Introduction
The World Health Organisation (WHO) defines anaemia as a haemoglobin (Hb) of less than 130 mg/mL in men and 120 mg/mL in non-pregnant women. Anaemia has a global prevalence of 25% [1]. In patients presenting for surgery the prevalence can be as high as 40% [2-4]. Anaemia can be acute or chronic; but becomes problematic when there are insufficient numbers of red blood cells to maintain physiological oxygen demand, the end result being compromised oxygen delivery [5,6].
The most common cause of anaemia is Iron Deficiency Anaemia (IDA). Other causes include B12 or folate deficiency, the anaemia of chronic inflammation and infection [5], however in the surgical patient acute anaemia can occur through blood loss and haemodilution. Patients who present for surgery with anaemia have increased risk of perioperative transfusion; this has led to a number of recent recommendations on the investigation and management of anaemia and iron deficiency in the perioperative setting [7-9].
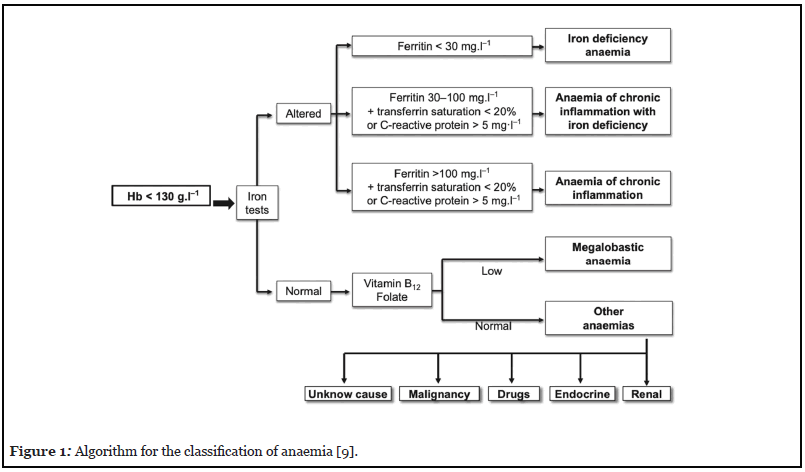
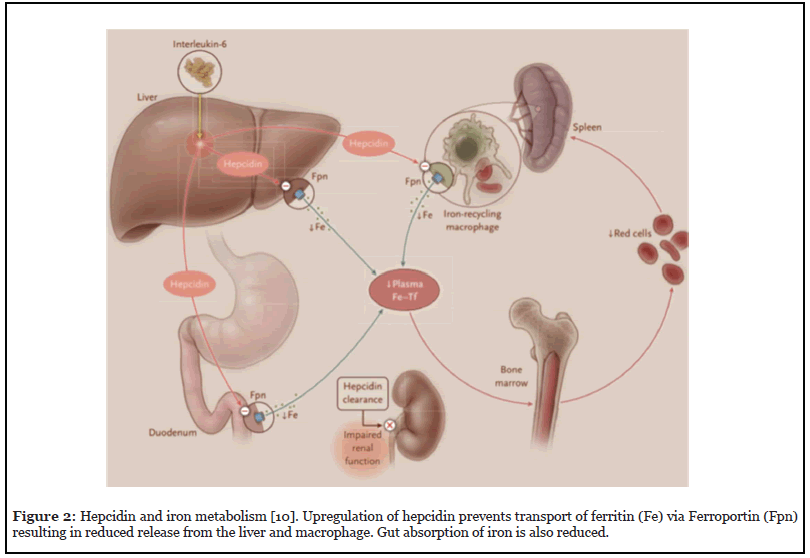
In patients requiring major surgery with an expected blood loss of more than 500 mls or a transfusion risk of greater than 10%, an international consensus statement suggests that women should have the same haemoglobin threshold as men, a minimum of 130 mg/mL [9]. An ideal algorithm presented by Munoz et al. (Figure 1) teases out the true or absolute iron deficiency (AID) over the anaemia of chronic inflammation including functional iron deficiency (FID) [9]. AID exists when there are insufficient iron stores, in converse to FID where sufficient stores are present but the body is unable to utilise them due to inflammation [6]. The inflammatory process in chronic disease including cardiovascular disease in the cardiac surgical patient leads to upregulation of the inhibitory hormone hepcidin, which prevents iron utilisation (Figure 2) [6,10–12].
The Association of Cardiothoracic Anaesthetists and Critical Care (ACTACC) published their audit findings of 19,033 patients from 12 UK cardiac surgical centres in 2016. They demonstrated 23-45% of patients presenting for cardiac surgery were anaemic [4]. Besser et al. showed the most common cause of anaemia to be iron deficiency in the same surgical cohort [13]. Anaemia was associated with increased mortality, morbidity, red cell concentrate (RCC) transfusion, postoperative complications, and prolonged length of stay (LOS) in the Intensive Care Unit (ICU) and hospital [4,14,15].
Patients undergoing cardiac surgery with pre-operative anaemia have a higher incidence of RCC transfusion and massive transfusion compared to non-anaemic patients. Multiple studies show that independent of anaemia; blood transfusion is associated with increased patient length of stay, morbidity and mortality [4,16-18].
