Abstract
Cardiovascular diseases (CVDs) remain a leading cause of morbidity and mortality worldwide. Effective prevention and management strategies are essential to reduce the burden of CVDs. This review summarizes recent advances in the prevention and management of CVDs. In terms of prevention, lifestyle modifications, such as diet, exercise, and smoking cessation, remain important strategies. Pharmacological interventions, including statins, antiplatelet agents, and antihypertensive medications, have also shown benefits. Innovative approaches, such as genetic testing and risk stratification, targeted prevention strategies, and the use of mobile health technology, are being explored. In terms of management, acute management strategies, such as reperfusion therapy for myocardial infarction, acute heart failure management, and stroke management, have improved outcomes. Chronic management strategies, including medical therapy for heart failure, arrhythmia management, and device therapy for heart failure, have also shown benefits. Innovative approaches, such as precision medicine and personalized treatment, the use of artificial intelligence in diagnosis and treatment, and advances in traditional Chinese and regenerative medicine, are being investigated. While recent advances in the prevention and management of CVDs are promising, challenges remain in implementing these strategies, including cost-effectiveness, access to care, and resistance to change in clinical practice. Continued research and innovation are essential to reduce the burden of CVDs and improve patient outcomes.
Keywords
Pharmacological interventions, Genetic testing, Personalized medicine, Cardiovascular diseases, Artificial intelligence, Nanotechnology, Telemedicine
Background
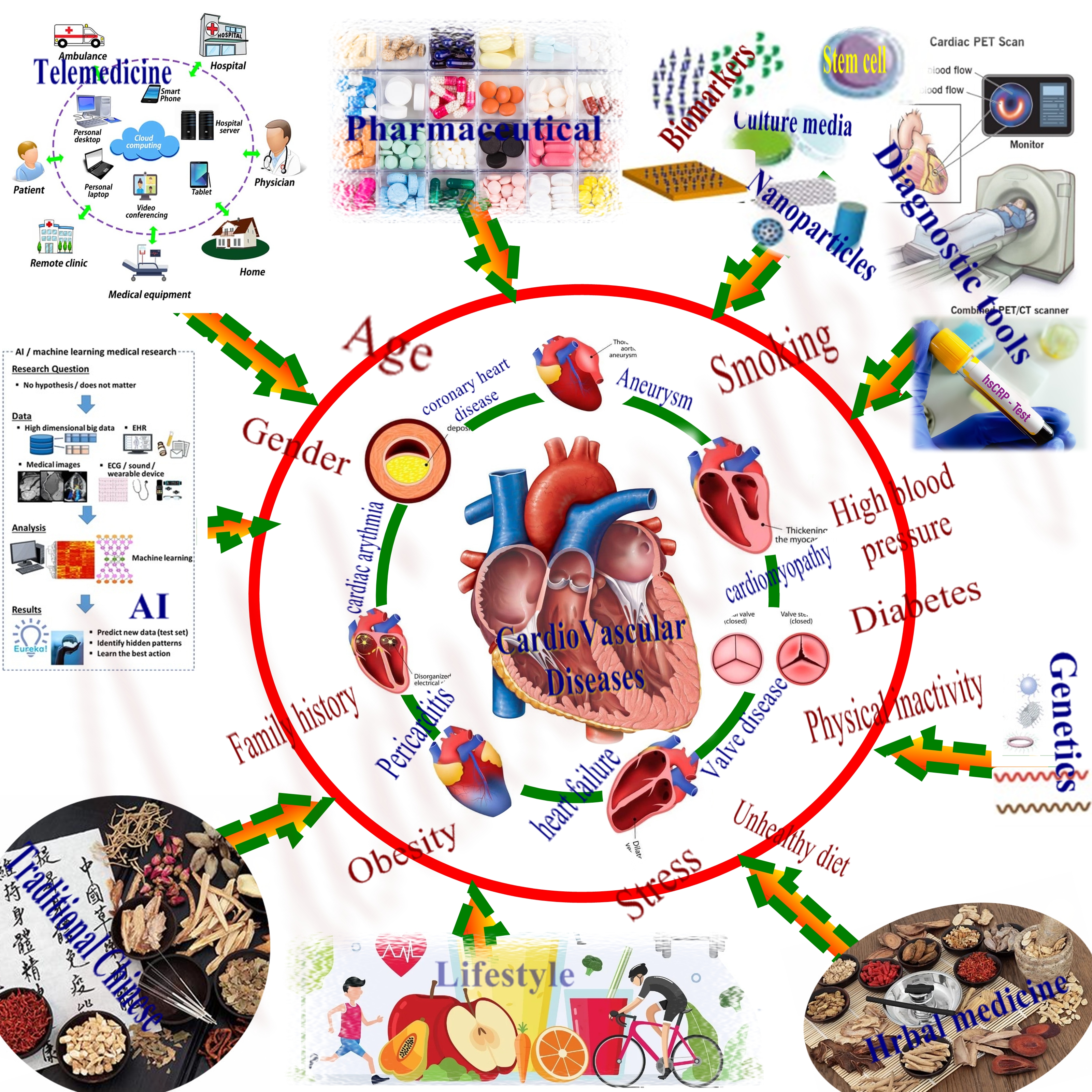
Cardiovascular diseases (CVDs) are a group of disorders that affect the heart and blood vessels, and they are a leading cause of mortality and morbidity worldwide. CVDs include coronary artery disease (CAD), heart failure, stroke, and hypertension. CAD is the most common form of CVD and occurs when the coronary arteries become narrowed or blocked, resulting in reduced blood flow to the heart. Heart failure occurs when the heart cannot pump enough blood to meet the body's needs. Stroke occurs when blood flow to the brain is disrupted, leading to brain damage and possibly death. Hypertension, or high blood pressure, is a major risk factor for CVDs and can lead to heart attack, stroke, and heart failure [1]. Risk factors for CVDs include age, gender, family history, tobacco use, high blood pressure, high cholesterol, diabetes, obesity, physical inactivity, and poor diet. The prevalence of CVDs is increasing globally, largely due to aging populations and lifestyle factors. Effective prevention and management strategies are essential to reduce the burden of CVDs [2]. These strategies include lifestyle modifications, such as diet, exercise, and smoking cessation, as well as pharmacological interventions, such as statins, antiplatelet agents, and antihypertensive medications. Innovative approaches, such as genetic testing and risk stratification, targeted prevention strategies, and the use of mobile health technology, are also being explored [3].
Cardiovascular diseases (CVDs) can be treated using a variety of conventional and novel treatment approaches. Conventional treatment approaches include the use of medications, such as beta-blockers, ACE inhibitors, and statins, which can lower blood pressure, improve heart function, and reduce cholesterol levels. In addition, surgical interventions, such as angioplasty, stenting, and coronary artery bypass grafting, can be used to improve blood flow to the heart. Novel treatment approaches include regenerative therapies, such as stem cell therapy, which can help repair damaged heart tissue and improve heart function. Gene therapy, which can modify gene expression to prevent or treat heart disease, is also an emerging approach. In addition, digital health technologies, such as wearables and telemedicine, are being used to monitor patients and improve the delivery of care [4].
