, early marriage, lower socioeconomic strata, multiparity, and gestational age are a few of these [31].
Up to 40% of pregnant women with untreated asymptomatic bacteriuria gradually develop acute pyelonephritis, which has all the accompanying deleterious effects on the mother and the fetus [32]. In this meta-analysis study, the proportion of pregnant women who developed asymptomatic bacteriuria was 61%. Significantly lower findings were observed in previous findings in Ethiopia [33]. The magnitude of asymptomatic bacteriuria found in this study is significantly higher compared to findings in study done in Africa (11.1% versus 61%) [34]. Moreover, the prevalence of asymptomatic bacteriuria was found high in this study compared to studies done in Latin America (18.45%) [35]. The difference from latter study may be due to difference in study populations. In this study, the prevalence of symptomatic UTI was found to be 22%. This finding is higher compared to other study findings done in Ethiopia (6%) [33]. The possible explanation for this is, most of the study participants in the present study (93.8%) were in the 2nd and 3rd trimesters of pregnancy. The risk of acquiring bacteriuria during pregnancy increases with gestational age from 0.8% in the 12th week to 1.93% at the end of pregnancy [12].
In this meta-analysis, Gram negative bacteria accounted for 78% of culture-positive urine samples. However, Gram positive accounts for a relatively small proportion. This might be because Gram negative bacteria have a special structure called pilus adhesions that aid in attachment to uroepithelial cells and shield the bacteria from urine lavage, allowing for tissue invasion and multiplication that leads to invasive infection and pregnancy-related pyelonephritis. The Proportion of Gram-negative bacteria found in this study is high compared to findings in studies done in Ethiopia (64% versus 78%) [33] and another study done in Ethiopia by Getaneh et al., (55% versus 78%) [36].
In this meta-analysis, E. coli was the first most common bacterial isolate, which accounts for 43% of all isolates. Many studies reported E. coli as the first most common bacteria isolated from the urine culture of pregnant women [27,28,32,37–39]. The second common etiologic agent of bacteriuria isolated from pregnant women was Staphylococcus aureus which accounts for 20% of all isolated bacteria. Unlike this, the study done in Ethiopia by showed that the second common etiologic agent of bacteriuria isolated from pregnant women was CONs which accounts for 22% of all isolated bacteria [32]. K. pneuomonae which accounts for 16% of all isolated bacteria, was the third most frequent bacteria in this study. However, some studies showed that K. pneuomonae is the second common etiologic agent of bacteriuria isolated from pregnant [37–39]. Bacterial causative agent of UTIs might vary geographically and even over time within a population [40].
In this meta-analysis, overall MDR was observed among 91% of all isolates. However, the proportion of MDR was found slightly high in this study compared to studies done in Ethiopia (89%) [32]. The increased frequency of ESBL-producing isolates by various gram-negative bacterial isolates was the cause of the higher record of MDR that was reported from this study. This makes sense because a large number of the isolates that produce these enzymes are also resistant to trimethoprim, quinolones, and aminoglycosides. Additionally, plasmids frequently co-express other resistance mechanisms [26].
Strength and limitations
The main strength of this meta-analysis is that it was the first to be reported from East Africa. The authors used a pre-specified protocol for search strategy, data abstraction, and quality assessment. The included studies were low risk of bias based on JBI quality assessment checklist. Moreover, we employed subgroup analysis based on study country and year of publication and sensitivity analysis to identify the small study effect and the risk of heterogeneity. In this meta-analysis, there is also significant publication bias, which is graphically assessed using funnel plots and statistically checked for the presence of a small study effect using the Egger test, affecting the interpretation of the findings. Furthermore, the reader should be aware that the protocol for this study was made public and registered on Prospero (with registration number:--), which could lead to avoid bias.
Conclusion
This meta analysis's findings indicated that pregnant women in the East Africa had substantial bacteriuria. E coli was the most common bacterial isolate. The second most common etiologic agent of bacteriuria isolated from pregnant women was Staphylococcus aureus. K. pneuomonae was the third most frequent bacteria in this study.
The overall observed MDR was high. The high prevalence of bacteriuria in the East Africa’s pregnant women serves as a warning to clinicians to check for the disease at least once during antenatal care using a urine culture, and to intervene if the results turn out positive. Given the high prevalence of drug resistance, frequent epidemiological surveillance of antibiotic resistance is necessary, along with the implementation of effective infection control and stewardship programs.
Acknowledgements
Not applicable.
Funding
The authors received no specific funding for this work.
Availability of Data and Materials
All relevant data are within the manuscript and its supporting information files.