Iron deficiency can exist with or without anaemia. Recent evidence in patients with iron deficiency but no anaemia shows that these patients share the same risk as patients with anaemia [19-21].
The increase in demand, limited supply and increase in cost of donated blood has led to implementation of strategies to reduce these problems [8,9,16,22,23]. This has been particularly poignant in the COVID 19 pandemic where access for and availability of donors has been limited across the world [24-26]. Patient Blood Management (PBM) is the collective term given to these recommendations and is essential to blood conservation. The three pillars of PBM are shown in Figure 3 [27].
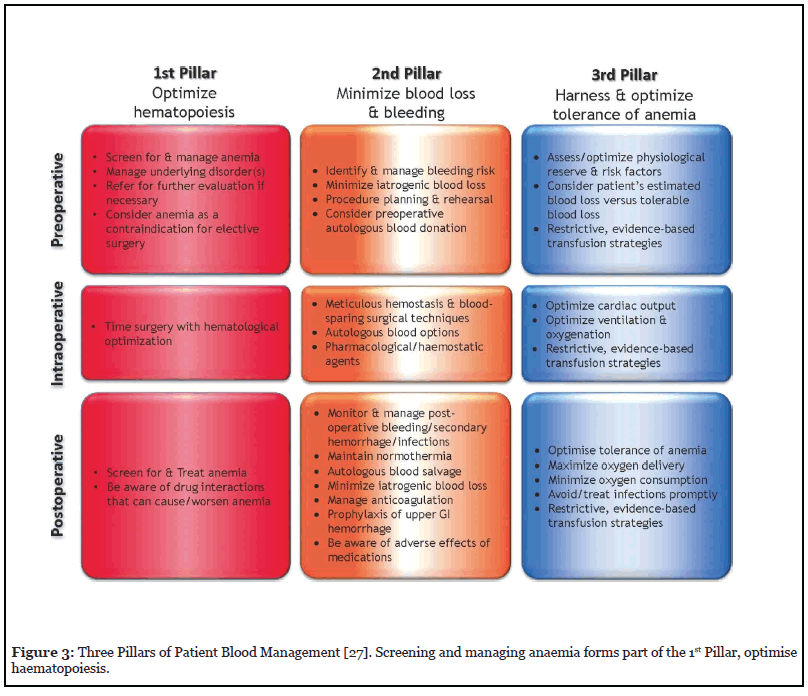
Diagnosis and treatment of perioperative iron deficiency anaemia sits within the first pillar.
The National Institute of Health and Clinical Excellence (NICE) guidance (NG24) recommends that patients with iron deficiency anaemia are supplemented with iron preoperatively [28]. This is supported by various guidelines and the international consensus statement, which recommends that iron deficient patients, anaemic or not, undergoing surgery with expected blood loss greater than 500 ml, should be supplemented with iron [9,23,29].
In response to UK and international guidance, perioperative departments across the NHS have developed their own pathways. This narrative compares and contrasts some of the better-established protocols in cardiac surgery and appraises recently published evidence in the cardiac and non-cardiac surgical settings.
The Cardiff Pathway – Evans et al., 2021
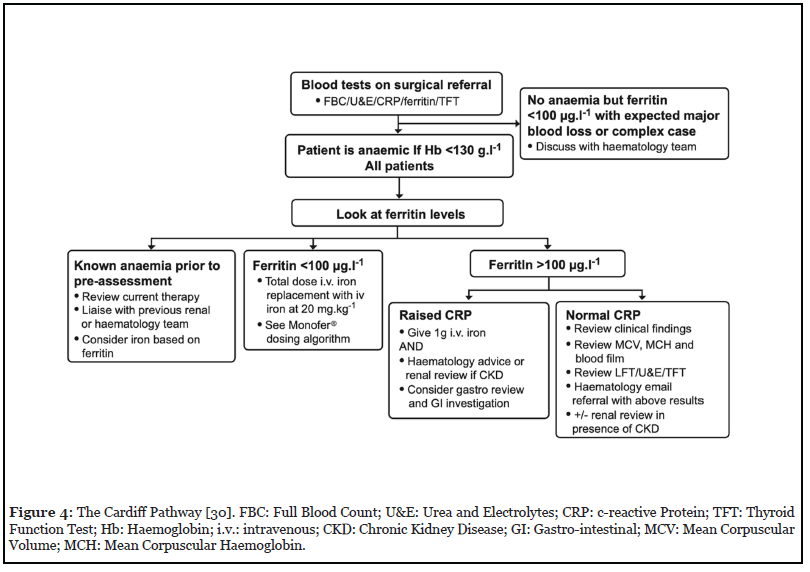
Evans et al. published The Cardiff Pathway in May 2021 in Anaesthesia [30]. This pathway, as part of a Welsh NHS quality improvement project identified a need to treat patients with anaemia and iron deficiency in the cardiac surgical setting. The aim of the pathway was to correct IDA in the pre-operative period with the potential to increase pre-operative haemoglobin, reduce peri-operative allogenic transfusion and improve patient outcome. The pathway was delivered through staff and patient education sessions.
