Abstract
Background: The histone deacetylase (HDAC) family consists of epigenetic modifiers that demonstrate anti-inflammatory activities, and hence they might have a substantial role in auto-inflammatory disorders including rheumatoid arthritis (RA). Therefore, the aim of this study is to investigate the expression of HDAC1, HDAC2, and HDAC11 genes in peripheral blood mononuclear cells (PBMCs) of RA cases compared to healthy controls.
Methods: In the current study, PBMCs were isolated after blood collection from 48 RA patients and 50 healthy controls. Afterward, RNA extraction and cDNA synthesis was performed. The expression of target genes was investigated utilizing the real-time polymerase chain reaction (PCR) with a relative quantification method.
Results: Overexpression of HDAC1 (Fold change= 1.91, P-value <0.001), HDAC2 (Fold change= 1.47, P-value=0.002), and HDAC11 (Fold change= 7.77, P-value <0.001) were observed in PBMCs of RA individuals in comparison to healthy subjects. Besides, a strong correlation was found between the expressions of these three genes in PBMCs.
Conclusion: Regarding the histones and non-histone proteins that are targeted by HDACs and are involved in inflammatory responses, the significant increase in the expression of HDAC1, HDAC2, and HDAC11 suggests that these genes may have an important role in RA pathogenesis.
Keywords
HDAC1, HDAC2, HDAC11, Gene expression, Immunogenetic, Rheumatoid arthritis
Key Points
- RA (Rheumatoid Arthritis) is an inflammatory disease of synovial joints.
- Histone deacetylases (HDACs) are a superfamily of enzymes that contribute to inflammatory gene expression.
- It is hypothesized that HDACs have been involved in RA pathogenesis by targeting histones and non-histone proteins like p53 and consequently regulating the expression of inflammatory and apoptotic genes in RA patients.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease defined by chronic inflammation, synovial hyperplasia, and joint swelling [1]. The disease primarily involves the small joints in the hands and feet [2]. Its prevalence in Western societies is 0.5 to 1 percent of the overall population and affects women 3 to 4 times more than men [3]. So far, a number of genetic factors, that contribute to the susceptibility to autoimmune diseases such as RA, have been identified, however, genetic association studies cannot explain the exact cause of autoimmune diseases alone and it seems that environmental and epigenetic factors trigger autoimmunity in genetically predisposed persons [4,5]. The studies of twins have confirmed the importance of epigenetic factors in disease pathogenesis rather than genetic contributions [6]. It has been observed that in many cases of identical twins, there is no similarity in the incidence of RA [5,6]. Epigenetic modifications are associated with inherited changes that do not alter the gene sequence but affect transcriptional regulation [7]. The epigenetic factors importance in various diseases has been reported [7].
Histone modification is one of the epigenetics factors, which includes histone methylation, acetylation, and deacetylation. The level of histone acetylation performs a vital regulatory position in gene expression by determining the chromatin condensation and status in the chromosomal region [8]. Histone acetylation level is determined by the coordination between histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities and determines the level of gene transcription. Acetylation of histone tail lysine residues by HAT is related to enhanced gene expression. While histone hypoacetylation following the activity of HDAC enzymes leads to gene silencing [8,9]. Four different classes of HDACs are known based on phylogenetic analyses and sequence homologies of yeast HDACs. Class I HDACs (HDACs 1–3 and 8), and class II HDACs (HDACs 4-7, 9, and 10) take a significant part in the lysine deacetylation of N-terminal tails of histones. Class III includes the sirtuin protein family (Sirt1–7) which is dependent on nicotinamide adenine dinucleotide (NAD) as a cofactor. HDAC11 is the only member of the class IV HDACs. HDAC11 has similarities in the catalytic domain with class I and class II HDACs. It has a role in the stability of the CDT1 DNA replication factor and regulation of interleukin 10 expressions. Class I, II, and IV HDACs are zinc-dependent amidohydrolase enzymes [8,10,11]. The effect of HDAC activity on the pathogenesis of RA is generally obscure, but it is clear that HDACs are implicated in RA pathogenesis by targeting both histone and non-histone proteins. Non-histone proteins targeted by HDACs include P53 which is activated in the RA synovial fibroblasts (RASFs) and NF-κB which participates in the proinflammatory pathways [12]. Due to the increased synovial activity of HDACs in RA patients compared to controls, which is further increased by inflammatory stimulation, it is suggested that some members of the Zn-dependent HDACs play substantial roles in RA pathogenesis [13,14]. The level of cytoplasmic TNF-α was associated with nuclear HDAC activity, which was significantly higher in RA patients compared to those with osteoarthritis (OA) and normal controls. RA synovial tissue exhibited higher levels of HDAC1 mRNA expression compared to both OA and normal controls, and this was positively correlated with TNF-α mRNA expression. In RA, nuclear HDAC1 protein levels were elevated in contrast to OA synovial tissue. Stimulation with TNF-α notably increased nuclear HDAC activity and HDAC1 mRNA expression at 24 hours, and HDAC1 protein expression at 48 hours in RASFs [14].
