Keywords
Epilepsy surgery, Cognitive outcomes, Seizure freedom, Electrocrotocography
Introduction
Epilepsy has a worldwide prevalence of about 50 million [1]. Seizure medications provide adequate control in two thirds of these patients but about a third are refractory to multiple medications and need surgery or other treatments [2]. Resective surgery remains curative for epilepsy patients although seizure freedom varies depending on seizure onset zone and imaging. Highest freedom rates of 65% are seen in temporal onset epilepsy but decline after 5-10 years post-surgery with 2-3% patients experiencing seizure recurrence every year [3]. There is limited data about seizure freedom after 10-20 years post-surgery. Lesional imaging correlates with greater probability of seizure freedom but has poor correlation in patients needing intracranial EEG evaluation [4]. Neuropsychiatric deficits like word finding difficulty, memory loss, depression, psychoses are seen in patients and may worsen or improve after surgery [5].
We analyzed our institutional outcomes for resective surgery over a period of 20 years post op. We studied effects of electrocortigraphy, imaging and follow-up on outcomes after resective surgery. We evaluated the incidence of neuropsychiatric deficits as well. We wished to compare our institutional trends with known outcomes and establish new data for outcomes with follow-up up to 20 years. We compared outcomes between patients evaluated with scalp and intracranial EEG.
Methods
We retrospectively analyzed surgery patients at the University of Pennsylvania. We included patients with at least 2 years follow-up beginning in 1996 - Dec 2015. We included patients with resective surgeries like lobectomies and / or patients that had intracranial EEG evaluation using grids or strips with / without resective surgery. Patients with only callosotomies, VNS implantations and laser ablations were excluded. Some patients had VNS / RNS implantations in addition to resections and were included in the final analysis. Patients undergoing stereo EEG were not included as we had recently started performing it and did not have sufficient follow-up. These patients underwent evaluations using MRI, PET, neuropsychological testing and electrocorticography using grids and strips or old depths. 142 consecutive patients were analyzed from 1996 till 2015. The patient demographics are presented in Table 1.
| Gender | 69 Males, 73 Females |
|---|---|
| Median follow up | 8-13 years (range 2-20 years) |
| Scalp EEG only | 66 patients |
| Scalp and Intracranial EEG evaluation | 76 patients |
| Deceased | 2 patients – none immediately post - op |
Table 1: Demographics.
Patients’ visits were retrospectively reviewed to assess seizure incidence, Engel scores, medication dosages, cognitive deficits. Imaging & EEG results were reviewed.
We analyzed outcomes using Engel scores. Outcomes were analyzed based on type of EEG evaluation. We studied patients with intermediate follow-up ranging from 2-10 years and long-term follow-up ranging from 10-20 years. Intracranial EEG patients were evaluated using grids, strips or older depths. Patients were grouped based on the lobe of onset. Some patients were excluded from lobar analysis due to multilobar or bilateral involvement. We performed separate analyses of temporal onset patients with scalp EEG evaluation only since they formed majority of patients evaluated by scalp EEG and represent textbook patients for surgery. Patients were also grouped based on imaging. We created separate groups of patients with positive or negative MRI and PET studies in any combination with a separate group that only had a MRI and not PET. Cognitive and psychiatric deficits were grouped together.
| Numbers | Engel 1 | Engel 2 | Engel 3 | Engel 4 | Total |
|---|---|---|---|---|---|
| Scalp EEG only | 42 | 7 | 10 | 7 | 66 |
| Scalp and Intracranial EEG | 25 | 15 | 11 | 25 | 76 |
Table 2: Results.
| Group (patient cohort) | Number of patients (Male + Female) | Outcome (Engel Scores) | Follow up duration (in years) | Number of Anti-seizure meds (avg. range) |
|---|---|---|---|---|
| 1A (scalp EEG with good outcomes) | 49 (22M + 27F) | Engel 1 & 2 | 3-20 yrs | 1-2 meds |
| 1B (scalp EEG with Poor outcomes) | 17 (10M + 7F) | Engel 3 & 4 | 3-20 yrs | 2-3 meds |
| 2A (intracranial EEG with good outcomes) | 40 (24M + 16F) | Engel 1 & 2 | 2-17 yrs | 1-3 meds |
| 2B (intracranial EEG with poor outcomes) | 36 (13M + 23F) | Engel 3 & 4 | 2-20 yrs | 2-4 meds |
Table 3: Distribution of Patients.