The Cardiff Pathway (Figure 4), was one of the first in the UK to define anaemia as Hb <130 mg/mL in both men and women. The pathway defines iron deficiency as a ferritin <100 ng/mL as per the international consensus statement [9]. In their centre, all patients undergoing elective cardiac surgery were screened from blood tests taken at surgical referral and those with iron deficiency anaemia were invited to attend an outpatient clinic for administration of intravenous iron in the form of ferric derisomaltose (Monofer) at a dose of 20 mg/kg, prior to surgery.
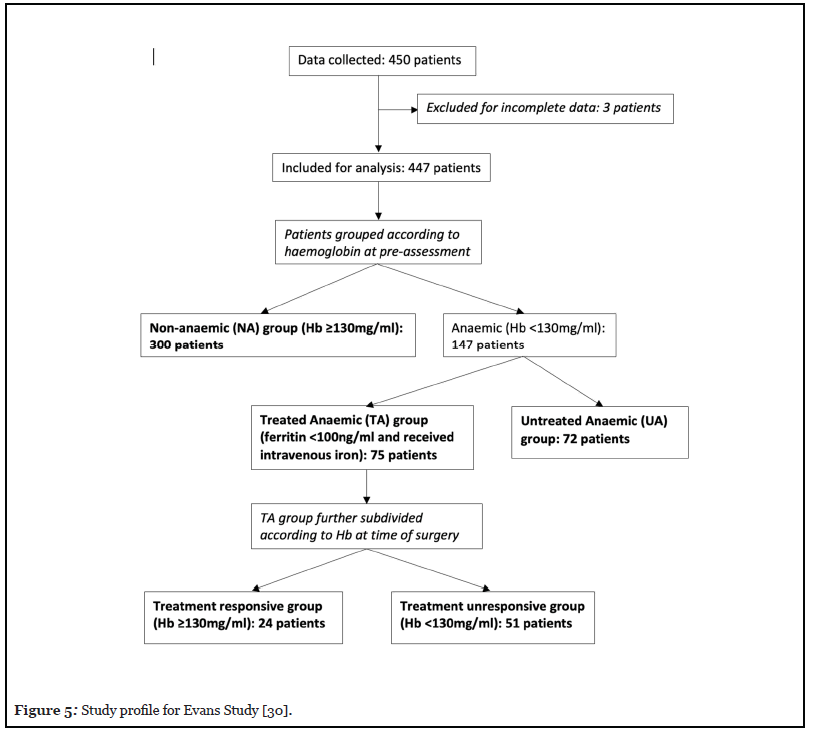
Data was collected retrospectively. All patients underwent cardiac surgery with the same PBM strategies which included tranexamic acid, cell salvage, and a hospital wide single unit transfusion policy with a trigger of Hb <80 mg/ mL.450 patients had data collected; three patients were excluded due to incomplete data sets. The remaining 447 patients were analysed in groups according to their Hb at preassessment (summarised in Figure 5). Of the 447 patients; 300 had Hb ≥ 130 mg/ml and formed the Non- Anaemic (NA) group and 147 were anaemic (Hb<130mg/ mL). Seventy-five of the anaemic patients had IDA (defined by the Cardiff pathway) and received intravenous iron; the Anaemic Treated (AT) group. The remaining 72 formed the Untreated Anaemic (UA) group. The reasons for not treating the UA group are summarised in Table 1.
| Reason for not treating UA group | Number of patients (total 72) |
|---|---|
| Did not meet pathway criteria for treatment | 22 |
| Unable to attend due to geographical distance | 1 |
| Insufficient time prior to surgery | 5 |
| Patient declined offer of intravenous iron | 1 |
| Surgeons’ preference not to give intravenous iron | 43 |
Table 1: Reasons for not treating Untreated Anaemic (UA) group [30].
Trends emerged between the groups; anaemic patients were older, more likely to be female, have a greater operative risk, which was calculated using the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II tool, and lower BMI than the non-anaemic group.
In group AT, the median (IQR [range]) time between administration of iron treatment and surgery was 42 (8-72 [7-262]) days. This group was further subdivided into those who achieved Hb ≥ 130 mg/mL by the day of surgery, termed ‘treatment responsive’ (24/75, 32%) and those who did not (Hb <130 mg/mL), termed ‘treatment unresponsive’ (51/75, 68%).
Patients who received intravenous iron were more likely to increase their Hb between surgical referral and operation (61/75, 81% of patients in AT group) when compared to the patients who did not receive iron (58/372, 16%). There was a mean increase in Hb of 17 mg/mL in the treatment responsive group. Despite not achieving a Hb ≥ 130mg/ ml by the time of surgery, the treatment unresponsive still had a mean increase in Hb of 6 mg/mL. Group NA and group UA showed a mean decrease in Hb of 2 mg/mL. In addition to this, 21/300 (7%) of the NA group became anaemic whilst waiting for surgery. 13 of these patients were non-anaemic and iron deficient at presentation. To our knowledge this finding has not been described in any other published data.
RCC transfusion was significantly lower in group NA over groups AT and UA. When the AT group was split into treatment responsive and treatment unresponsive, the treatment responsive group had similar transfusion requirements to the NA group (46% transfused ≥ 1 unit RCC vs 37%) and significantly lower than the treatment unresponsive group (78% transfused ≥ 1 unit RCC) and UA group (78% transfused ≥ 1 unit RCC). Group NA and treatment responsive patients were less likely to be transfused ≥ 3 units of RCC (16% and 17% respectively) when compared to the treatment unresponsive and untreated anaemics (23% and 28% respectively).