Author’s Contribution
MG, DE, TE, and AM: Writing-original draft, conceptualization, and investigation; LW, NK, and ET: Conducted the formal data analysis, writing-review, and editing; MH, MM and AA: Search strategy development, formal data analysis, writing-review, and editing. All authors read and approved the final manuscript for publication.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
References
2. Kolawole AS, Kolawole OM, Kandaki-Olukemi YT, Babatunde SK, Durowade KA, Kolawole CF. Prevalence of urinary tract infections (UTI) among patients attending Dalhatu Araf Specialist Hospital, Lafia, Nasarawa state, Nigeria. International journal of medicine and medical sciences. 2009 May;1(5):163–7.
3. Okonko IO, Ijandipe LA, Ilusanya OA, Donbraye-Emmanuel OB, Ejembi J, et al. Incidence of urinary tract infection (UTI) among pregnant women in Ibadan, South-Western Nigeria. African Journal of Biotechnology. 2009;8(23): 6649–57.
4. Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010 Mar;60(3):213–6.
5. Vanga A, Malhotra V, Ripley K, Khardori N. Controversies in Treating Asymptomatic Bacteriuria and Urinary Tract Infection: A Case Based Review of Antibiotic Use in Renal Transplant Patients and its Impact on the Development of Resistance. Indian J Pediatr. 2020 Jan;87(1):51–5.
6. Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD007855.
7. Jacobs KM, Thomas-White KJ, Hilt EE, Wolfe AJ, Waters TP. Microorganisms Identified in the Maternal Bladder: Discovery of the Maternal Bladder Microbiota. AJP Rep. 2017 Jul;7(3):e188–96.
8. Bates J, Thomas-Jones E, Pickles T, Kirby N, Gal M, Bongard E, et al. Point of care testing for urinary tract infection in primary care (POETIC): protocol for a randomised controlled trial of the clinical and cost effectiveness of FLEXICULT™ informed management of uncomplicated UTI in primary care. BMC Fam Pract. 2014 Nov 25;15:187.
9. Kripke C. Duration of therapy for women with uncomplicated UTI. Am Fam Physician. 2005 Dec 1;72(11):2219.
10. McCormick T, Ashe RG, Kearney PM. Urinary tract infection in pregnancy. The Obstetrician & Gynaecologist. 2008 Jul;10(3):156–62.
11. Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest. 2008 Oct;38 Suppl 2:50–7.
12. Blomberg B, Olsen BE, Hinderaker SG, Langeland N, Gasheka P, Jureen R, et al. Antimicrobial resistance in urinary bacterial isolates from pregnant women in rural Tanzania: implications for public health. Scand J Infect Dis. 2005;37(4):262–8.
13. Laudano JB. Ceftaroline fosamil: a new broad-spectrum cephalosporin. J Antimicrob Chemother. 2011 Apr;66 Suppl 3:iii11–8.
14. Pitout JD, Nordmann P, Laupland KB, Poirel L. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005 Jul;56(1):52–9.
15. Assefa A, Asrat D, Woldeamanuel Y, G/Hiwot Y, Abdella A, Melesse T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia. Ethiop Med J. 2008 Jul;46(3):227–35.
16. Alemu A, Moges F, Shiferaw Y, Tafess K, Kassu A, Anagaw B, et al. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. 2012 Apr 25;5:197.
17. Ferede G, Yismaw G, Wondimeneh Y, Sisay Z. The prevalence and antimicrobial susceptibility pattern of bacterial uropathogens isolated from pregnant women. Eur J Exp Biol. 2012;2(5):1497–502.
18. Tadesse S, Kahsay T, Adhanom G, Kahsu G, Legese H, G/Wahid A, et al. Prevalence, antimicrobial susceptibility profile and predictors of asymptomatic bacteriuria among pregnant women in Adigrat General Hospital, Northern Ethiopia. BMC Res Notes. 2018 Oct 19;11(1):740.
19. Wang H, Barisic I, Loane M, Addor MC, Bailey LM, Gatt M, et al. Congenital clubfoot in Europe: A population-based study. Am J Med Genet A. 2019 Apr;179(4):595–601.
20. Abu D, Abula T, Zewdu T, Berhanu M, Sahilu T. Asymptomatic Bacteriuria, antimicrobial susceptibility pattern and associated risk factors among pregnant women attending antenatal care in Assosa General Hospital, Western Ethiopia. BMC Microbiol. 2021 Dec 16;21(1):348.
21. Taye S, Getachew M, Desalegn Z, Biratu A, Mubashir K. Bacterial profile, antibiotic susceptibility pattern and associated factors among pregnant women with Urinary Tract Infection in Goba and Sinana Woredas, Bale Zone, Southeast Ethiopia. BMC Res Notes. 2018 Nov 8;11(1):799.