Combining conventional and novel treatment approaches can provide a comprehensive and personalized approach to treating CVDs. However, more research is needed to validate the effectiveness of these emerging therapies and to further improve patient outcomes. By continuing to invest in research and innovation, we can improve the treatment and management of CVDs and ultimately reduce the global burden of these diseases [5]. Another novel treatment approach for CVDs is the use of advanced medical devices, such as implantable cardiac devices, which can monitor and regulate heart function. Pacemakers and implantable cardioverter defibrillators (ICDs) are commonly used to treat arrhythmias, while left ventricular assist devices (LVADs) can be used to support heart function in patients with advanced heart failure [6].
Effective prevention and management of CVDs are critical to reducing the global burden of these conditions. CVDs are major causes of morbidity and mortality, with high economic costs related to healthcare utilization and lost productivity. Prevention strategies, such as lifestyle modifications and pharmacological interventions, can reduce the risk of developing CVDs and delay disease progression. Effective management of CVDs can improve patient outcomes, reduce the risk of complications and hospitalizations, and prolong survival. In addition to improving individual health outcomes, effective prevention and management of CVDs can also have broader societal benefits. By reducing the burden of CVDs, healthcare systems can allocate resources more efficiently and effectively, and the economic burden of CVDs can be reduced, leading to increased productivity and economic growth [7]. Recent years have seen significant advances in the prevention and management of CVDs. These advances include the development of innovative technologies and therapies, as well as new approaches to prevention and management. In terms of prevention, lifestyle modifications remain important strategies, with a growing emphasis on personalized approaches to care. Pharmacological interventions, including statins, antiplatelet agents, and antihypertensive medications, have also shown benefit in reducing the risk of CVDs [8]. Innovative approaches, such as genetic testing and risk stratification, targeted prevention strategies, and the use of mobile health technology, are being explored. In terms of management, acute management strategies, such as reperfusion therapy for myocardial infarction, acute heart failure management, and stroke management, have improved outcomes. Chronic management strategies, including medical therapy for heart failure, arrhythmia management, and device therapy for heart failure, have also shown benefit. Innovative approaches, such as precision medicine and personalized treatment, the use of artificial intelligence in diagnosis and treatment, and advances in regenerative medicine, are being investigated.
Precision medicine is another emerging approach to treating CVDs, which involves tailoring treatment to a patient's unique genetic makeup, lifestyle, and environment. This approach can help identify patients who are at higher risk of developing CVDs and provide more targeted and personalized therapies [9]. Preventive interventions, such as lifestyle modifications, can also play a critical role in the treatment of CVDs. Healthy lifestyle modifications, including regular exercise, healthy diet, and smoking cessation, can help prevent the development of CVDs and improve overall cardiovascular health. Early detection and management of risk factors, such as hypertension and diabetes, are also essential in preventing the development of CVDs [10]. Despite advancements in prevention and management strategies, challenges remain in the effective treatment and prevention of CVDs. These challenges include the high cost of medications and interventions, limited access to care in some regions, and resistance to change in clinical practice [11]. As the population ages, there is a growing need for innovative approaches to CVD management, including the development of emerging therapies and technologies. Gene therapy, nanotechnology, and immunotherapy are among the emerging therapies being studied for the treatment of CVDs [12].
While innovative diagnostics and therapies hold promise to prolong survival, we must thoughtfully assess impacts on quality of life and disease trajectory, not merely mortality. As patient advocates, we cannot ignore lived experience and personhood in the quest to extend lifespan. Progress must empower prevention so communities can pursue health on their terms. Although technology presents tools to combat disease, it does not supplant the need for social support and equitable access so all people can build resilience against avoidable risks in a dignified, empowered manner.
Prevention of Cardiovascular Diseases
Prevention of cardiovascular diseases (CVDs) is essential to reduce the global burden of these diseases. CVDs are largely preventable through lifestyle modifications, such as healthy diet, regular physical activity, and avoidance of tobacco and excessive alcohol consumption [13].
Novel diagnostic tools for cardiovascular diseases (CVDs)
Early and accurate diagnosis is essential for effective treatment and improved patient outcomes. Recent advances in diagnostic tools, such as artificial intelligence (AI) and machine learning, biomarkers, cardiovascular imaging, wearable technology, and point-of-care testing, have the potential to improve the accuracy and speed of diagnosis for CVDs [14].
Artificial intelligence and machine learning: AI and machine learning algorithms can analyze large amounts of data from multiple sources, such as electronic health records, imaging studies, and genetic testing, to identify patterns and predict the risk of CVDs. These tools can also assist in interpreting complex imaging data, such as echocardiograms or angiograms, and provide more accurate and timely diagnoses. Several studies have shown that AI and machine learning algorithms can improve the accuracy of CVD diagnosis, particularly for conditions such as coronary artery disease, heart failure, and arrhythmias [15].
Biomarkers: Biomarkers are measurable substances in the blood, urine, or tissues that can indicate the presence of a disease or the risk of developing a disease. Recent advances in biomarker research have identified several promising biomarkers for CVDs, such as high-sensitivity troponin, which can detect small amounts of cardiac damage and help diagnose acute coronary syndrome [16]. Other promising biomarkers include BNP, which can indicate heart failure, and galectin-3, which has been associated with the risk of heart failure and cardiovascular death [17].
Cardiovascular imaging: There have been several advances in cardiovascular imaging, such as 3D echocardiography, cardiac MRI, and PET imaging, which can provide detailed information on the structure and function of the heart and blood vessels [18]. These imaging techniques can detect early signs of CVDs and provide more accurate diagnoses. For example, 3D echocardiography has been shown to improve the accuracy of left ventricular ejection fraction measurement, which is an important parameter in the diagnosis and management of heart failure [19].
Point-of-care testing: Point-of-care testing refers to diagnostic tests that can be performed at a patient's bedside or in a clinical setting, providing rapid results [20]. Recent advances in point-of-care testing have led to the development of portable devices that can measure biomarkers, such as troponin or BNP, and provide rapid diagnoses of CVDs. These devices have the potential to improve the speed and accuracy of diagnosis, particularly in emergency settings [21].
Limitations and challenges of Novel diagnostic tools
Cost: Some of the newer diagnostic tools, such as AI and machine learning algorithms, and some of the advanced imaging techniques, can be costly and may not be accessible to all patients. This can limit their use in certain settings or populations.
Implementation: The implementation of new diagnostic tools can be challenging, particularly in busy clinical settings where time and resources may be limited. There may also be a need for additional training for healthcare professionals to effectively use these tools [22].
Privacy and security: The use of AI and machine learning algorithms, as well as wearable technology, raises concerns about privacy and security of patient data. Ensuring the protection of patient data and maintaining patient confidentiality is essential.
Validation: While many of the new diagnostic tools show great promise, more research is needed to validate their effectiveness and accuracy. This is particularly important for biomarkers and other diagnostic tests that are not yet widely used in clinical practice [23].
Reimbursement: The reimbursement policies for these new diagnostic tools are still evolving, and this may limit their use in some healthcare systems. Ensuring that these tools are reimbursed appropriately will be important to encourage their adoption and use in clinical practice [24].