The treatment unresponsive group had a median LOS of 11 days compared to 8 days in group NA. A trend towards decreased 30-day mortality in the treatment responsive group was demonstrated when compared to treatment unresponsive and untreated anaemics, however the study was not designed to evaluate this outcome.
There are a number of limitations to this study being retrospective in nature and underpowered to show a difference in mortality as an outcome measure. Due to limited patient blood sampling, it was not possible to draw firm conclusions about change in Hb pre-operatively. It is possible that some patients may have had surgery before a maximum increase in their Hb had occurred and others may have passed their maxima. The Cardiff Pathway, defined IDA as Hb <130 mg/mL and Ferritin <100 ng/mL. At the time of the study period, transferrin saturations (TSATs) were not part of the protocol. Without this it is difficult to distinguish the IDA from chronic inflammatory anaemia with iron deficiency.
Evans et al. made the following novel conclusions from their analysis. A single dose of intravenous iron at 20 mg/ kg prior to cardiac surgery corrected IDA (Hb ≥ 130mg/ml) by the time of surgery in one third of patients. Correction of IDA results in reduced transfusion requirements when compared to anaemic patients and a similar transfusion picture to non-anaemic patients. Some non-anaemic patients become anaemic in the pre-operative period and non-anaemic iron deficiency may increase this risk.
Quarterman et al. Pathway
Quarterman et al. have also successfully implemented a pre-operative anaemia pathway and published a retrospective study of their findings [31]. This pathway shows similarities to the Cardiff Pathway in that anaemia is defined as Hb <130 mg/mL in both men and women and the same treatment administered i.e., a single dose of 20 mg/kg of intravenous iron. The protocols differ in that Quarterman’s protocol defines IDA as ferritin <30 ng/mL or ferritin <100 ng/mL and transferrin saturations (TSATs) <20%. The time frame from treatment to the day of surgery was much shorter in the Quarterman study.
Quarterman found no reduction in transfusion between the non-anaemic group and anaemic treated groups in the initial analysis.
The anaemic treated and untreated groups were more likely be female, older and undergo a combined procedure which are known risk factors for increased blood transfusion [32].
The mean change in Hb before surgery in the anaemic treated group was +8 mg/mL in the Quarterman study. Evans et al. showed that by dividing patients into a treatment responsive and unresponsive subset, a mean rise of 17 mg/mL could be demonstrated. Explanations for the difference in these responses could be that the Quarterman pathway treated more severely iron deficient anaemic patients with a median time of 21 days between treatment and surgery, half the time as the Evans study. Quarterman and colleagues performed a subgroup analysis on severity of anaemia and response to intravenous iron and found that those who had a lower Hb, had a larger increase in Hb from treatment, suggesting those who have severe IDA respond better to intravenous iron. The authors were able to separate the type of IDA being treated, allowing them to perform a subgroup analysis comparing change in Hb and outcome. FID appeared to have a higher transfusion requirement, however numbers in the subgroups were small making it difficult to draw firm conclusions.
The authors included data on post-operative complications. The treated anaemic group had a higher incidence of renal replacement therapy and stroke compared to the non-anaemic group. The authors commented that the anaemic patients included in their study had a higher prevalence of diabetes and previous stroke which may account for the difference in this outcome and may have influenced transfusion practice. Both of these retrospective observational studies have limitations making it hard to draw firm conclusions on the benefits of intravenous iron to treat pre-operative anaemia in cardiac surgery and supports the need for a prospective randomised controlled trial.
In response to a letter [33], Quarterman et al. presented a subgroup analysis on the treated anaemic patients who were non-anaemic by the time of surgery and those who remained anaemic [34]. Of the one hundred and ninety patients treated, 74 (38.9%) patients achieved non-anaemic status by surgery. Comparison of the treatment responsive group and treatment unresponsive groups showed that the former had a reduction in patients transfused RCC (48.7% vs 67.7%), similar to the findings of Evans. They also report a reduced incidence of post-operative renal replacement therapy in the treatment responsive group. However, 48.7% of patients transfused in the treated anaemic group was still significantly higher than the nonanaemic group (25.5%). The difference in definition of iron deficiency in the Quarterman study (ferritin <30 ng/mL) and Evans’s study (ferritin <100 ng/mL) could account for this difference, as patients with more severe iron deficiency may have impaired post-operative haemoglobin recovery.
PREVENTT Trial – Richards et al., 2020
Multiple studies and meta-analyses emphasise a need for a well-designed prospective randomised controlled trial looking at the efficacy and cost effectiveness of intravenous iron in cardiac surgery. In non-cardiac surgery, the PREVENTT trial was published in 2020 [35].