The overexpression of HDAC1 is a defining feature of RA-SF. In RA-SF, HDAC1 plays two separate roles: it promotes cell proliferation and at the same time inhibits the production of MMP-1. Furthermore, HDAC2 plays a vital role in the proliferation and programmed cell death of RA-SF. Blocking HDAC2 might be a practical approach to reduce RA-SF proliferation while maintaining MMP production unchanged since both HDAC1 and HDAC2 have similar effects on RA-SF proliferation [13].
HDAC inhibitors showed anti-rheumatic properties. Besides, previous studies have exhibited that HDAC inhibitors show anti-inflammatory characteristics and can suppress the expression of inflammatory genes like IL-6, IL-8, TNFα, IL1β, and TGFβ [15,16]. Therefore it is suggested that the anti-rheumatic properties of HDAC inhibitors are achieved by suppressing proinflammatory cytokines and chemokines in RASFs [12]. Studies carried out in the last 20 years have demonstrated positive results regarding the impact of HDACi therapies on the advancement of RA [17,18].
Most studies focused on HDACs genes expression in the synovial fibroblasts (SFs) of rheumatoid arthritis patients [13,19]. Studies suggest that disruptions in HDAC activity could contribute to the abnormal gene expression seen in RA. Further exploration of the complex relationship between HDACs and RA could reveal potential therapeutic targets and novel treatment strategies for this complex autoimmune disorder. However, due to the importance of immune cell regulation in RA pathogenesis, this study aimed to evaluate the expression of the genes HDAC1, HDAC2, and HDAC11 in peripheral blood mononuclear cells (PBMCs) of people with RA opposed to healthy controls.
Material and Methods
Participants
Forty eight RA patients (over 20 years old) who were diagnosed with active RA based on the American College of Rheumatology (ACR) and 50 sex-matched healthy controls without any autoimmune disease neither in themselves nor in their first-degree relatives were included in this study. All participants were recruited from the Rheumatology Research Center (RRC) of Shariati Hospital (Table 1). After obtaining informed consent, 4 milliliters of venous blood were drawn from each participant. This study was approved by the Ethics Committee of Tehran Medical University (IR.TUMS.VCR.REC.1398.885).
Isolation of PBMCs and total RNA extraction
The isolation of PBMCs from whole blood samples was done by Ficoll-Hypaque gradient centrifugation (Sigma, St. Louis, Missouri, USA 690PB-100A). Isolated cells were used for total RNA extraction by the High Pure RNA Isolation Kit (Roche, Mannheim, Germany). Extracted RNA Concentration and purity were confirmed by NanoDrop ND-2000C (Thermo Fisher Scientific, Waltham, MA, USA). RNA with 260/280 ratios below 1.8 and upper 2.0 was discarded.
cDNA synthesis and qPCR
To synthesize first-strand cDNA from isolated RNA, the CellAmp Direct RNA Preparation Kit for Real-Time PCR (Takara bio) was used. Gene expression analysis was performed by real-time PCR (StepOne Plus Real-Time PCR System; Applied Biosystems, USA) utilizing RealQ Plus 2x Master Mix Green (Ampliqon, DENMARK). Repeated experiments were conducted for each sample and the mean cycle threshold (Ct) value was calculated. The beta-2-microglobulin (B2m) gene was used for normalization. The fold-change method (2-ΔCt) was used to analyze the expression of HDAC1, HDAC2, and HDAC11 genes.
Statistical analysis
Quantitative and categorical variables were described as mean ± SD (and Median(Q1, Q3)) and number (%), respectively. After assessing the normality distribution of continuous variables by Shapiro-Wilk test, the Student’s t or Mann-Whitney U tests were used for comparison means between groups. To assess the association between categorical variables, the Chi-square and Fisher exact tests were used. Also, Spearman's correlation test was used to determine the association between continuous variables. To adjust confounder factors, the multiple regression model was used. The P-Values <0.05 were considered statistically significant. All analyses were performed by R software version 4.2.3 and SPSS version 27.
