Abstract
Our recently published research article “Vγ2+ γδ T cells in the presence of anti-CD40L control surgical inflammation and promote skin allograft survival” revealed that the Vγ2+ subset of γδ T cells, which otherwise are known primarily for its proinflammatory function, regulate the survival of skin allografts in the presence of anti-CD40L. Upon depletion of Vγ2+ γδ T cells, tolerogen DST (donor-specific transfusion) plus anti-CD40L induced skin allograft survival was significantly reduced. Tolerogen treatment increased the frequency of CD39+Vγ2+ regulatory γδ T cells and suppressed IFN-γ-producing effector Vγ2+ γδ T cells in the spleen and allograft. Tolerized Vγ2+ γδ T cells inhibited differentiation of inflammatory T helper type 1 (Th1) cells. Furthermore, the adoptive transfer of regulatory Vγ2+ γδ T cells prolonged the survival of allografts in untreated wild-type and TCRδ-/- mice. Our study highlights the critical role γδ T cells in transplant tolerance and paves the way for future research toward exploring regulatory γδ T cells.
Keywords
Gamma-delta T cells, Transplantation Tolerance, Co-stimulation, Allograft, Surgery
Abbreviations
AML: Acute Myeloid Leukemia; CNS: Central Nervous System; DST: Donor-Specific Transfusion; EAE: Experimental Autoimmune Encephalomyelitis; GVHD: Graft-Versus-Host Disease; GVT: Graft-Versus-Tumor; HSCT: Hematopoietic Allogeneic Stem Cell Transplantation; LN: Lymph Node; Th1: T helper type 1; Treg: Regulatory CD4 T cell
Introduction
Gamma-delta (γδ) T cells belong to a rare population of T cells in mice lymphoid tissue and human peripheral blood. On the contrary, the epithelial and mucosal barriers are enriched with γδ T cell population, where they perform unique immune functions, help in tissue repair, and maintain homeostasis. These tissue-resident γδ T cells possess the characteristics of innate and adaptive immune cells, enabling them to act as a bridge between the two. γδ T cells exist as various independent subsets which are shown to perform distinct functions during disease conditions. The molecular intricacies involved in the development, differentiation, and distribution of γδ T cell subsets are covered by us and others [1]. γδ T cells constitute about 1-10% of T cells in circulation and are highly enriched on epithelial and mucosal sites [2,3]. γδ T cells have also been shown to perform effector functions during infection, cancer, and autoimmunity, which have been thoroughly reviewed in recent publications [4,5]. The role of γδ T cells is very well explored in infection, cancer, and autoimmunity but well explored in transplantation. It has been perceived that γδ T cells may have a possible function in graft-versus-tumor (GVT) without driving the graft-versus-host disease (GVHD) in hematopoietic allogeneic stem cell transplantation (HSCT) [6]. Minculescu et al. showed a protective role of γδ T cells in relapse-free survival and GVHD in patients with a high frequency of γδ T cells in circulation [7]. In newly diagnosed acute myeloid leukemia (AML) patients, an increased frequency of CD25+CD127loVδ2+ γδ T cells was reported in the bone marrow of patients and show an immunosuppressive function [8]. Proteasome inhibitor Bortezomib is shown to enhance the cytotoxic function of ex-vivo expanded γδ against AML and T-cell acute lymphoblastic leukemia (T-ALL) [9]. γδ T cells perform their functions by direct cytotoxic effect through death receptor (FAS-FASL, TRAIL-TRAIL receptor or through perforin/granzyme) or by secreting the soluble molecules such as IFN-γ, TNF-α, IL-4, IL-13, IL-17, and IL-22 [2,5]. γδ T cell subsets are also proposed to be used as adaptive cellular therapy in some cancers [10-12]. The role of some of the γδ T cell subsets is listed in Table 1. The function of various subsets of γδ T cells and the effect of various tolerogenic regimens on the γδ T cells in the transplantation still need to be explored.