This well-designed multicentre, prospective randomised controlled trial assessed whether the pre-operative administration of intravenous iron in anaemic patients prior to major elective abdominal surgery reduced blood transfusion and mortality. Anaemia was defined as Hb <130 mg/mL for men and <120 mg/mL for women but no consideration was given to whether the patient was iron deficient or not. Anaemic patients were treated with a single dose of 1000 mg intravenous iron or placebo. In the intravenous iron group, the mean rise in Hb preoperatively was 4.7 mg/mL with a median 15 days between randomisation and surgery. Results showed there was no difference between the two groups in transfusion rate or mortality. Other outcomes such as complications, length of stay and quality of life were similar between the two groups. There were two positive findings suggesting benefit of intravenous iron. Firstly, there was a reduction in readmission at 8 weeks post-surgery in the treatment group (13%) compared to placebo (22%). Secondly, recovery of Hb post-operatively was improved at 8 weeks and 6 months in the intravenous iron group.
The rise in Hb prior to surgery and percentage of patients who achieved non-anaemic status was smaller than that observed in the retrospective cardiac surgery studies [30,31]. There are a few possible reasons, the time before surgery was much shorter in the PREVENTT trial (median 15 days) and patients received a single dose of 1000 mg intravenous iron rather than a weight-appropriate dose. The reduced dosing and short time frame possibly limit the response seen in Richards’ study. The underlying cause for surgery, (e.g. cancer, inflammatory bowel disease) resulting in chronic inflammation and upregulation of hepcidin may limit efficacy of the iron therapy [35-37]. Finally, the PREVENTT trial treated all anaemic patients, including non-iron deficient patients. The authors acknowledge that this is a limitation to the study and performed a subgroup analysis of iron deficiency anaemia patients (ferritin <100 ng/mL). There was no difference in outcomes between treated and placebo group, but the study was underpowered to show this.
The PREVENTT trial did not divide the treatment group into those that achieved non-anaemic status by surgery and those that did not. When assessing these outcomes, it would be interesting to know if those that did achieve non-anaemic status had reduced blood transfusion requirements and better quality of life outcomes. Trials have shown that treatment with intravenous iron improves quality of life and functional capacity in heart failure patients with iron deficiency whether anaemic or not [38-40]. A retrospective study comparing ferric gluconate and ferric carboxymaltose treatment in IDA patients following cardiac surgery showed improved exercise capacity at time of discharge in the latter treatment group. There was no control group present in this study, so it is difficult to ascertain whether the improvement may have been due to correction of the underlying cardiac disease [41].
Discussion
Evans and colleagues implemented a pre-operative anaemia pathway in cardiac surgery and demonstrated that treating iron deficiency anaemia with a single dose of intravenous iron achieved a Hb ≥ 130mg/mL in one third of patients. This group also showed reduced transfusion requirements when compared to anaemic patients who are not treated or did not respond adequately to treatment. The long-term outcome benefits such as improvement in morbidity and post-operative complications were not described in this study which describes the effectiveness of intravenous iron treatment and mortality only.
Good quality prospective randomised clinical trials are lacking in cardiac surgery [42]. A double blinded randomised clinical trial comparing placebo against pre-operative oral iron and intravenous iron sucrose did not show any difference in transfusion requirements or correction of anaemia post-operatively. This study was small in design and one of the main limitations was the time before surgery – 5 to 6 days [43]. The PROTECT trial, another small prospective randomised trial, treated nonanaemic patients pre-operatively with 1000mg of iron isomaltoside or placebo. The treated group had higher post-operative Hb when compared to the placebo group but transfusion requirements remained the same. This study treated non-anaemic patients, did not use a weightbased dose of iron and the study was underpowered to show a difference in transfusion outcome [44].
Trials looking at the efficacy of combined intravenous iron therapy and erythropoietin show promise. Cladellas et al. compared no treatment with preoperative erythropoietin and intravenous iron sucrose in anaemic patients one month before valve replacement surgery. They found a decrease in blood transfusion and shorter length of stay in the treatment group [45]. Recently a prospective randomised trial looking at short term treatment with erythropoietin, ferric carboxymaltose, B12 and folate preoperatively in patients with isolated iron deficiency or anaemia in cardiac surgery reduced post-operative blood transfusion [46].
The successful implementation of pre-operative anaemia pathways and treatment of IDA patients prior to surgery in NHS systems fraught with difficulties has to be commended [30,31]. In 2015, only 25% of 3,793 anaemic patients undergoing elective surgery in England, were treated for their anaemia pre-operatively and barriers to PBM strategies included time pressures and lack of staff education [47]. A multicentre study in 2019 demonstrated that 64% (7/11) of cardiac surgical centres in the UK were able to set up patient pathways to diagnose and treat IDA patients with intravenous iron [48]. From the seven centres with pathways set up, 136 anaemic patients were identified and 47% treated before surgery. The study highlighted several barriers to implementation such as limited time before surgery, lack of staff, logistical and institutional difficulties. Those who were successfully treated preoperatively had a significant increase in Hb (8.4 mg/mL) prior to surgery when compared to the anaemic patients who were not treated. The study did not look at patients who achieved an Hb ≥ 130 mg/mL and if there was a difference in transfusion requirements.
The correction of anaemia pre-operatively forms a small part of PBM. In Western Australia, a government driven healthcare wide PBM programme is established practice. Staff education is one of the many drivers to help apply the programme [49]. During education sessions, staff were surveyed; 82% said the information was new to them and 69% would go on to change their practice. The result of this PBM programme reduced patient mortality, morbidity, blood product transfusion and cost. A single centre in the United States of America, has also demonstrated reduced blood product transfusion, complications, length of stay in hospital and costs since introducing PBM strategies in 2007 [50]. There have been meta-analyses, which support the positive impact of PBM programmes on patient outcome and reduction in healthcare cost. A more recent meta-analysis has questioned if PBM strategies have benefit other than reducing blood product transfusion and highlight the need for high quality trials to assess the true benefit of PBM [51-53].