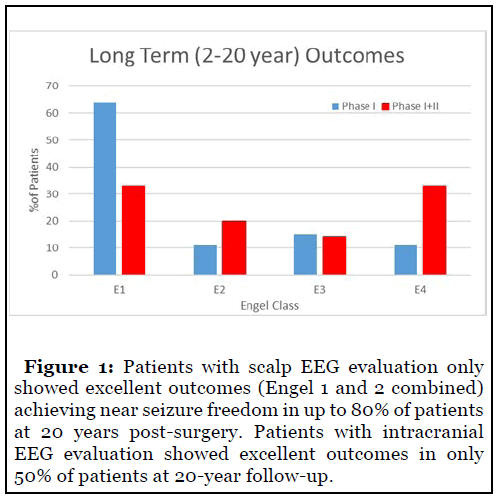
Resective surgery showed high rates of seizure freedom over prolonged follow-up in our patient population (Figure 1). Seizure freedom in the patients with scalp EEG evaluations only was 64% over 20 years. Engel 1 and 2 combined outcomes were seen in nearly 80% of patients. Engel 1 outcomes in the intracranial population were 33% although Engel 1 and 2 together slightly exceeded 50%. Engel 4 outcomes in the intracranial population included 33% of patients.
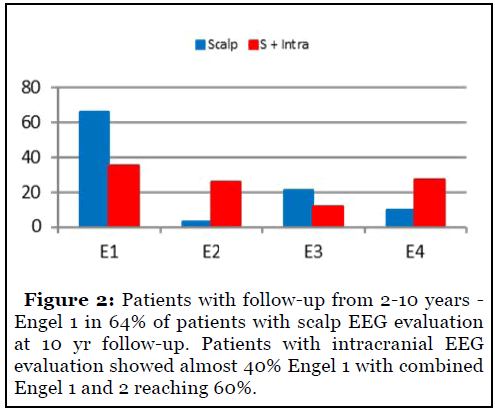
Intermediate follow-up included patients with followup ranging from 2-10 years (Figure 2). Engel 1 outcomes in 65% of patients with scalp EEG at 10-year followup. Patients with intracranial EEG showed almost 40% seizure freedom with combined Engel 1 and 2 reaching 60%.
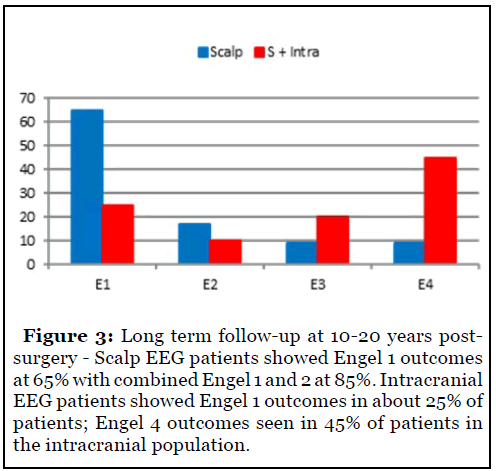
Long-term follow-up for patients seen over 10-20 years was also studied (Figure 3). Scalp EEG patients showed persistence of Engel 1 outcomes at 65% for greater than 10 years with combined Engel 1 and 2 approaching 85%. Intracranial EEG patients showed Engel 1 outcomes in about 25% patients. Engel 4 outcomes were noted in 45% patients in the intracranial population.