|
Subset of γδ T cells
|
Tissue localization
|
Cytokines
|
Functions
|
|---|---|---|---|
|
Vγ1 |
Lymphoid tissue |
IFN-γ, TNF-α, IL-17, IL-4, IL-13 |
Inhibits innate immune response against Listeria monocytogenes, inhibits Treg development, promotes airway hyperactivity |
|
Vγ2 (Vγ4) |
Lymphoid tissue and lung |
IL-17, IFN-γ, TNF-α |
Promotes inflammation in the CNS during EAE, regulates the induction of Tregs, helps in producing anti-collagen IgG and IgG2a antibodies, hampers IgE response, promotes virus-mediated lung inflammation |
|
Vγ5 |
Skin epidermis |
IGF-1, KGF-1, KGF-2, IFN-γ, TNF-α, IL-22 |
Promotes wound healing and tissue repair provides protection again skin cancer, inhibits GVHD |
|
Vγ6 |
Placenta, uterus, testes, tongue, lung, and kidney |
IL-17, IFN-γ, TNF-α, IL-22, TGF-β |
Prevents intrauterine infection, regulates nephritis, inhibits lung fibrosis, promotes bacterial clearance |
|
Vγ7 |
Intestinal mucosa |
IFN-γ, TNF-α |
Promotes epithelial homeostasis, maintains intestinal barrier integrity, suppresses colitis |
Vγ2+ γδ T cells in Costimulatory Blockade-induced Skin Allograft Survival
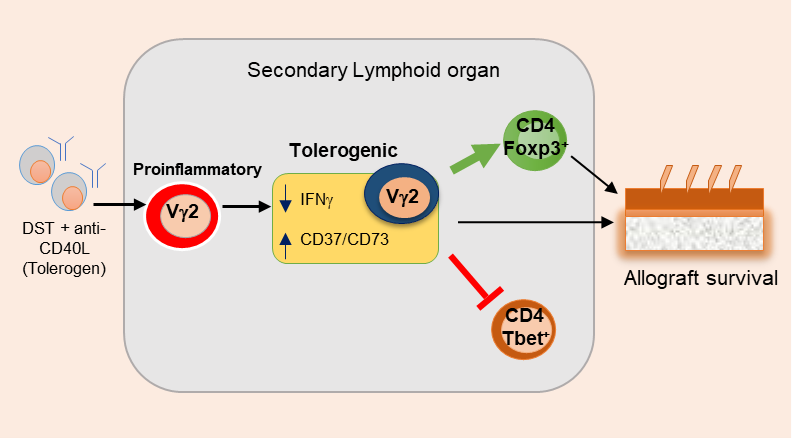
Recently, Giri et al., showed that treatment of DST and anti-CD40L promotes regulatory Vγ2+ γδ T cells and prolongs skin allograft survival [13]. Murine γδ T cells can recognize surface-expressed proteins directly without the major histocompatibility complex molecules. Because our results showed that the depletion of Vγ2+ T cells under tolerogenic conditions affects allograft survival, but not the syngeneic grafts. This suggest that Vγ2+ cells’ actions are mediated toward alloantigen and not towards the nonspecific injury associated with transplantation protocols. Further investigation is still required to establish the molecular nature of γδ T cells specificity towards alloantigen. Depleting Vγ2+ γδ T cells led to increased MHC-IIhighF4/80+ macrophage migration, which induces efficient alloantigen presentation and T cell priming. TCR and CD40L signaling are reported to promote the proliferation of γδ T cells and the expression of IFN-γ in an IL-2–independent manner [14]. Hence, the blockade of CD40L signaling impacted IFN-γ producing ability of γδ T cells and supported the proliferation of a specific subset that showed enhanced regulatory functions. During bacterial and virus infection, IFN-γ produced by γδ T cells is reported to promote CD4+ T cells’ differentiation toward the Th1 lineage [15,16]. We also observed that naive Vγ2+ γδ T cells could independently induce the differentiation of Th1 cells, which was suppressed in the presence of tolerized Vγ2+ γδ T cells (Figure 1). In support, in-vivo depletion of Vγ2+ γδ T cells in tolerogen-treated mice significantly increased the expression of IFN-γ in CD4+ T cells, CD8+ T cells, and Vγ1.1+ γδ T cells. Extracellular adenosine triphosphate produced during tissue damage or surgery during transplantation is pro-inflammatory. The membrane-associated enzymes CD39 and CD73 convert inflammatory adenosine triphosphate to noninflammatory adenosine. CD39+/CD73+ γδ T cells function as regulatory cells [17]. In our study, we observed increased CD39+ γδ T cells in the LN and the skin allografts of tolerized recipients (Figure 1). CD4+Foxp3+ Tregs are critical for the induction of tolerance, and their increased frequency correlates to improved allograft survival [18]. We observed that depletion of Vγ2+ γδ T cells in tolerogen-treated mice reduced