Conclusions
The work undertaken by Evans, Quarterman and Richards generates more questions for future research rather than answering the question – does pre-operative intravenous iron reduce the need for blood transfusion in elective surgery? In Evans’ and Quarterman’s studies, for those patients who achieved non-anaemic status by the time of surgery, intravenous iron was effective at reducing transfusion requirement, but likely the truly iron deficient patients achieved the best response [30,31,34].
For patients who did not respond adequately to intravenous iron, further research is required to investigate why. One of the barriers to treating anaemia pre-operatively is time. [47,48]. In clinical practice the time to optimise anaemia is dictated by the urgency of surgery. The balance of the risk associated with anaemia should be balanced against the risk of waiting. In cardiac surgery, there is a risk of complications and morbidity should patients wait longer than required [54]. If time is prioritised, there is a subgroup of patients who are unlikely to respond to intravenous iron due to chronic inflammation [36,37]. A study in ICU patients showed that high levels of hepcidin may be an indicator of poor response to intravenous iron [55]. A small randomised controlled trial looking at non-responders to iron therapy in anaemic patients also found an association with high levels of hepcidin and poor response [56]. Once these patients are identified, an appropriate treatment should be given to best correct the anaemia pre-operatively or aid Hb recovery post operatively. Combination treatment with erythropoietin could be a possible solution [46].
The small proportion of patients who become anaemic in the time they wait for surgery are exposed to the same risk and outcome as anaemic patients. Two-thirds of these patients had existing iron deficiency in the absence of anaemia. There is an argument these patients would benefit from pre-operative iron to prevent reduction in Hb before surgery and provide substrate for red cell production following perioperative blood loss and haemodilution. Studies have shown that non-anaemic iron deficient patients have increased transfusion requirement and poorer outcome when undergoing cardiac and major non-cardiac surgery [19-21]. Guidelines also support the treatment of these patients if they are undergoing surgery with expected blood loss of greater than 500 ml [9].
Recommendations
From the current available evidence, the following recommendations should be put forward for the preoperative treatment of iron deficiency anaemia in cardiac surgery.
1. Both men and women should be considered anaemic with a Hb <130 mg/mL.
2. Anaemia should be identified early in the perioperative pathway. Screening for anaemia at surgical referral would allow the maximum time for treatment prior to surgery.
3. Definitions for AID and FID should be included in all pathways, and the use of TSATs implemented.
4. A weight-based dose of intravenous iron therapy (as per manufacturer recommendations) should be given to all patients if treatment is recommended.
5. The target for correction should be ≥ 130 mg/mL to benefit from reduced transfusion requirements.
6. Multi-disciplinary team meetings to discuss risk and benefit of delayed surgery or undergoing sub-optimal anaemia treatment should be held for anaemic patients with a short timeframe to surgery.
7. Development of standardised care across all healthcare settings to allow all patients benefit.
It is important to remember the success of implementing anaemia management pre-operatively is a small part of patient blood management. The authors recommend all pillars of this multimodal approach should be introduced to show maximum benefit for patients in all healthcare settings.
Conflicts of Interest
CE has been the Principal Investigator in the UK CAVIAR study and is the current PI on the RCT ITACS. She undertaken consulting and speaking roles for Pharmacosmos.
There was no funding in the preparation of this article
Acknowledgements
Mabel Phillips - Preassessment nurse University Hospital of Wales.
References
2. Clevenger B, Richards T. Pre-operative anaemia. Anaesthesia. 2015;70(s1):20-e8.
3. Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, Soweid A, Hoballah JJ, Taher AT, Jamali FR. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. The Lancet. 2011;378(9800):1396–407.
4. Klein AA, Collier TJ, Brar MS, Evans C, Hallward G, Fletcher SN, Richards T. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK – the first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia. 2016;71(6):627–35.
5. World Health Organisation. Vitamin and mineral nutrition information system (VMNIS) haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Internet]. 2011 [cited 2021 Feb 17]. Available from: https://www.who.int/vmnis/indicators/ haemoglobin/en/
6. Cleland SR, Thomas W. Iron homeostasis and perioperative management of iron deficiency. BJA Educ. 2019;19(12):390–7.
7. Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. British Journal of Surgery. 2015;102:1314–24.
8. Clevenger B, Mallett SV, Klein AA, Richards T. Patient blood management to reduce surgical risk. British Journal of Surgery. 2015;102:1325–37.
9. Muñoz M, Acheson AG, Auerbach M, Besser M, Habler O, Kehlet H, Liumbruno GM, Lasocki S, Meybohm P, Baikady RR, Richards T, Shander A, So-Osman C, Spahn DR, Klein AA. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017;72(2):233–47.
10. Ganz T. Anemia of Inflammation. New England Journal of Medicine. 2019; 381:1148-1157
11. Camaschella C. Iron deficiency. Blood. 2019;133(1):30– 9.
12. Wunderer F, Traeger L, Sigurslid HH, Meybohm P, Bloch DB, Malhotra R. The role of hepcidin and iron homeostasis in atherosclerosis. Pharmacological Research. 2020 Mar 1;153:104664.