While there are limitations and challenges associated with the latest advances in novel diagnostic tools for CVDs, the potential benefits of these tools are significant. Addressing these challenges will be essential to ensure that these tools are effective, accessible, and safe for patients.
Lifestyle modifications
Lifestyle modifications are a critical component of the prevention and management of cardiovascular diseases (CVDs). These modifications include changes to diet, physical activity, and smoking habits, as well as efforts to manage stress and maintain a healthy weight [25]. Lifestyle modifications have been shown to reduce the risk of developing CVDs and can also help manage existing conditions (Table 1). By adopting healthy lifestyle habits, individuals can improve their overall cardiovascular health and reduce the burden of CVDs on both themselves and society as a whole [26]. Lifestyle and mind-body therapies should play a larger role alongside medications or procedures when managing cardiovascular conditions. Stress reduction techniques like meditation and yoga, dietary changes emphasizing whole foods and plants, supplemental herbs like hawthorn and rauwolfia serpentina, and increased physical activity have tremendous potential to aid prevention and slow disease progression. These holistic modalities deserve greater emphasis considering meaningful quality of life improvements observed and favorable safety profile compared to pharmacological options.
|
Lifestyle Modification |
Effects on CVD Risk Factors |
|
Smoking cessation |
Reduces risk of atherosclerosis, thrombosis, hypertension, heart attack, stroke |
|
Healthy diet (e.g. Mediterranean diet, DASH diet) |
Reduces blood pressure, LDL cholesterol, inflammation; improves lipid profile |
|
Regular exercise and physical activity |
Lowers blood pressure, improves cholesterol profile, reduces inflammation, aids weight loss |
|
Weight management |
Reduces blood pressure, cholesterol levels, inflammation; improves insulin sensitivity |
|
Moderate alcohol intake |
Increases HDL cholesterol; reduces inflammation |
|
Stress management |
Lowers blood pressure and heart rate |
|
Adequate sleep |
Reduces inflammation, improves insulin sensitivity and lipid metabolism |
Diet and nutrition: A healthy diet is defined as one that is low in saturated and trans fats, added sugars, and sodium, and high in fruits, vegetables, whole grains, lean proteins, and healthy fats. Such a diet can help lower blood pressure, reduce high cholesterol levels, and maintain a healthy weight, all of which are risk factors for CVDs [27]. Specific dietary patterns, such as the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH) diet, have been shown to reduce the risk of CVDs [28]. These diets emphasize whole foods, such as fruits, vegetables, whole grains, and lean proteins, and limit processed foods and added sugars [29].
In addition to dietary patterns, specific nutrients and supplements have also been studied for their potential benefits in reducing the risk of CVDs [30]. Omega-3 fatty acids, found in fatty fish and supplements, have been shown to reduce triglyceride levels and lower the risk of heart disease [31]. Plant sterols, found in certain margarines and supplements, can also help lower cholesterol levels [32].
Exercise and physical activity: In addition to a healthy diet, regular exercise and physical activity are important lifestyle modifications that can help prevent cardiovascular diseases (CVDs) [33]. Exercise has been shown to have multiple benefits for heart health, including reducing blood pressure, improving cholesterol levels, and maintaining a healthy weight [34]. The American Heart Association recommends at least 150 minutes of moderate-intensity aerobic exercise or 75 minutes of vigorous-intensity aerobic exercise per week for adults to maintain cardiovascular health [35]. Examples of moderate-intensity activities include brisk walking, cycling, and swimming, while examples of vigorous-intensity activities include running, hiking, and playing sports [36]. Resistance training, such as weightlifting or using resistance bands, can also provide additional benefits for heart health by improving muscle strength and endurance [37]. Incorporating physical activity into daily routines, such as taking the stairs instead of the elevator or walking instead of driving, can also help increase overall physical activity levels [38].
Smoking cessation: Smoking is a significant risk factor for cardiovascular diseases (CVDs) and quitting smoking is one of the most important lifestyle modifications in preventing CVDs. Smoking damages the blood vessels, increases blood pressure, and raises the risk of blood clots, all of which can contribute to the development of CVDs [39]. Quitting smoking has immediate and long-term benefits for cardiovascular health. Within just a few hours of quitting smoking, blood pressure and heart rate begin to decrease. Within a few months, circulation improves and lung function increases [40]. Over time, the risk of heart disease, stroke, and other CVDs decreases. Smoking cessation interventions can include behavioral counseling, nicotine replacement therapy, prescription medications, or a combination of these approaches [41]. Behavioral counseling can help individuals develop strategies to cope with cravings and withdrawal symptoms, while nicotine replacement therapy and prescription medications can help reduce cravings and withdrawal symptoms [42].
Pharmacological interventions
Pharmacological interventions, including medications such as statins, antiplatelet agents, and antihypertensive drugs, play a critical role in the prevention and management of cardiovascular diseases (CVDs) [43]. These medications can help reduce the risk of developing CVDs and can also help manage existing conditions. Pharmacological interventions are often used in combination with lifestyle modifications to provide optimal cardiovascular health benefits [44]. Despite their effectiveness, there can be challenges associated with the high cost of some medications and potential side effects. Therefore, it is important for healthcare providers to carefully evaluate each patient's individual needs and risks when prescribing pharmacological interventions for CVDs [45].
Statins: Statins are a class of medications that work by reducing the production of cholesterol in the liver, which in turn lowers the level of low-density lipoprotein (LDL), or "bad" cholesterol, in the blood. By lowering LDL cholesterol levels, statins can help reduce the risk of heart attack, stroke, and other CVDs [46]. Statins are typically prescribed for individuals who have an elevated risk of CVDs, such as those with high cholesterol levels, diabetes, or a history of heart disease. The American College of Cardiology and the American Heart Association recommend statin therapy for individuals with a 10-year risk of CVDs greater than 7.5% [47]. While statins are generally safe and well-tolerated, they can have side effects, including muscle pain, liver damage, and an increased risk of type 2 diabetes. Healthcare providers should monitor patients on statin therapy to ensure that they are tolerating the medication well and that their cholesterol levels are adequately controlled [48].
Antiplatelet agents: Antiplatelet agents are another type of pharmacological intervention used in the prevention of cardiovascular diseases (CVDs). These medications work by inhibiting the formation of blood clots, which can reduce the risk of heart attack and stroke [49].
Aspirin is the most commonly used antiplatelet agent and is typically prescribed for individuals who have had a prior heart attack or stroke, or who are at high risk of these events [50]. Aspirin works by inhibiting the activity of platelets, which are small cells in the blood that play a key role in blood clotting [51]. Other antiplatelet agents, such as clopidogrel, prasugrel, and ticagrelor, may be prescribed for individuals who are unable to tolerate aspirin or who require more potent antiplatelet therapy [52]. While antiplatelet agents are generally safe and effective, they can have side effects, including bleeding and gastrointestinal irritation. Healthcare providers should monitor patients on antiplatelet therapy to ensure that they are tolerating the medication well and that their risk of bleeding is minimized [53].