the percentage of CD4+Foxp3+ Tregs in the spleen. A study using the autoimmune keratitis model also reported that the differentiation of Foxp3+Tregs in the animal is dependent on γδ T cells, and TCRδ–/–mice have a reduced number of peripheral CD4+CD25+Foxp3+ Tregs [19]. However, the direct interaction between γδ T cells and Tregs and the molecular mechanism promoting the tolerance need detailed investigation. Emerging reports also suggest the involvement of γδ T cells during transplant rejection and spontaneous tolerance. IL-17 produced by γδ T cells is shown to mediate allograft rejection by inducing neutrophil recruitment and CD4+Th17 differentiation [20]. IL-17 produced by Vγ4+ γδ T cells facilitated the accumulation of mature DCs and priming of effector T cells, causing the rejection of H-Y minor antigen-mismatched skin allograft [21]. Consequently, the depletion of γδ T cells prolonged the allograft survival by reducing IL-17-mediated pathology and enhancing the accumulation of CD4+Foxp3+ Tregs into the allograft [18,22]. Activated V γ2+ γδ T cells have been shown to produce high levels of IFN-γ and perforin, thereby preventing the development of B16 melanoma in mice. γδ Tregs were also reported to suppress acute Graft-versus-host disease (GVHD) during allogeneic peripheral blood stem cell transplantation [23]. Regulatory γδ T cells secrete IL-4 and IL-10, which suppress Th1 differentiation of CD4 T cells, prolonging the survival of kidney and skin allografts [24,25].
Conclusion and Future Perspective
γδ T cells are relatively new to be recognized for their non-redundant functions across many disease conditions. But their unique potential for performing effector and regulatory functions has made them a potent target for clinical research. However, detailed studies on crosstalk with other immune cells at the site of inflammation and in secondary lymphoid tissues need to be investigated. Although the regulatory function of γδ T cells is reported in several publications, detailed signals and molecular interaction that brings the regulatory plasticity are unknown and need future investigation. Our study strengthens the role of regulatory γδ T cells, which might prove helpful in designing better strategies for tolerance and immunosuppression. Clinical trials with γδ T cell therapy against cancer are ongoing and establishing their important contributions to human health.
Acknowledgements
SG received Senior Research Fellowship from the Indian Council of Medical Research, Government of India. GL received grants from the Department of Biotechnology (grant numbers BT/PR15533/MED/30/1616/2015 and BT/PR14156/BRB/10/1515/2016) and the Department of Science and Technology (DST/SJF/LSA-01/2017-18), Government of India.
References
2. Giri S, Lal G. Differentiation and functional plasticity of gamma-delta (γδ) T cells under homeostatic and disease conditions. Mol Immunol. 2021;136:138-149.
3. Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014; 40(4):490-500.
4. Paul S, Shilpi, Lal G. Role of gamma-delta (γδ) T cells in autoimmunity. J Leukoc Biol. 2015;97(2):259-271.
5. Paul S, Lal G. Regulatory and effector functions of gamma-delta (gammadelta) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int J Cancer. 2016;139(5):976-985.
6. Arruda LCM, Gaballa A, Uhlin M. Impact of gammadelta T cells on clinical outcome of hematopoietic stem cell transplantation: systematic review and meta-analysis. Blood Adv. 2019;3(21):3436-3448.