13. Hung M, Ortmann E, Besser M, Martin-Cabrera P, Richards T, Ghosh M, Bottrill F, Collier T, Klein AA. A prospective observational cohort study to identify the causes of anaemia and association with outcome in cardiac surgical patients. Heart. 2015;101(2):107–12.
14. Hung M, Besser M, Sharples LD, Nair SK, Klein AA. The prevalence and association with transfusion, intensive care unit stay and mortality of pre-operative anaemia in a cohort of cardiac surgery patients*. Anaesthesia. 2011;66(9):812–8.
15. Kim C, Connell H, McGeorge A, Hu R. Prevalence of preoperative anaemia in patients having first-time cardiac surgery and its impact on clinical outcome. A retrospective observational study. Perfusion. 2015;30(4):277–83.
16. Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased Mortality, Postoperative Morbidity, and Cost After Red Blood Cell Transfusion in Patients Having Cardiac Surgery. Circulation. 2007;116(22):2544–52.
17. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. The Annals of Thoracic Surgery. 2002;74(4):1180–6.
18. Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and bloodcomponent transfusion in isolated coronary artery bypass grafting*. Critical Care Medicine. 2006;34(6):1608–16.
19. Rössler J, Schoenrath F, Seifert B, Kaserer A, Spahn GH, Falk V, Spahn DR. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: a prospective study. British Journal of Anaesthesia. 2020;124(1):25–34.
20. Piednoir P, Allou N, Driss F, Longrois D, Philip I, Beaumont C, Montravers P, Lasocki S. Preoperative iron deficiency increases transfusion requirements and fatigue in cardiac surgery patients: a prospective observational study. [Miscellaneous Article]. Journal of Anaesthesiology. 2011;28(11):796–801.
21. Miles LF, Sandhu RN, Grobler AC, Heritier S, Burgess A, Burbury KL, Story DA. Associations between nonanaemic iron deficiency and outcomes following surgery for colorectal cancer: An exploratory study of outcomes relevant to prospective observational studies. Anaesth Intensive Care. 2019;47(2):152–9.
22. Scott BH, Seifert FC, Grimson R. Blood transfusion is associated with increased resource utilisation, morbidity and mortality in cardiac surgery. Annals of Cardiac Anaesthesia. 2008;11(1):15.
23. Pagano D, Milojevic M, Meesters MI, Benedetto U, Bolliger D, von Heymann C, Jeppsson A, Koster A, Osnabrugge RL, Ranucci M, Ravn HB, Vonk ABA, Wahba A, Boer C. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. European Journal of Cardio-Thoracic Surgery. 2018;53(1):79–111.
24. Baron DM, Franchini M, Goobie SM, Javidroozi M, Klein AA, Lasocki S, Liumbruno GM, Muñoz M, Shander A, Spahn DR, Zacharowski K, Meybohm P. Patient blood management during the COVID–19 pandemic: a narrative review. Anaesthesia. 2020;75(8):1105–13.
25. Gehrie EA, Frank SM, Goobie SM. Balancing Supply and Demand for Blood during the COVID-19 Pandemic. Anesthesiology. 2020;133(1):16–8.
26. Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, Germain M, Goldman M, Massey E, Prati D, Shehata N, So-Osman C, Thachil J. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. The Lancet Haematology. 2020;7(10):e756– 64.
27. Shander A, Javidroozi M, Perelman S, Puzio T, Lobel G. From Bloodless Surgery to Patient Blood Management. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2012;79(1):56–65.
28. National Institute for Health Care Excellence. Blood transfusion. NICE Guideline (NG24). 2017;
29. Butcher A, Richards T. Cornerstones of patient blood management in surgery. Transfusion Medicine.2018;28(2):150–7.
30. Evans CR, Jones R, Phillips G, Greene G, Phillips M, Morris-Clarke R. Observational study of pre-operative intravenous iron given to anaemic patients before elective cardiac surgery. Anaesthesia. 2021 EPub 29 January. https://doi/abs/10.1111/anae.15396
31. Quarterman C, Shaw M, Hughes S, Wallace V, Agarwal S. Anaemia in cardiac surgery – a retrospective review of a centre’s experience with a pre-operative intravenous iron clinic. Anaesthesia. 2020. EPub 5 November. https://doi/ abs/10.1111/anae.15271
32. Klein AA, Collier T, Yeates J, Miles LF, Fletcher SN, Evans C, Richards T. The ACTA PORT-score for predicting perioperative risk of blood transfusion for adult cardiac surgery. British Journal of Anaesthesia. 2017;119(3):394– 401.
33. Méndez E, Colomina MJ. Pre-operative optimisation with intravenous iron in cardiac surgery: some considerations. Anaesthesia. 2021. EPub 19 January. https://doi/abs/10.1111/anae.15366
34. Quarterman C, Agarwal S. Pre-operative optimisation with intravenous iron in cardiac surgery: a reply. Anaesthesia. 2021. EPub 23 February. https://doi/ full/10.1111/anae.15448
35. Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Chau M, Macdougall IC, Murphy G, Swinson R, Collier T, Van Dyck L, Browne J, Bradbury A, Dodd M, Evans R, Brealey D, Anker SD, Klein A. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. The Lancet. 2020;396(10259):1353–61.