Antihypertensive medications: Antihypertensive medications are a class of pharmacological interventions used in the prevention of cardiovascular diseases (CVDs). These medications work by reducing blood pressure, which is a major risk factor for CVDs, including heart attack, stroke, and heart failure [54]. There are several classes of antihypertensive medications, including diuretics, beta-blockers, ACE inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers, and renin inhibitors. These medications work by different mechanisms, but all aim to reduce blood pressure by relaxing blood vessels, decreasing blood volume, or reducing the activity of the renin-angiotensin-aldosterone system [55]. Antihypertensive medications are typically prescribed for individuals with high blood pressure, which is defined as a blood pressure reading of 130/80 mmHg or higher. The American College of Cardiology and the American Heart Association recommend antihypertensive therapy for individuals with a blood pressure reading of 140/90 mmHg or higher [56]. While antihypertensive medications are generally safe and effective, they can have side effects, including dizziness, fatigue, and sexual dysfunction. Healthcare providers should monitor patients on antihypertensive therapy to ensure that they are tolerating the medication well and that their blood pressure is adequately controlled [57].
Innovative approaches of prevention
Innovative approaches are being explored for the prevention and management of cardiovascular diseases (CVDs). These approaches include genetic testing and risk stratification, targeted prevention strategies, and the use of mobile health technology. Emerging therapies and technologies such as gene therapy, nanotechnology, and immunotherapy are also being studied. Advances in precision medicine, personalized treatment, and the use of artificial intelligence in diagnosis and treatment offer new opportunities for individualized treatment plans and improved patient outcomes [58].
Genetic testing and risk stratification: Genetic testing and risk stratification are innovative approaches to preventing cardiovascular diseases (CVDs). These approaches aim to identify individuals who are at higher risk of developing CVDs based on their genetic makeup and other risk factors [59]. Genetic testing can identify inherited genetic variants that increase the risk of CVDs, such as familial hypercholesterolemia or inherited arrhythmias. By identifying individuals with these genetic variants, healthcare providers can initiate targeted screening and early intervention to reduce the risk of CVDs [60]. Risk stratification is a process of assessing an individual's risk of developing CVDs based on multiple risk factors, including age, sex, blood pressure, cholesterol levels, and family history [61]. Innovative risk stratification tools, such as the American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equations, can provide a more accurate assessment of an individual's risk of developing CVDs and guide treatment decisions [62]. Personalized risk stratification, which combines genetic testing and risk factor assessment, can provide an even more accurate assessment of an individual's risk of developing CVDs. This approach can help identify individuals who may benefit from more aggressive prevention strategies, such as earlier initiation of pharmacological therapy or lifestyle modifications [63].
Targeted prevention strategies: Targeted prevention strategies are another innovative approach to preventing cardiovascular diseases (CVDs). These strategies aim to identify and target specific risk factors for CVDs in individuals who are at higher risk. One example of a targeted prevention strategy is the use of statins for primary prevention in individuals with elevated levels of LDL cholesterol. This approach targets a specific risk factor for CVDs and has been shown to be effective in reducing the risk of heart attack and stroke in individuals who are at higher risk due to their cholesterol levels [64]. Another example of a targeted prevention strategy is the use of novel biomarkers, such as high-sensitivity C-reactive protein (hsCRP), to identify individuals who are at higher risk of developing CVDs. Elevated levels of hsCRP have been associated with an increased risk of CVDs, and targeted interventions, such as statin therapy or lifestyle modifications, can be initiated in these individuals to reduce their risk [65]. Genetic testing and risk stratification, as discussed in the previous section, can also be used as targeted prevention strategies to identify individuals who are at higher risk of developing CVDs and to initiate personalized prevention strategies [66].
Use of mobile health technology: The use of mobile health (mHealth) technology is an innovative approach to preventing cardiovascular diseases (CVDs). mHealth technology includes mobile apps, wearable devices, and other digital tools that can help individuals monitor their health and make informed decisions about their lifestyle and treatment options [67]. mHealth technology can be used to promote healthy behaviors, such as physical activity and healthy eating, by providing personalized coaching, tracking progress, and offering motivational support. For example, mobile apps can provide personalized exercise plans, track calorie intake, and offer healthy recipes and meal plans [68]. Wearable devices, such as fitness trackers and smartwatches, can monitor physical activity, heart rate, and sleep patterns, providing individuals with real-time feedback on their health status. These devices can also provide reminders to take medication, monitor blood pressure, and track other health metrics [69]. mHealth technology can also facilitate remote monitoring and management of chronic conditions, such as hypertension and diabetes, allowing healthcare providers to monitor patients' health status and adjust treatment plans as needed [70].
Management of Cardiovascular Diseases
Management strategies can include acute management approaches, such as reperfusion therapy for myocardial infarction, acute heart failure management, and stroke management [71]. Chronic management strategies, including medical therapy for heart failure, arrhythmia management, and device therapy for heart failure, have also shown benefit. Lifestyle modifications and pharmacological interventions are often used in combination with management strategies to provide optimal cardiovascular health benefits [72]. Innovative approaches such as precision medicine, personalized treatment, and the use of artificial intelligence in diagnosis and treatment are being investigated to improve the effectiveness of CVD management [73].
Acute management
Acute management of cardiovascular diseases involves immediate medical interventions aimed at stabilizing the patient's condition and preventing further damage to the heart or brain. This may include medications, medical procedures, and lifestyle modifications tailored to the specific condition and severity of the patient's symptoms [74]. Effective acute management is critical for improving patient outcomes and reducing the risk of complications or long-term disability. Healthcare providers must stay up-to-date on the latest guidelines and best practices to ensure the best possible outcomes for their patients [75].
Reperfusion therapy for myocardial infarction: Reperfusion therapy is an essential component of the acute management of myocardial infarction (MI), also known as a heart attack. Reperfusion therapy aims to restore blood flow to the blocked coronary artery and limit the damage to the heart muscle [76]. The two main types of reperfusion therapy for MI are fibrinolytic therapy and percutaneous coronary intervention (PCI). Fibrinolytic therapy involves the administration of medications, such as alteplase or tenecteplase, that dissolve the blood clot causing the MI [77]. PCI involves the insertion of a catheter into the blocked coronary artery to open the blockage and restore blood flow [78]. PCI is the preferred reperfusion therapy for most patients with MI if it can be performed in a timely manner. Studies have shown that PCI is associated with better outcomes than fibrinolytic therapy, including lower rates of mortality, recurrent MI, and stroke [79]. The promise of therapies such as remote ischemic preconditioning, hypothermia, and pharmacological agents like cyclosporine A and metoprolol in reducing myocardial ischemia-reperfusion injury. Fibrinolytic therapy is typically reserved for patients who are unable to undergo PCI due to logistical or clinical reasons, such as delays in transport to a PCI-capable hospital or contraindications to PCI [80].