7. Minculescu L, Marquart HV, Ryder LP, Andersen NS, Schjoedt I, Friis LS, et al. Improved Overall Survival, Relapse-Free-Survival, and Less Graft-vs.-Host-Disease in Patients With High Immune Reconstitution of TCR Gamma Delta Cells 2 Months After Allogeneic Stem Cell Transplantation. Front Immunol. 2019;10:1997.
8. Liang S, Dong T, Yue K, Gao H, Wu N, Liu R, et al. Identification of the immunosuppressive effect of gammadelta T cells correlated to bone morphogenetic protein 2 in acute myeloid leukemia. Front Immunol. 2022;13:1009709.
9. Story JY, Zoine JT, Burnham RE, Hamilton JAG, Spencer HT, Doering CB, et al. Bortezomib enhances cytotoxicity of ex vivo-expanded gamma delta T cells against acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Cytotherapy. 2021;23(1):12-24.
10. Zakeri N, Hall A, Swadling L, Pallett LJ, Schmidt NM, Diniz MO, et al. Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma. Nat Commun. 2022;13(1):1372.
11. He W, Hu Y, Chen D, Li Y, Ye D, Zhao Q, et al. Hepatocellular carcinoma-infiltrating gammadelta T cells are functionally defected and allogenic Vdelta2(+) gammadelta T cell can be a promising complement. Clin Transl Med. 2022;12(4):e800.
12. Barros MS, de Araujo ND, Magalhaes-Gama F, Pereira Ribeiro TL, Alves Hanna FS, Tarrago AM, et al. gammadelta T Cells for Leukemia Immunotherapy: New and Expanding Trends. Front Immunol. 2021;12:729085.
13. Giri S, Meitei HT, Mishra A, Lal G. Vgamma2(+) gammadelta T Cells in the Presence of Anti-CD40L Control Surgical Inflammation and Promote Skin Allograft Survival. J Invest Dermatol. 2022;142(10):2706-2714.e3.
14. Ramsdell F, Seaman MS, Clifford KN, Fanslow WC. CD40 ligand acts as a costimulatory signal for neonatal thymic gamma delta T cells. J Immunol. 1994;152(5):2190-2197.
15. Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373(6511):255-257.
16. Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165(8):4174-4181.
17. Otsuka A, Hanakawa S, Miyachi Y, Kabashima K. CD39: a new surface marker of mouse regulatory γδ T cells. J Allergy Clin Immunol. 2013;132(6):1448-1451.
18. Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311-1318.
19. 19. Huang Y, Yang Z, Huang C, McGowan J, Casper T, Sun D, et al. γδ T Cell-Dependent Regulatory T Cells Prevent the Development of Autoimmune Keratitis. J Immunol (Baltimore, Md : 1950). 2015;195(12):5572-5581.
20. Zhu H, Li J, Wang S, Liu K, Wang L, Huang L. gammadelta T cell receptor deficiency attenuated cardiac allograft vasculopathy and promoted regulatory T cell expansion. Scand J Immunol. 2013;78(1):44-49.
21. Li Y, Huang Z, Yan R, Liu M, Bai Y, Liang G, et al. Vgamma4 gammadelta T Cells Provide an Early Source of IL-17A and Accelerate Skin Graft Rejection. J Invest Dermatol. 2017;137(12):2513-2522.
22. Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30(2):235-240.
23. Xuan L, Wu X, Qiu D, Gao L, Liu H, Fan Z, et al. Regulatory gammadelta T cells induced by G-CSF participate in acute graft-versus-host disease regulation in G-CSF-mobilized allogeneic peripheral blood stem cell transplantation. J Transl Med. 2018;16(1):144.
24. Gorczynski RM, Chen Z, Hoang Y, Rossi-Bergman B. A subset of gamma delta T-cell receptor-positive cells produce T-helper type-2 cytokines and regulate mouse skin graft rejection following portal venous pretransplant preimmunization. Immunology. 1996;87(3):381-389.
25. Zhou J, Appleton SE, Stadnyk A, Lee TD, Nashan BA. CD8+ gammadelta T regulatory cells mediate kidney allograft prolongation after oral exposure to alloantigen. Transpl Int. 2008;21(7):679-687.