Results
Patients and controls
Forty eight RA patients (44 females and 4 males, age: 47 ± 13.05 years) and 50 healthy controls (45 females and 5 males, age: 42.53 ± 7.07 years) were examined in this study. Characteristics of study subjects, including age, sex, ethnicity, and erythrocyte sedimentation rate (ESR), are presented in (Table 1). As expected, the difference in ESR level between the two groups is strongly significant (P-value <0.001).
|
Characteristics |
RA patients n=48 |
Healthy control n=50 |
P-value
|
|
|
Age (Year) |
47.00 ± 13.05 |
42.53 ± 7.07 |
0.040+ |
|
|
Sex |
Male |
4 (8%) |
5 (10%) |
0.999* |
|
Female |
44 (92%) |
45 (90%) |
||
|
Ethnicity |
Persian |
15 (31%) |
26 (52%) |
0.038** |
|
Turk |
23 (48%) |
12 (24%) |
||
|
Other |
10 (21%) |
12 (24%) |
||
|
ESR |
25.36 ± 24.15 |
5.29 ± 5.38 |
< 0.001++ |
|
|
ESR: Erythrocyte Sedimentation Rate; RA: Rheumatoid Arthritis; Data indicated as Mean ± SD and n(%) for countitive and categorical variables, respectively; *Calculated based on Fisher exact test; **Calculated baed on Chi-square test; +Calculated baed on Independent t-test; ++ Calculated based on Mann-whitney test. Median (Q1,Q3) of ESR are 18.00 (8.00, 33.00) and 3.00 (1.00, 8.00) in RA patients and Controls, respectively. |
||||
HDAC gene expression in PBMCs from RA patients
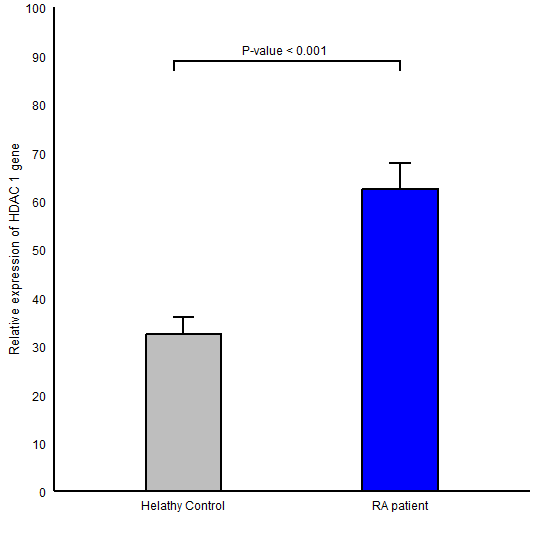
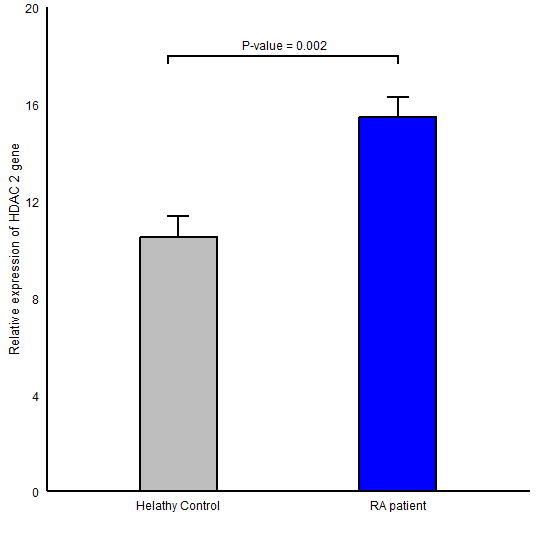
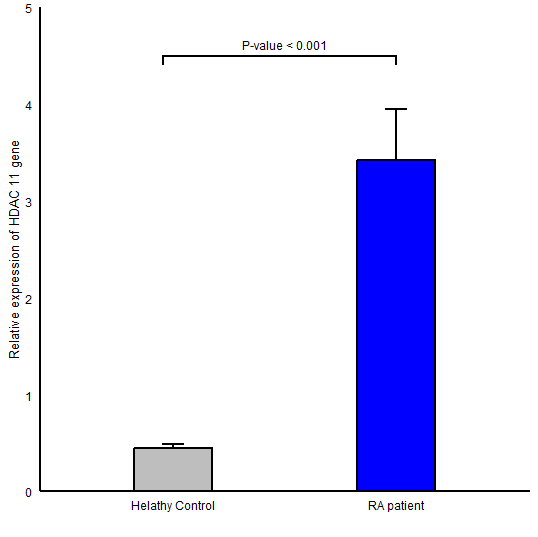
The expression level of HDAC1 in RA group (Mean ± SD =62.41±37.51) in comparison to healthy controls (Mean ± SD =32.58 ± 24.59) was significantly higher (P-value <0.001; (Figure 1)). Also, a statistically significant difference was found for HDAC2 gene expression between RA (Mean ± SD =15.46 ± 5.80) and control (Mean ± SD =10.51 ± 6.25, P-value =0.002; (Figure 2)) groups. We observed an elevation in the expression level of HDAC11 gene (HDAC11 gene of RA and control groups was 3.42 ± 3.61 , 0.44 ± 0.30 respectively and P-value <0.001; (Figure 3)).