Acute heart failure management: Acute heart failure (AHF) is a medical emergency that requires prompt evaluation and management to improve outcomes and prevent complications. The management of AHF involves the identification and treatment of the underlying cause of heart failure, as well as the optimization of hemodynamic status and symptom relief [81]. The initial management of AHF may include oxygen therapy, diuretic therapy, and vasodilator therapy, such as nitroglycerin or nitroprusside, to improve symptoms and reduce fluid overload. Inotropic agents, such as dobutamine or milrinone, may also be used to improve cardiac output and hemodynamic stability. In addition to pharmacological management, non-pharmacological interventions, such as ultrafiltration or mechanical circulatory support, may be considered in severe cases of AHF. The management of AHF also involves the identification and treatment of comorbidities, such as hypertension, diabetes, and renal dysfunction, which can exacerbate heart failure and contribute to poor outcomes [82].
Stroke management: Stroke is a medical emergency that requires prompt evaluation and management to improve outcomes and prevent complications. The management of stroke involves the identification of the type of stroke, the identification and treatment of the underlying cause of the stroke, and the optimization of neurological function and prevention of complications. The two main types of stroke are ischemic stroke and hemorrhagic stroke. Ischemic stroke is caused by a blockage in a blood vessel supplying the brain, while hemorrhagic stroke is caused by bleeding in the brain. The initial management of ischemic stroke may include the administration of intravenous tissue plasminogen activator (tPA) within 3-4.5 hours of symptom onset, which can dissolve the clot causing the stroke and restore blood flow to the brain. Mechanical thrombectomy, which involves the removal of the clot using a catheter inserted through the groin, may be considered in certain cases [83]. The management of hemorrhagic stroke involves the identification and treatment of the underlying cause of the bleeding, such as hypertension or aneurysm. Blood pressure control, reversal of anticoagulant therapy, and management of intracranial pressure are also important components of the management of hemorrhagic stroke [84]. After the acute management of stroke, ongoing management involves the prevention of complications, such as pneumonia, deep vein thrombosis (DVT), and pressure ulcers, as well as the initiation of secondary prevention measures, such as antiplatelet or anticoagulant therapy, blood pressure control, and lifestyle modifications [85].
Chronic management
Chronic management of cardiovascular diseases refers to the ongoing medical care and lifestyle modifications aimed at preventing further damage to the heart and improving the patient's long-term prognosis [86]. This may include medications to control blood pressure and cholesterol levels, lifestyle changes such as diet and exercise, and regular monitoring and follow-up with healthcare providers. Effective chronic management is essential for preventing complications and reducing the risk of future cardiovascular events. Healthcare providers must work closely with patients to develop personalized management plans that address their unique needs and risk factors [87].
Medical therapy for heart failure: Medical therapy is an essential component of the chronic management of heart failure. The goals of medical therapy for heart failure are to improve symptoms, prevent disease progression, and reduce the risk of hospitalization and mortality. The main classes of medications used in the medical therapy of heart failure include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid receptor antagonists (MRAs). These medications work by reducing the workload on the heart, improving cardiac function, and reducing fluid accumulation [88]. In addition to these medications, other classes of medications may also be used in the management of heart failure, such as diuretics, which reduce fluid overload, and digoxin, which improves cardiac output. The choice of medications for the management of heart failure depends on the underlying cause of the heart failure, the severity of the heart failure, and the patient's comorbidities and medication tolerability. Healthcare providers should work with patients to develop a personalized treatment plan that optimizes medical therapy and minimizes the risk of adverse effects. Lifestyle modifications, such as salt restriction, weight management, and physical activity, are also important components of the chronic management of heart failure. These lifestyle modifications can improve symptoms, reduce hospitalization, and improve quality of life in patients with heart failure [89].
Arrhythmia management: Arrhythmia management is an important component of the chronic management of cardiovascular diseases. Arrhythmias are abnormal heart rhythms that can lead to symptoms such as palpitations, dizziness, and syncope, and can increase the risk of stroke and sudden cardiac death. The management of arrhythmias depends on the type of arrhythmia, the severity of symptoms, and the underlying cause of the arrhythmia. Treatment options for arrhythmias include medications, electrical cardioversion, catheter ablation, and implantable devices such as pacemakers and defibrillators. Antiarrhythmic medications, such as beta-blockers, calcium channel blockers, and sodium channel blockers, are commonly used to manage arrhythmias by slowing down the heart rate or restoring normal sinus rhythm. Electrical cardioversion, which involves the delivery of a synchronized electrical shock to the heart, can be used to convert certain types of arrhythmias to normal sinus rhythm [90]. Catheter ablation is a procedure that involves the insertion of a catheter through a blood vessel to the heart to destroy the areas of the heart that are responsible for the arrhythmia. This procedure is commonly used to treat atrial fibrillation and other types of supraventricular tachycardia. Implantable devices, such as pacemakers and defibrillators, are used to manage arrhythmias by regulating the heart rate or delivering electrical shocks to the heart to restore normal rhythm and prevent sudden cardiac death [91].
Device therapy for heart failure: Device therapy is an important component of the chronic management of heart failure. Device therapy includes implantable devices, such as cardiac resynchronization therapy (CRT) devices, implantable cardioverter-defibrillators (ICDs), and ventricular assist devices (VADs), which can improve cardiac function and reduce the risk of hospitalization and mortality in patients with heart failure [92]. CRT devices are used to treat heart failure with reduced ejection fraction (HFrEF) by improving the coordination of the heart's contractions and improving cardiac function. CRT devices consist of a pacemaker-like device that is implanted under the skin and leads that are placed in the heart to stimulate the heart's chambers to contract in a synchronized manner [93]. ICDs are used to prevent sudden cardiac death in patients with heart failure by delivering electrical shocks to the heart to restore normal rhythm. ICDs can also be combined with CRT devices in patients with HFrEF and conduction abnormalities [94]. VADs are used to support the failing heart in patients with end-stage heart failure who are not candidates for heart transplantation. VADs are implanted in the heart and can either assist the heart's function or replace the function of the heart entirely. The choice of device therapy depends on the patient's clinical characteristics, underlying comorbidities, and the severity of heart failure. Device therapy is typically reserved for patients with severe heart failure who have failed to respond to medical therapy alone [95].
Innovative approaches of management
Innovative approaches to the management of cardiovascular diseases involve the use of new technologies, therapies, and treatment strategies that go beyond traditional approaches. These may include novel drug therapies, minimally invasive surgical procedures, and advanced imaging and diagnostic techniques. Additionally, emerging fields such as precision medicine and digital health offer new opportunities for personalized and data-driven approaches to cardiovascular care [96].
Precision medicine and personalized treatment: Precision medicine and personalized treatment are innovative approaches that have the potential to revolutionize the management of cardiovascular diseases. Precision medicine involves the use of genetic, environmental, and lifestyle information to develop personalized treatment plans that are tailored to an individual's unique characteristics and needs. The development of new technologies, such as genomic sequencing and advanced imaging, has enabled healthcare providers to better understand the underlying mechanisms of cardiovascular diseases and develop personalized treatment plans that target specific molecular pathways [97]. For example, genetic testing can identify individuals who are at increased risk of developing cardiovascular diseases due to inherited genetic mutations. This information can be used to develop personalized treatment plans that target these specific genetic mutations, such as the use of PCSK9 inhibitors in individuals with familial hypercholesterolemia. Advanced imaging technologies, such as MRI and CT angiography, can provide detailed information about the structure and function of the heart and blood vessels, enabling healthcare providers to develop personalized treatment plans that target specific areas of the heart or blood vessels [98].