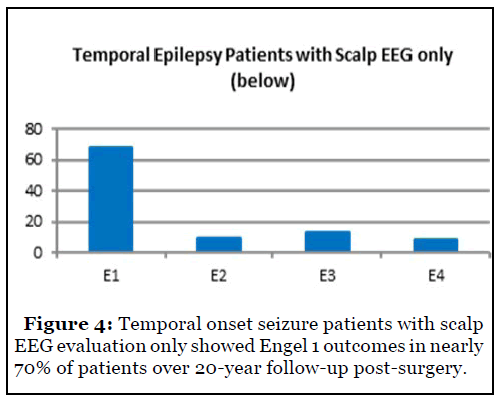
Temporal onset seizure patients with scalp EEG evaluation only showed excellent outcomes with Engel 1 outcomes seen in nearly 70% of patients over 20-year follow-up (Figure 4).
Analysis of outcomes with respect to lobe of onset showed excellent outcomes for temporal onset seizures with Engel 1 outcomes seen in nearly 60% patients. Frontal onset seizures showed poor outcomes with Engel 4 outcomes in 50% patients (Figure 5).
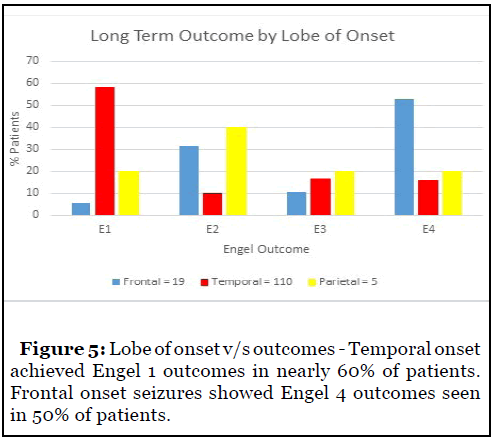
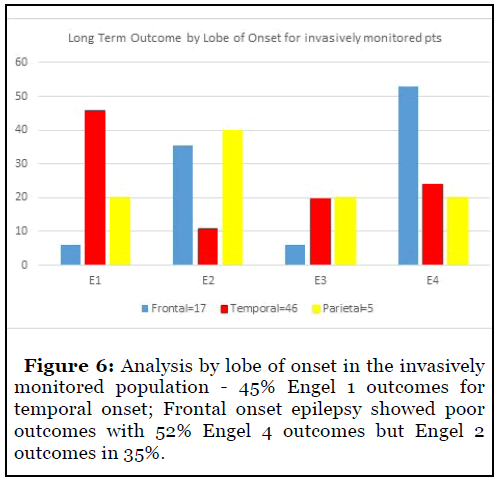
Analysis by lobe of onset in the invasively monitored population showed 45% Engel 1 outcomes in the temporal onset cohort. Frontal onset epilepsy showed poor outcomes with 52% patients with Engel 4 outcomes although 35% patients achieved Engel 2 outcomes (Figure 6).
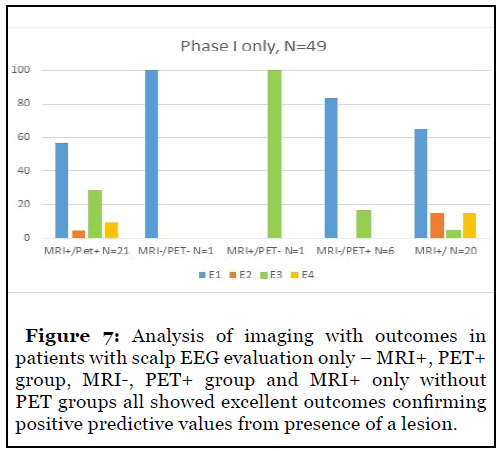
Analysis of imaging in patients with scalp EEG evaluation only showed excellent outcomes in patients with MRI and PET positivity. MRI negative, PET positive patients showed good outcomes as well supporting the benefits of PET in MRI negative patients. Patients with positive MRI findings only without PET showed good outcomes supporting the usefulness of lesional imaging and confirming positive predictive values for good outcomes in lesional patients (Figure 7).