36. Schaefer B, Meindl E, Wagner S, Tilg H, Zoller H. Intravenous iron supplementation therapy. Molecular Aspects of Medicine. 2020;75:100862.
37. Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260–72.
38. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan B-A, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. New England Journal of Medicine. 2009;361(25):2436–48.
39. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM., Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG., Poole-Wilson PA., Anker SD., Ponikowski P. Effect of Intravenous Iron Sucrose on Exercise Tolerance in Anemic and Nonanemic Patients With Symptomatic Chronic Heart Failure and Iron Deficiency. Journal of the American College of Cardiology. 2008;51(2):103–12.
40. Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira- Barretto AC, de Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. IRON-HF study: A randomized trial to assess the effects of iron in heart failure patients with anemia. International Journal of Cardiology. 2013;168(4):3439–42.
41. Nugara C, Vitale G, Caccamo G, Sarullo S, Giallauria F, Di Franco A, Vitale S, Sarullo FM. Effect of intravenous iron replacement therapy on exercise capacity in iron deficient anemic patients after cardiac surgery. Monaldi Archives for Chest Disease. 2020;90(1).
42. Tankard KA, Park B, Brovman EY, Bader AM, Urman RD. The Impact of Preoperative Intravenous Iron Therapy on Perioperative Outcomes in Cardiac Surgery: A Systematic Review. J Hematol. 2020;9(4):97–108.
43. Garrido-Martín P, Nassar-Mansur MI, de la Llana- Ducrós R, Virgos-Aller TM, Fortunez PMR, Ávalos- Pinto R, Jimenez-Sosa A, Martínez-Sanz R. The effect of intravenous and oral iron administration on perioperative anaemia and transfusion requirements in patients undergoing elective cardiac surgery: a randomized clinical trial. Interact Cardiovasc Thorac Surg. 2012;15(6):1013–8.
44. Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sanguinis. 2015;109(3):257–66.
45. Cladellas M, Farré N, Comín-Colet J, Gómez M, Meroño O, Bosch MA, Vila J, Molera R, Segovia A, Bruguera J. Effects of Preoperative Intravenous Erythropoietin Plus Iron on Outcome in Anemic Patients After Cardiac Valve Replacement. The American Journal of Cardiology. 2012;110(7):1021–6.
46. Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, Kaserer A, Hegemann I, Hofmann A, Maisano F, Falk V. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. The Lancet. 2019;393(10187):2201–12.
47. Grant-Casey J, Allard S, Atterbury C, Highton D, Lowe D, Mortimer A, Richards T, Robinson M, Wilan P. 2015 Audit of Patient Blood Management in Adults undergoing elective, scheduled surgery. National Comparative Audit of Blood Transfusion; 2015.
48. Klein AA, Chau M, Yeates JA, Collier T, Evans C, Agarwal S, Richards T. Preoperative intravenous iron before cardiac surgery: a prospective multicentre feasibility study. British Journal of Anaesthesia. 2020;124(3):243– 50.
49. Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, Hamdorf J, Gallagher T, Koay A, Geelhoed GC, Farmer SL. Improved outcomes and reduced costs associated with a health-system–wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–58.
50. Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55(5):1075–81.
51. Meybohm P, Straub N, Füllenbach C, Judd L, Kleinerüschkamp A, Taeuber I, Zacharowski K, Choorapoikayil S. Health economics of Patient Blood Management: a cost-benefit analysis based on a metaanalysis. Vox Sanguinis. 2020;115(2):182–8.
52. Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Füllenbach C, Freedman J, Waters JH, Farmer S, Leahy MF, Zacharowski K, Meybohm P, Choorapoikayil S. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Metaanalysis. Annals of Surgery. 2019;269(5):794–804.
53. Roman MA, Abbasciano RG, Pathak S, Oo S, Yusoff S, Wozniak M, Qureshi S, Lai FY, Richards T, Yao G, Estcourt L, Murphy GJ. Patient blood management internebtions do not lead to important clinical benefits or cost effectiveness for major surgery: a network meta-analysis. British Journal of Anaesthesia. 2021;126(1):149-156.
54. Koomen EM, Hutten BA, Kelder JC, Redekop WK, Tijssen JGP, Kingma JH. Morbidity and mortality in patients waiting for coronary artery bypass surgery. European Journal of Cardio-Thoracic Surgery. 2001;19(3):260–5.
55. Litton E, Baker S, Erber W, Farmer S, Ferrier J, French C, Gummer J, Hawkins D, Higgins A, Hofmann A, De Keulenaer B, McMorrow J, Olynyk JK, Richards T, Towler S, Trengove R, Webb S, Chapman A, Jenkinson E, Palermo AM, Roberts B, on behalf of the IRONMAN Study investigators, the Australian and New Zealand Intensive Care Society Clinical Trials Group. Hepcidin predicts response to IV iron therapy in patients admitted to the intensive care unit: a nested cohort study. Journal of Intensive Care. 2018;6(1):60.
56. Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. American Journal of Hematology. 2013;88(2):97–101.