Limitations and challenges of precision medicine and personalized treatment
One of the main limitations of precision medicine and personalized treatment is the availability and quality of data [99]. To develop personalized treatment plans, healthcare providers need access to high-quality data on an individual's genetic, environmental, and lifestyle factors. However, this data is not always available or reliable, and there may be gaps in our understanding of the complex interactions between these factors [100]. Another challenge is the cost and complexity of implementing precision medicine and personalized treatment. Developing personalized treatment plans requires specialized expertise and resources, such as genetic testing and advanced imaging technologies. These resources may not be available in all healthcare settings, and the cost of these technologies may limit access to personalized treatment for some patients. There are also concerns about the ethical and legal implications of precision medicine and personalized treatment. For example, there may be concerns about the privacy and security of patient data, as well as concerns about the potential for these technologies to exacerbate existing disparities in healthcare access and outcomes [101]. Finally, while precision medicine and personalized treatment offer many benefits, it is important to recognize that these approaches are not a replacement for standard medical care. Healthcare providers should continue to use evidence-based guidelines and treatments as the foundation of their clinical practice, while incorporating personalized treatment plans where appropriate [102].
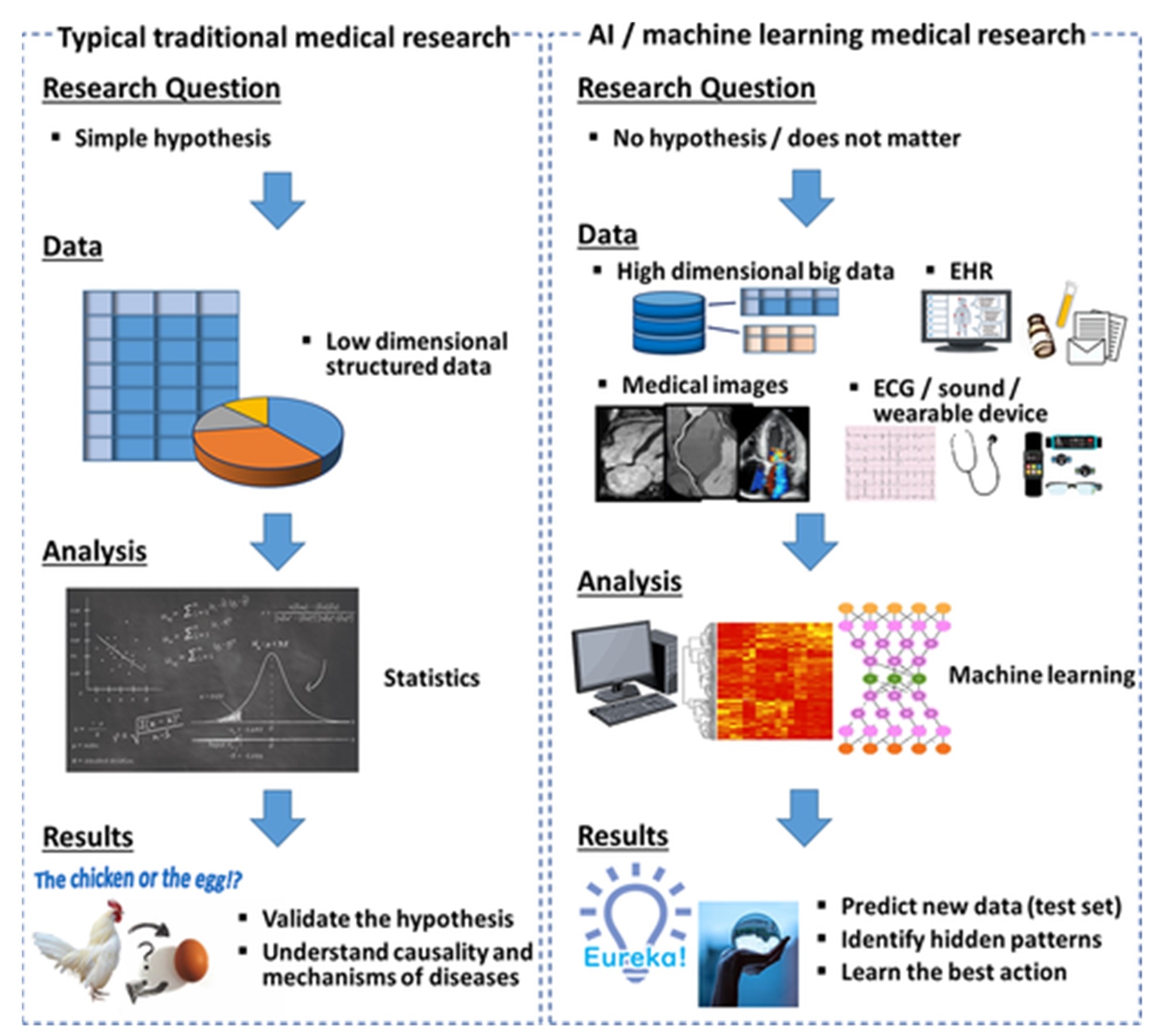
Use of artificial intelligence in diagnosis and treatment: The use of artificial intelligence (AI) in the diagnosis and treatment of cardiovascular diseases is an emerging field with tremendous potential. AI involves the use of computer algorithms and machine learning to analyze large amounts of data and identify patterns and relationships that may not be apparent to human observers. AI has the potential to improve the accuracy and speed of diagnosis, enabling healthcare providers to identify cardiovascular diseases earlier and develop personalized treatment plans that are tailored to an individual's unique characteristics [103]. For example, AI can be used to analyze imaging data, such as echocardiograms or angiograms, to identify subtle changes in heart structure and function that may be indicative of a cardiovascular disease. AI can also be used to analyze large amounts of patient data, such as electronic health records or genetic data, to identify risk factors and develop personalized treatment plans [104]. As depicted in Figure 1, conventional hypothesis-driven medical studies first formulate a hypothesis and subsequently test it using statistical analysis. In contrast, medical investigations leveraging AI techniques can be hypothesis-free and primarily data-driven in their approach. Compared to traditional statistical approaches limited to handling structured datasets, AI methods exhibit proficiency in managing diverse data types, including unstructured inputs like images, waveforms, and electronic health records (EHRs). While validation of hypotheses and elucidating causality and underlying mechanisms are the main objectives in conventional medical research, studies employing AI aim to accurately predict new data observations and uncover hidden patterns within the information. In summary, Figure 1 provides a useful comparison between the workflows, data types, goals, and analytic methods used in traditional statistics-based medical studies versus those utilizing modern AI tools, highlighting their complementary roles in advancing biomedicine [105].
Figure 1. Comparison of Traditional Statistics and AI in Medical Research [105].