22. Gessese YA, Damessa DL, Amare MM, Bahta YH, Shifera AD, Tasew FS, et al. Urinary pathogenic bacterial profile, antibiogram of isolates and associated risk factors among pregnant women in Ambo town, Central Ethiopia: a cross-sectional study. Antimicrob Resist Infect Control. 2017 Dec 29;6:132.
23. Hamdan HZ, Ziad AH, Ali SK, Adam I. Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum North Hospital. Ann Clin Microbiol Antimicrob. 2011 Jan 18;10:2.
24. Kaduma J, Seni J, Chuma C, Kirita R, Mujuni F, Mushi MF, et al. Urinary Tract Infections and Preeclampsia among Pregnant Women Attending Two Hospitals in Mwanza City, Tanzania: A 1:2 Matched Case-Control Study. Biomed Res Int. 2019 Mar 27;2019:3937812.
25. Nteziyaremye J, Iramiot SJ, Nekaka R, Musaba MW, Wandabwa J, Kisegerwa E, et al. Asymptomatic bacteriuria among pregnant women attending antenatal care at Mbale Hospital, Eastern Uganda. PLoS One. 2020 Mar 19;15(3):e0230523.
26. Loh K, Sivalingam N. Urinary tract infections in pregnancy. Malays Fam Physician. 2007 Aug 31;2(2):54–7.
27. Getaneh T, Negesse A, Dessie G, Desta M, Tigabu A. Prevalence of Urinary Tract Infection and Its Associated Factors among Pregnant Women in Ethiopia: A Systematic Review and Meta-Analysis. Biomed Res Int. 2021 Dec 1;2021:6551526.
28. Mwang'onde BJ, Mchami JI. The aetiology and prevalence of urinary tract infections in Sub-Saharan Africa: a Systematic Review. Journal of Health & Biological Sciences. 2022 Nov 22;10(1):1–7.
29. Girma A, Aemiro A, Workineh D, Tamir D. Magnitude, Associated Risk Factors, and Trend Comparisons of Urinary Tract Infection among Pregnant Women and Diabetic Patients: A Systematic Review and Meta-Analysis. J Pregnancy. 2023 Jul 28;2023:8365867.
30. Chelkeba L, Fanta K, Mulugeta T, Melaku T. Bacterial profile and antimicrobial resistance patterns of common bacteria among pregnant women with bacteriuria in Ethiopia: a systematic review and meta-analysis. Arch Gynecol Obstet. 2022 Sep;306(3):663–86.
31. Emiru T, Beyene G, Tsegaye W, Melaku S. Associated risk factors of urinary tract infection among pregnant women at Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia. BMC Res Notes. 2013 Jul 25;6:292.
32. Kladenský J. Uroinfekce v gravidite--kdy lécit, jak lécit a cím lécit [Urinary tract infections in pregnancy: when to treat, how to treat, and what to treat with]. Ceska Gynekol. 2012 Apr;77(2):167–71.
33. Awoke N, Tekalign T, Teshome M, Lolaso T, Dendir G, Obsa MS. Bacterial Profile and asymptomatic bacteriuria among pregnant women in Africa: A systematic review and meta analysis. EClinicalMedicine. 2021 Jun 9;37:100952.
34. Hailu M, Mulugeta G, Asrat D. Prevalence and antimicrobial resistance pattern of bacterial isolates among children suspected for septicemia and urinary tract infections at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Int J Sci Eng Res. 2016;7(10):1431–44.
35. de Souza HD, Diório GRM, Peres SV, Francisco RPV, Galletta MAK. Bacterial profile and prevalence of urinary tract infections in pregnant women in Latin America: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2023 Nov 8;23(1):774.
36. Belete MA, Saravanan M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005-2016. Infect Drug Resist. 2020 May 18;13:1465–77.
37. Getaneh T, Negesse A, Dessie G, Desta M, Tigabu A. Prevalence of Urinary Tract Infection and Its Associated Factors among Pregnant Women in Ethiopia: A Systematic Review and Meta‐Analysis. Biomed Res Int. 2021 Dec 1;2021:6551526.
38. Azami M, Jaafari Z, Masoumi M, Shohani M, Badfar G, Mahmudi L, et al. The etiology and prevalence of urinary tract infection and asymptomatic bacteriuria in pregnant women in Iran: a systematic review and Meta-analysis. BMC Urol. 2019 May 30;19(1):43.
39. Pallett A, Hand K. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother. 2010 Nov;65 Suppl 3:iii25–33.
40. Kibret M, Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed. 2014 Feb;4(2):164–8.