Our results (Figures 1-3) show that the expression of HDAC1, HDAC2, and HDAC11 genes are significantly up-regulated in PBMCs from RA group in comparison to healthy individuals (Fold chenges was 1.91, 1.47, and 7.77 respectively). Also, by adjusting the effects of age, ESR, and ethnicity, the difference in HDAC gene expression between the diseased and healthy groups was significant.
Figure 1. Comparison of HDAC1 gene expression between RA patients and healthy controls in peripheral blood mononuclear cells; Data were reported as mean ± SEM (P ≤ 0.001).
Figure 2. Comparison of HDAC2 gene expression between RA patients and healthy controls in peripheral blood mononuclear cells; Data were reported as mean ± SEM (P ≤ 0.001).
Figure 3. Comparison of HDAC11 gene expression between RA patients and healthy controls in peripheral blood mononuclear cells; Data were reported as mean ± SEM (P ≤ 0.001).
Expression correlation between HDAC genes
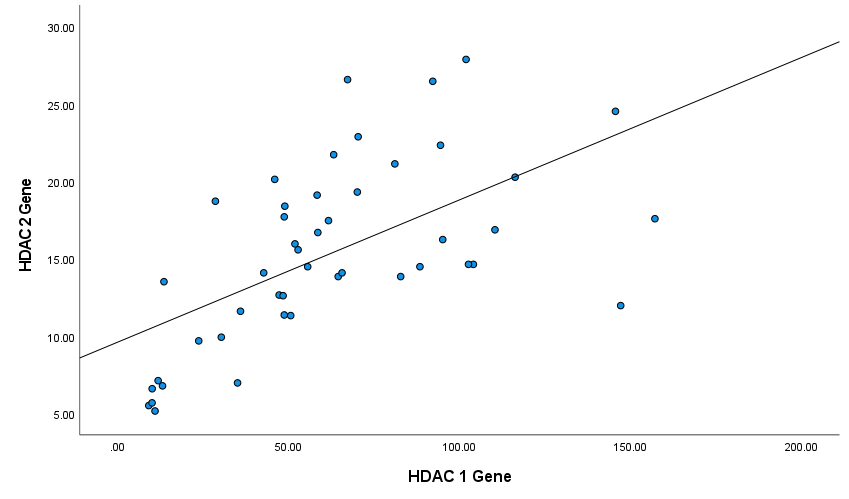
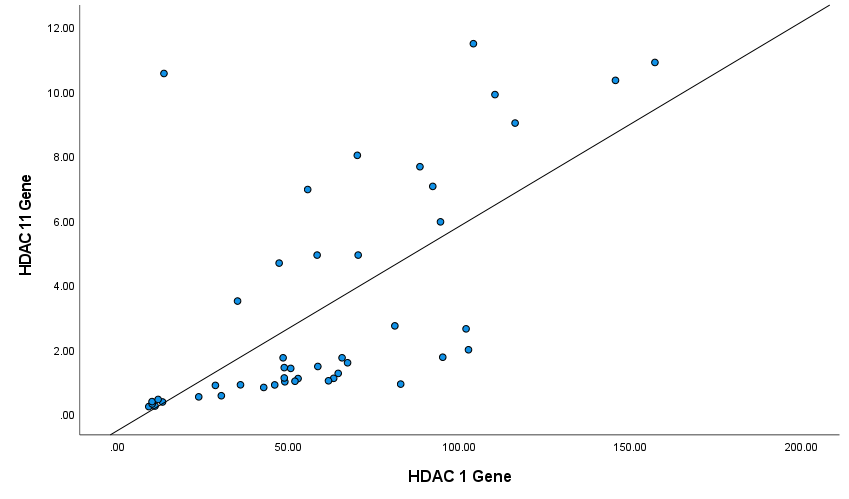
Next, we analyzed the correlation between the expression levels of HDAC genes in patients' PBMCs by the Spearman's test. Figures 4-6 show Spearman's correlation of the expression levels of HDAC1 and HDAC2 and HDAC11genes in RA group’s PBMCs respectively. HDAC1 expression was positively correlated with HDAC2 and HDAC11 expression levels (Spearman's correlation coefficient was 0.66 and 0.53 respectively, P-values<0.001). Also, HDAC2 and HDAC11 expressions have a significant positive correlation (Spearman's correlation coefficient was 0.75, P-values<0.001; (Figure 5)).