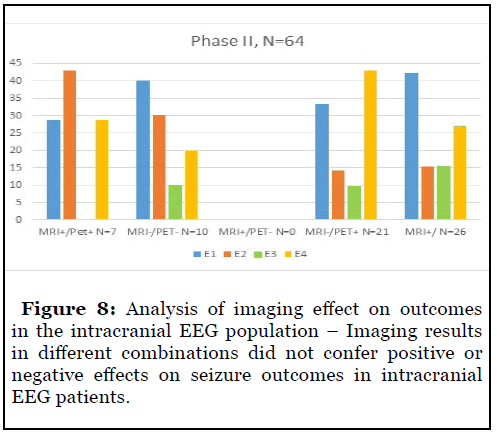
The positive predictive value of imaging was absent in the intracranial EEG population as patients with positive and negative MRI and PET studies in different combinations appeared to show similar outcomes with Engel 1 outcomes ranging from 30-40% across groups. There were a higher number of Engel 3 and 4 outcomes seen in this population of patients as compared to the scalp EEG population reflecting greater complexity and disease activity in them. (Figure 8).
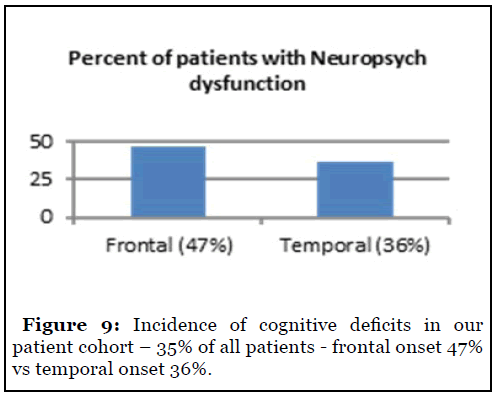
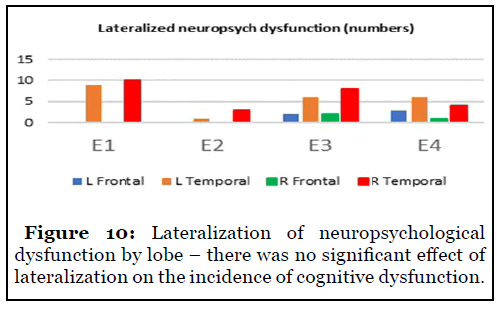
49/142 (35%) patients complained of neuropsychological complaints. Frontal onset conferred a higher risk of deficits as compared to temporal onset – 47% vs 36% (Figure 9). There was no significant difference in lateralization among groups to suggest a greater risk of deficits between the left and right hemispheres (Figure 10).
Discussion & Conclusions
Surgical resection for epilepsy is a well-known curative treatment although most studies have analyzed patients with 1-10 year follow-up. There has been recent emphasis to study patients for longer periods of follow-up.
We decided to study resective surgeries only as these had been performed for a long time and were the historical gold standard and provided us with sufficient followup. Our institutional experience with laser ablations has been more recent and we did not have sufficient numbers or duration of follow-up to power the study and enable comparison with resections.
Our intracranial EEG patients were evaluated using grids, strips or short depths for evaluation. We excluded stereo EEG patients as our institutional experience is recent and offers short follow-up period.
Prior studies have shown seizure freedom of 45-60% with follow-up of 1-10 years. Our experience is consistent with and possibly better than these findings. Our scalp EEG population showed Engel 1 outcomes in 64% patients and remained consistent in patients with follow-up of 20 years. Most studies note a seizure recurrence rate of 2-3% of patients per year after 5 years, which was not seen in our study [3]. Prior studies have noted aggressive seizure medication withdrawal in their patient populations. We taper down on seizure medications in a conservative manner and many patients have remained on low doses of 1-2 medications rather than going off them all. Our scalp EEG group had consistent seizure freedom rates between those with less than 10-year follow-up and 10-20 year follow-up which would reflect the result of continued medications.