Limitations and challenges of AI
One of the main limitations of AI is the quality and availability of data. AI algorithms require large amounts of high-quality data to be trained and validated, and the quality and consistency of this data can impact the accuracy and reliability of AI predictions. In addition, there may be biases in the data, such as underrepresentation of certain populations, which can limit the generalizability of AI predictions. Another limitation of AI is the interpretability of the results. AI algorithms often make predictions based on complex mathematical models that may be difficult to interpret or explain. This can make it challenging for healthcare providers to understand the underlying mechanisms of the AI predictions and may limit their ability to incorporate these predictions into clinical decision-making. There are also concerns about the ethical and legal implications of AI in healthcare. For example, there may be concerns about the privacy and security of patient data, as well as concerns about the potential for AI to exacerbate existing disparities in healthcare access and outcomes [106].
Advances in regenerative medicine: Regenerative medicine is an innovative approach to the treatment of cardiovascular diseases that has the potential to revolutionize the field of cardiology. Regenerative medicine involves the use of stem cells, gene therapy, tissue engineering, and other novel techniques to repair or regenerate damaged or diseased tissues and organs [107]. Recent advances in regenerative medicine have shown promise in the treatment of cardiovascular diseases. For example, stem cell therapies have been shown to improve heart function and reduce the risk of adverse events in patients with heart failure. Gene therapy has also shown promise in the treatment of inherited cardiovascular diseases, such as familial hypercholesterolemia [108]. Tissue engineering approaches, such as the development of bioengineered blood vessels and heart tissues, offer the potential for personalized, regenerative therapies that can replace damaged or diseased tissues with functional, healthy tissues [109]. Table 2 outlines the unique characteristics of the major stem cell types being investigated for cardiac repair and regeneration along with their potential therapeutic applications. Differences in origin, potency, and functional properties lead to varied approaches for treating cardiovascular diseases.
|
Stem Cell Type |
Characteristics |
Potential Applications |
|
Embryonic stem cells |
Pluripotent; can differentiate into any cell type |
Generation of cardiomyocytes for transplantation |
|
Induced pluripotent stem cells |
Derived from adult cells genetically reprogrammed into a pluripotent state |
Patient-specific cardiomyocytes for transplantation; disease modeling |
|
Bone marrow mononuclear cells |
Mixed population including hematopoietic and mesenchymal stem cells |
Intracoronary infusion after MI to reduce remodeling |
|
Mesenchymal stem cells |
Multipotent; immunomodulatory properties |
Allogeneic transplantation; reduction of inflammation |
|
Cardiac progenitor cells |
Reside in heart niches; multipotent |
Activation of endogenous regeneration; transplantation |
|
Cardiosphere-derived cells |
Derived from cardiac biopsies; clonogenic |
Transplantation to improve cardiac function after MI |
Limitations and challenges of regenerative medicine
One of the main challenges of regenerative medicine is the complexity of the cardiovascular system. The heart and blood vessels are complex organs with multiple cell types and structures, and repairing or regenerating these tissues requires a deep understanding of their biology and function. Another challenge is the safety and efficacy of regenerative medicine techniques. While there have been promising preclinical and early clinical studies, more research is needed to fully understand the safety and efficacy of these approaches, particularly in larger patient populations and over longer periods of time [110]. There are also challenges related to the cost and scalability of regenerative medicine techniques. Developing and scaling up these technologies can be expensive and time-consuming, and the cost of these therapies may limit access for some patients [111]. Finally, there are regulatory and ethical challenges associated with the use of regenerative medicine techniques. Ensuring the safety and efficacy of these approaches requires rigorous regulatory oversight and adherence to ethical standards, such as informed consent and protection of patient privacy.
Advances in Traditional Chinese Medicine: Traditional Chinese Medicine (TCM) has been used for centuries to treat various health conditions, including cardiovascular diseases (CVDs) [112]. While the effectiveness of TCM for treating CVDs has not been extensively studied, there is some evidence to suggest that certain TCM treatments may be beneficial [113].
Acupuncture is a form of Traditional Chinese Medicine (TCM) that has been used for centuries to treat various health conditions, including cardiovascular diseases (CVDs). Acupuncture involves the insertion of fine needles into specific points on the body to stimulate energy flow and promote healing. In recent years, there has been growing interest in the use of acupuncture as a complementary therapy for CVDs. Some evidence suggests that acupuncture can help reduce blood pressure, improve heart function, and decrease the risk of cardiovascular events in patients with hypertension. Additionally, acupuncture has been found to improve exercise tolerance and quality of life in patients with heart failure [114].
Herbal medicine Herbal medicine has been used for centuries in Traditional Chinese Medicine (TCM) to treat various health conditions, including cardiovascular diseases (CVDs). Several herbs, including ginseng, hawthorn, and danshen, have been found to have cardioprotective effects [115]. For example, danshen has been shown to help improve blood flow and reduce the risk of recurrent myocardial infarction. Hawthorn extract has been found to help improve blood lipid levels and reduce the risk of atherosclerosis [116].
TCM practices such as Tai Chi and Qigong (TCQ) are popular mind-body interventions that combine martial arts and meditative movements. TCQ emphasizes posture, movement, breath, and mindfulness and is a gentle exercise that can play a critical role in preserving and promoting healthy cognitive aging [117].
The potential role of brain-derived neurotrophic factors (BDNFs) in promoting cognitive function by facilitating neurogenesis and synaptic plasticity in the cerebral cortex and hippocampus is highlighted in the article [118]. Exercise, such as TCQ, has the potential to affect the synthesis and release of hippocampal BDNFs, which can delay cognitive decline in older adults. A randomized controlled trial found that TCQ may improve cognitive function and mental well-being in people with mild dementia, and a meta-analysis suggested TCQ's potential to improve cognitive function in middle-aged and older adults with mild cognitive impairment [119]. It is important to note that while TCM may be used as a complementary therapy for CVDs, it should not be used as a substitute for standard medical treatment. If you have CVDs, it is important to work with a healthcare provider to determine the best course of treatment for your individual needs. Additionally, it is important to seek out a qualified and experienced TCM practitioner if you are interested in trying TCM for your cardiovascular health.
Future Directions and Challenges
The future directions for cardiovascular diseases (CVDs) are promising, with new developments in precision medicine, regenerative therapies, and digital health technologies. However, there are still challenges that need to be addressed, such as disparities in access to care, rising healthcare costs, and the need for more effective prevention strategies. In addition, there is a need for more research to better understand the underlying mechanisms of CVDs and to develop more targeted and personalized treatments. In order to address these challenges and take advantage of the opportunities presented by emerging technologies, there is a need for collaboration among healthcare professionals, researchers, policymakers, and patients. By working together, we can improve the prevention, diagnosis, and treatment of CVDs and reduce the global burden of these diseases [120].
Emerging therapies and technologies
Emerging therapies and technologies for cardiovascular diseases (CVDs) are rapidly transforming the landscape of CVD treatment and management [121]. From gene editing to tissue engineering, and from new drug therapies to advanced medical devices, there are a variety of promising approaches that are being developed to improve patient outcomes and reduce the burden of CVDs [122]. These emerging therapies and technologies have the potential to revolutionize the way we diagnose, treat, and prevent CVDs. In this context, it is important to keep up-to-date with the latest developments in this field in order to improve patient outcomes and reduce the global burden of CVDs [123].