Figure 4. Correlation between HDAC1 and HDAC2 genes among patients (Spearman's correlation coefficient = 0.66, P ≤ 0.001).
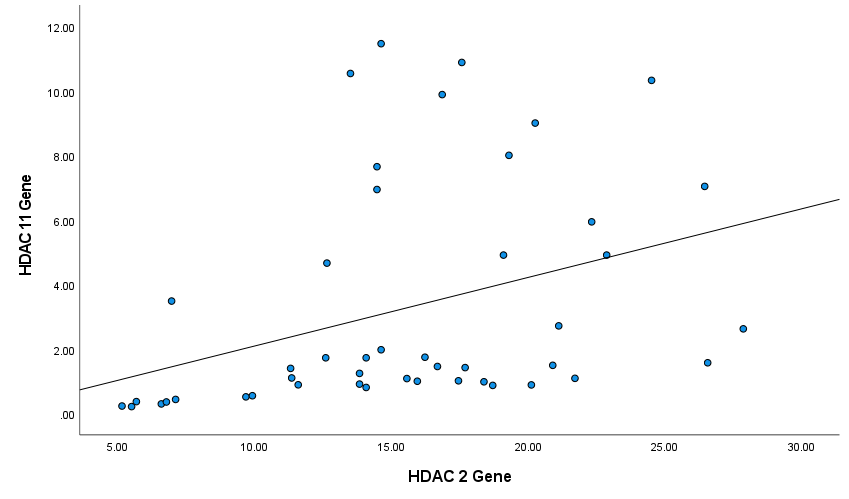
Figure 5. Correlation between HDAC2 and HDAC11 genes among patients (Spearman's correlation coefficient = 0.75, P ≤ 0.001).
Figure 6. Correlation between HDAC11 and HDAC1 genes among patients (Spearman's correlation coefficient = 0.53, P ≤ 0.001).
Discussion
In this study, we evaluated the mRNA levels of HDAC1, HDAC2, and HDAC11 in mononuclear cells isolated from RA cases and healthy controls. Based on our results, the gene expression of all 3 HDAC chromatin-modifying enzymes that can regulate the expression of inflammatory cytokines, was significantly higher in RA patients compared with healthy controls. In addition, correlation coefficients between expressions of these three genes are above 0.5 and represent a large expression relationship between them.
Research on the expression of histone deacetylases, particularly HDAC1, has largely focused on various types of cancers. Several reports have demonstrated elevated HDAC1 expression in breast, colon, gastric, and prostate cancers [19-21], implying a potential link between aberrant HDAC expression and tumorigenesis.
Studies on the expression of histone deacetylase genes in RA have been mostly performed on SFs from patients. In line with our results, the majority of them reported an elevated level and activity of HDAC enzyme in patients. Researchers found that HDAC1 expression and activity were significantly increased in SFs from RA patients compared to healthy and OA subjects. They also discovered a positive correlation between TNFα levels and HDAC1 expression in RA synovial tissue. HDAC2 mRNA levels did not show a significant difference in the study [14]. Another study on the expression and role of histone deacetylases1-11 in SFs from 8 RA and 10 OA patients indicates a significant HDAC1 expression and activity enhancement in RASFs compared to osteoarthritis synovial fibroblasts (OASFs). Transcript levels of other HDACs were not significantly different between patients and controls. Increased HDACs expression in RASFs may contribute to the progression of their inflammatory and tumor-like features. Therefore, it is shown that HDAC1 knocking down with siRNAs is associated with a decrease in cell proliferation and an increase in apoptosis of RASFs [13].