Temporal epilepsy patients had nearly 70% seizure freedom while a high number of them had excellent outcomes – 80% with combined Engel 1 + 2. This suggests that selected temporal onset patients with concordant studies make an excellent population for surgery with high probability for seizure freedom. This was seen up to 20 years after resection suggesting that long term outcomes for this population is encouraging and better than previously reported [3].
Temporal onset patients with intracranial evaluation have lower seizure freedom rates as compared to the scalp group (45% vs 65%) but show significant seizure reduction (55-60% combining Engel 1 and 2). We explain this by presence of a larger epileptogenic network with temporal mimicking clinically or electrographically, bilateral involvement, discordant imaging or multifocal onset with temporal manifestations or combination of factors.
Frontal onset patients had poorer outcomes despite intracranial EEG – 52% patients with Engel 4 outcomes. Significant improvement is achieved in many patients – 35% patients with Engel 2 outcomes. Stereo EEG and newer techniques may improve these numbers as certain recent series have suggested [6].
Eight patients were not localized despite use of intracranial EEG and excluded from lobar analysis. These patients qualify for stereotactic EEG evaluation in future. 5 patients had parietal onset and variable outcomes, each Engel group showing 20-25% of patients. Occipital onset seizures were not seen in our study.
Lesional imaging has been previously correlated with higher seizure freedom and MRI and PET have been shown to have excellent predictions, especially in concordance with scalp EEG [7,8]. Our study confirmed this with scalp EEG patients having high seizure freedom with MRI and PET positivity – 60-75% patients with Engel 1 outcomes. Patients with either MRI or PET positivity had high seizure freedom.
Lesional imaging had lower success rates for the intracranial patients as lesional and nonlesional patients had similar rates of seizure freedom ranging from 30- 40%. We believe that decreased predictive values were from widespread network dysfunction negating the ability of positive imaging to predict seizure freedom. Many patients also had dual pathology resulting in lesional imaging that was poorly representative of the epileptogenic network unlike the patients in the scalp only group. Electrocorticography was able to partially negate loss of predictive ability of positive imaging resulting in respectable seizure freedom rates of 30-40%.
We believe that discordance in imaging, extratemporal onset, multiple foci, bilateral involvement automatically qualify a patient for intracranial EEG and decrease the probability of seizure freedom due to inability to completely remove the extensive surgical network with resection. Intracranial patients were also likely to have Engel 4 outcomes despite positive imaging and there was absent correlation between imaging and outcomes.
Neuropsychological deficits are seen in 10 – 40% of surgical patients [9]. Our study suggested that the incidence of these deficits may be similar or slightly higher in some cohorts. Frontal onset was more likely to produce these deficits as compared to temporal onset – 47% vs 36%. We did not separately analyze neurocognitive and psychiatric difficulties and grouped them together.
Severity of epilepsy did not correlate with probability of such deficits as they occurred in patients with better outcomes as well. Lateralization of onset did not suggest higher risk of deficits as suggested by prior studies as we noted near exact incidence of cognitive deficits in both left and right onset [9].
We did not quantify magnitude of deficit but they were reportedly mild, did not need formal testing, and did not affect the patients’ quality of life significantly.
Four of our intracranial EEG patients had an implantation effect. They experienced seizure freedom after an intracranial evaluation and did not need resective surgery or other intervention. This is consistent with previously reported rates of 4-5% patients with intracranial evaluation showing an implantation effect. The implantation effect has been noted for 3-4 months but our 4 patients had a prolonged effect – 2-7 years [10].
Two of the patients in our study died from causes unrelated to their epilepsy. We did not note any cases of SUDEP in our study group.
We accept that the retrospective nature of study would predispose to loss to follow-up. Formal neuropsychological testing after surgery is not performed routinely and we may consider it in some patients but scheduling difficulties and high costs prevent regular testing in the post-operative period. Resective surgery remains the only curative treatment and follow-up of 20 years shows that seizure freedom rates are better than previously reported, albeit with conservative medication tapering. Selected temporal epilepsy patients have an excellent probability of seizure freedom and may be eligible for resective surgery without intracranial EEG evaluation. Frontal and extratemporal onset epilepsy is more likely to need intracranial evaluation and may still have lower seizure freedom rates after surgery despite presence of lesional imaging. Intracranial EEG evaluation lowers the probability of seizure freedom but may still help achieve respectable outcomes and improvement in quality of life. Good intracranial EEG localization partially offsets the loss of positive predictive value from positive imaging in nonlesional patients. Neuropsychiatric deficits are commonly seen in up to 50% of patients but are frequently mild and do not preclude surgery. Frontal onset increases the risk of neuropsychiatric deficits. 5% of patients undergoing an intracranial EEG evaluation may benefit from implantation effects which could last for months to years rather than weeks.
Stereotactic EEG techniques are expected to change approaches and outcomes. The use of newer techniques like laser ablation, RNS would also change options offered to patients. Surgical therapy for epilepsy remains underutilized and must be considered at the earliest due to its curative potential and minimal risk for significant deficits [11]. We hope to use newer techniques and design more studies in future to improve our understanding.
Conflicts of Interest
The authors have no relevant disclosures or conflicts of interest to declare.
Acknowledgements
The authors would like to thank Jackie Raab MSN for her assistance in organizing the patients’ records and assisting in retrieving them.
References
2. Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. New England Journal of Medicine. 2011 Sep 8;365(10):919-26.
3. Tanriverdi T, Poulin N, Olivier A. Life 12 years after temporal lobe epilepsy surgery: a long-term, prospective clinical study. Seizure. 2008 Jun 1;17(4):339-49.
4. Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Research. 2010 May 1;89(2-3):310-8.
5. Lee PS, Pardini J, Hendrickson R, Destefino V, Popescu A, Ghearing G, et al. Short-term neurocognitive outcomes following anterior temporal lobectomy. Epilepsy & Behavior. 2016 Sep 1;62:140-6.
6. Bonini F, McGonigal A, Scavarda D, Carron R, Régis J, Dufour H, et al. Predictive factors of surgical outcome in frontal lobe epilepsy explored with stereoelectroencephalography. Neurosurgery. 2018 Aug 1;83(2):217-25.
7. Vollmar C, Stredl I, Heinig M, Noachtar S, Rémi J.Unilateral temporal interictal epileptiform discharges correctly predict the epileptogenic zone in lesional temporal lobe epilepsy. Epilepsia. 2018 Aug;59(8):1577-82.
8. Menon RN, Radhakrishnan A, Parameswaran R, Thomas B, Kesavadas C, Abraham M, et al. Does F-18 FDG-PET substantially alter the surgical decision-making in drug-resistant partial epilepsy?. Epilepsy & Behavior. 2015 Oct 1;51:133-9.
9. Gallagher A, Jambaqué I, Lassonde M. Cognitive outcome of surgery. InHandbook of Clinical Neurology 2013 Jan 1 (Vol. 111, pp. 797-802). Elsevier.
10. Abou-Khalil B, Valentin A. The implant effect after intracranial electrode placement: Is transient clinical improvement explained by post-implantation electrophysiological changes? Clinical Neurophysiology. 2018 Mar;129(3):666-667.
11. Institute of Medicine (US) Committee on the Public Health Dimensions of the Epilepsies. England MJ, Liverman CT, Schultz AM, Strawbridge LM, editors. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington (DC): National Academies Press (US); 2012.