On the other hand, a study reported different results of histone deacetylase protein expression in RASFs compared to OASFs. The results of this report on 7 RA patients and 6 OA persons showed that the level of HDAC1 and 2 is decreased in SFs of RA patients compared to OA. This difference may be due to differences in patient condition and demographic features. For instance, three patients in the Huber study received a TNF-α blocker which may change the activity and expression of HDAC enzymes [15].
One of the few studies that were done on HDACs gene expression in PBMCs of individuals with rheumatoid arthritis, was on three DMARD (disease-modifying antirheumatic drugs) treatment-naive RA individuals and three healthy controls. Their results show a notable decrease in HDAC1 gene expression and a considerable increase in HDAC11 gene expression in RA patients. Data on the HDAC2 gene expression were not significant in that study [22]. One study on 48 DMARD-naive RA patients compared to matched healthy control showed downregulation in the expression and enzyme activity of class I HDAC in PBMCs of patients [23]. Some investigators examined HAT and HDAC activities in the PBMCs of a small group of patients diagnosed with RA (N = 8) and observed an increase in HDAC activity but no significant changes in HAT levels compared to healthy controls [24]. Another study reported altered HAT and HDAC activities in ankylosing spondylitis, with no major changes observed in RA [25]. However, in our study, a significant increase in the expression of HDAC 1, 2, and 11 was observed in patients' PBMCs. The conflicting interpretations from these studies may be attributed to factors such as sample size, matched control group in terms of age and sex, disease activity, and treatment regimens. In our investigation, we assessed the HDAC genes expression in PBMCs from a relatively larger group of RA patients (n = 48), and our findings led to different conclusions.
In the current study, the correlation analysis was conducted between expression levels of HDAC1, 2, and 11 in PBMCs of participants, and a remarkable positive correlation was observed between the expression of these three genes. In line with previous reports, our study indicates that alteration in HDACs expression and function is involved in RA pathogenesis. Previously, inhibition of HDAC activity has been shown to reduce inflammatory cytokine production in fibroblast-like synoviocytes (FLS) and PBMC. [26]. knockdown of HDAC1 increases TNF-α and IL-1β production by RASFs. Inflammatory cytokines stimulate RASFs to produce matrix metalloproteinases (MMPs), well-known degradation agents of the main components of the extracellular matrix of articular cartilage.
Besides, one of the most important features of RA-SF is a defect in apoptosis, which leads to the great hyperplasia of the RA synovium. It is shown that knocking down HDAC1, and 2 by siRNA can decrease cell proliferation and hyperplasia and increase apoptosis in RA-SF. On the other hand, in line with this phenomenon, p53, an apoptosis inducer, can be acetylated and deacetylated by p300 and HDACs, respectively [13]. When lysine acetylation of p53 is reduced, the lysine residue is ubiquitinated by the Mdm2 enzyme, leading to the degradation of p53 [27]. The expression level of HDAC genes in patients can be influenced by various clinical factors, including disease activity, treatment regimens, and comorbidities. However, in the current study, it was not possible to analyze and adjust for the effect of these factors. This limitation should be taken into consideration.
Conclusion
In the current study, we have compared the expression of 3 epigenetics modifier genes HDAC1, 2, and 11 in RA patients and healthy individuals. These findings support the hypothesis that HDACs have been involved in RA pathogenesis by targeting histones and non-histone proteins like p53 and consequently regulating the expression of inflammatory and apoptotic genes in RA patients. Therefore, the use of HDAC inhibitors can be considered a therapeutic solution.
Declarations
Ethics approval
This study was performed based on the Declaration of Helsinki guidelines and was approved by the Ethics Committee of Tehran University of Medical Sciences.
Consent to participate
The written informed consent was signed by all participants before enrolling in the study.
Availability of data and material
All data generated or analyzed during this study are available upon request.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
MMo, SP, SE, MAakht, EM, and MAakhl: Acquisition of data, drafting of the article, analysis and interpretation of data, and final approval of the article. AF, VH, EF, AH, AJ, MM: The conception and design of the study, revising the article critically, interpretation of data, and final approval of the article.
References
2. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011 Dec 8;365(23):2205-19.
3. Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005 Mar;4(3):130-6.
4. Kochi Y, Suzuki A, Yamamoto K. Genetic basis of rheumatoid arthritis: a current review. Biochem Biophys Res Commun. 2014 Sep 19;452(2):254-62.
5. MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000 Jan;43(1):30-7.
6. Silman AJ, MacGregor AJ, Thomson W, Holligan S, Carthy D, Farhan A, et al. Twin concordance rates for rheumatoid arthritis: results from a nationwide study. Br J Rheumatol. 1993 Oct;32(10):903-7.
7. Aslani S, Mahmoudi M, Karami J, Jamshidi AR, Malekshahi Z, Nicknam MH. Epigenetic alterations underlying autoimmune diseases. Autoimmunity. 2016;49(2):69-83.
8. Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007 Mar;17(3):195-211.
9. Kusaczuk M, Krętowski R, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol. 2016 Jan;37(1):931-42.
10. Liu SS, Wu F, Jin YM, Chang WQ, Xu TM. HDAC11: a rising star in epigenetics. Biomed Pharmacother. 2020 Nov;131:110607.
11. Park SY, Kim JS. A short guide to histone deacetylases including recent progress on class II enzymes. Exp Mol Med. 2020 Feb;52(2):204-12.
12. Das C, Kundu TK. Transcriptional regulation by the acetylation of nonhistone proteins in humans -- a new target for therapeutics. IUBMB Life. 2005 Mar;57(3):137-49.
13. Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S. Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts. J Rheumatol. 2009 Aug;36(8):1580-9.
14. Kawabata T, Nishida K, Takasugi K, Ogawa H, Sada K, Kadota Y, et al. Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis. Arthritis Res Ther. 2010;12(4):R133.
15. Huber LC, Brock M, Hemmatazad H, Giger OT, Moritz F, Trenkmann M, et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007 Apr;56(4):1087-93.
16. Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):2995-3000.
17. Klein K, Gay S. Epigenetic modifications in rheumatoid arthritis, a review. Curr Opin Pharmacol. 2013 Jun;13(3):420-5.
18. Kong S, Yeung P, Fang D. The class III histone deacetylase sirtuin 1 in immune suppression and its therapeutic potential in rheumatoid arthritis. J Genet Genomics. 2013 Jul 20;40(7):347-54.
19. Mutze K, Langer R, Becker K, Ott K, Novotny A, Luber B, et al. Histone deacetylase (HDAC) 1 and 2 expression and chemotherapy in gastric cancer. Ann Surg Oncol. 2010 Dec;17(12):3336-43.
20. Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004 May 1;59(2):177-89.
21. Zhang Z, Yamashita H, Toyama T, Sugiura H, Ando Y, Mita K, et al. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast*. Breast Cancer Res Treat. 2005 Nov;94(1):11-6.
22. Glant TT, Besenyei T, Kádár A, Kurkó J, Tryniszewska B, Gál J, et al. Differentially expressed epigenome modifiers, including aurora kinases A and B, in immune cells in rheumatoid arthritis in humans and mouse models. Arthritis Rheum. 2013 Jul;65(7):1725-35.
23. Li Y, Zhou M, Lv X, Song L, Zhang D, He Y, et al. Reduced Activity of HDAC3 and Increased Acetylation of Histones H3 in Peripheral Blood Mononuclear Cells of Patients with Rheumatoid Arthritis. J Immunol Res. 2018 Oct 3;2018:7313515.
24. Gillespie J, Savic S, Wong C, Hempshall A, Inman M, Emery P, et al. Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum. 2012 Feb;64(2):418-22.
25. Toussirot E, Abbas W, Khan KA, Tissot M, Jeudy A, Baud L, et al. Imbalance between HAT and HDAC activities in the PBMCs of patients with ankylosing spondylitis or rheumatoid arthritis and influence of HDAC inhibitors on TNF alpha production. PLoS One. 2013 Aug 15;8(8):e70939.
26. Angiolilli C, Kabala PA, Grabiec AM, Van Baarsen IM, Ferguson BS, García S, et al. Histone deacetylase 3 regulates the inflammatory gene expression programme of rheumatoid arthritis fibroblast-like synoviocytes. Ann Rheum Dis. 2017 Jan;76(1):277-85.
27. Reed SM, Quelle DE. p53 Acetylation: Regulation and Consequences. Cancers (Basel). 2014 Dec 23;7(1):30-69.