Gene therapy: Gene therapy is an emerging therapy that has the potential to revolutionize the treatment of cardiovascular diseases. Gene therapy involves the delivery of therapeutic genes to replace or modify genes that are defective or malfunctioning, with the goal of improving cardiovascular function and reducing the risk of adverse events [124]. There have been promising preclinical and early clinical studies of gene therapy for the treatment of cardiovascular diseases, particularly for inherited cardiovascular diseases such as familial hypercholesterolemia and hypertrophic cardiomyopathy. Gene therapy has also shown promise in the treatment of heart failure, with studies demonstrating improvements in heart function and exercise capacity. One of the advantages of gene therapy is its potential for personalized, targeted therapies that can address the underlying causes of cardiovascular diseases. Gene therapy can be tailored to an individual's unique genetic profile, offering the potential for more effective and precise treatments [125]. However, there are also challenges and limitations associated with gene therapy. These include issues related to safety, efficacy, and delivery of the therapeutic genes to the target tissues. Gene therapy also requires specialized expertise and resources, such as viral vectors for gene delivery, which may limit access to these therapies for some patients.
Gene editing: Gene editing is a promising emerging technology that has the potential to transform the treatment of cardiovascular diseases. Gene editing involves the precise modification of an individual's DNA to correct genetic mutations that cause cardiovascular diseases [126].
One of the advantages of gene editing is its potential for personalized, targeted therapies that can address the underlying causes of cardiovascular diseases. Gene editing can be tailored to an individual's unique genetic profile, offering the potential for more effective and precise treatments [127].
There have been promising preclinical and early clinical studies of gene editing for the treatment of cardiovascular diseases, particularly for inherited cardiovascular diseases such as Hypertrophic cardiomyopathy (HCM): HCM is a genetic condition that results in the thickening of the heart muscle, leading to heart failure and sudden cardiac death. Gene editing approaches, such as CRISPR-Cas9, are being investigated to correct the genetic mutations that cause HCM. Familial hypercholesterolemia (FH) is a genetic condition that results in high levels of low-density lipoprotein (LDL) cholesterol, which can lead to atherosclerosis and heart disease. Gene editing approaches are being investigated to correct the genetic mutations that cause FH and to reduce LDL cholesterol levels. Gene editing approaches are being investigated to improve heart function in patients with heart failure. For example, gene editing approaches can be used to modify the genes that regulate heart muscle contraction, leading to improved heart function. Gene editing approaches are being investigated to reduce inflammation in the walls of arteries and prevent the formation of atherosclerotic plaques. Gene editing approaches can be used to modify the genes that regulate the immune response and inflammation in the body. However, there are also challenges and limitations associated with gene editing. These include issues related to safety, efficacy, and delivery of the gene editing tools to the target tissues. Gene editing also requires specialized expertise and resources, such as CRISPR-Cas9 gene editing tools, which may limit access to these therapies for some patients. In addition, there are also ethical and regulatory challenges associated with gene editing, particularly around the use of germline gene editing, which involves modifying the DNA of eggs, sperm, or embryos. The use of germline gene editing is currently highly regulated and controversial, with many ethical and social implications that need to be carefully considered. Moreover, one of the challenges of gene editing is the potential for off-target effects. The gene editing tools, such as CRISPR-Cas9, can sometimes introduce unintended changes to the genome, which can have unforeseen consequences. Therefore, it is essential to develop more precise and efficient gene editing tools to minimize the risk of off-target effects [128]. Another challenge is the delivery of the gene editing tools to the target tissues. Effective delivery of the gene editing tools to the target tissues is critical for the success of gene editing therapies. The delivery of these tools can be challenging, particularly for tissues that are difficult to access, such as the heart [129].
Immunotherapy: Immunotherapy is an emerging therapy that has shown promise in the treatment of cardiovascular diseases. Immunotherapy involves the use of the body's immune system to target and destroy cancer cells, infectious agents, or other disease-causing agents. In the context of cardiovascular diseases, immunotherapy is being investigated as a potential therapy for atherosclerosis, which is a major contributor to heart disease. Atherosclerosis is characterized by the buildup of fatty plaques in the walls of arteries, which can lead to heart attacks and strokes. Immunotherapy is being investigated as a potential way to reduce inflammation in the walls of arteries and prevent the formation of these plaques [130].
Some examples of immunotherapy approaches for cardiovascular diseases
Monoclonal antibodies can be designed to target specific inflammatory molecules, such as interleukin-1 beta (IL-1β) or interleukin-6 (IL-6), which play a role in the development of atherosclerosis. Clinical trials have shown that monoclonal antibodies targeting these molecules can improve outcomes for patients with cardiovascular diseases. Adoptive cell transfer involves the transfer of immune cells that have been genetically engineered to target specific disease-causing agents [131]. For example, T cells can be engineered to target and destroy cells that contribute to atherosclerosis. Clinical trials of adoptive cell transfer for cardiovascular diseases are ongoing. Vaccines can be used to induce an immune response against disease-causing agents in cardiovascular diseases. For example, a vaccine targeting a protein called PCSK9, which plays a role in the development of atherosclerosis, has shown promising results in preclinical studies [132]. Immune checkpoint inhibitors are drugs that block inhibitory signals in the immune system, allowing the immune system to better target cancer cells or cells infected with viruses that contribute to cardiovascular diseases. Clinical trials of immune checkpoint inhibitors for cardiovascular diseases are ongoing [133]. While immunotherapy offers many potential benefits for the treatment of cardiovascular diseases, there are also challenges and limitations associated with these approaches. These include issues related to safety, efficacy, and cost.
A comprehensive understanding of cardiovascular pathology must take into account the multifaceted contributions of the immune system, including the roles of both innate and adaptive immune cells. Recent work has highlighted the heterogeneity and plasticity of monocytes, macrophages, dendritic cells, and T/B lymphocytes involved in atherosclerosis progression and complication [134]. CD4+ T cell interactions with monocytes/macrophages can exacerbate post-infarction cardiac inflammation and adverse remodeling [135]. High-risk immune phenotypes characterized by features like low natural killer cells, elevated pro-inflammatory monocytes, and reduced M2 macrophages correlate with poorer cardiac outcomes [136]. Furthermore, the concepts of trained immunity and innate immune memory help explain epigenetic reprogramming in vessel wall monocytes/macrophages that sustains long term pro-atherogenic inflammation after certain exposures [137]. Continued investigation of the metabolic regulation, phenotypic heterogeneity, genetic modulation, and intercellular crosstalk of immune cells in the cardiovascular milieu will shed light on promising directions for therapeutically targeting immune pathways. Integrating insights from both basic and translational immunology studies in CVD models is imperative for a complete picture of disease mechanisms.
Nanotechnology: Nanotechnology is an emerging field that has shown promise in the treatment of cardiovascular diseases. Nanotechnology involves the design and manipulation of materials at the nanoscale, typically between 1 and 100 nanometers in size [138]. In the context of cardiovascular diseases, nanotechnology is being investigated as a potential way to deliver drugs or other therapeutic agents to the site of disease, such as atherosclerotic plaques or damaged heart tissue. Nanoparticles can be designed to target specific cells or tissues in the body, allowing for more precise delivery of therapeutic agents and reducing the risk of side effects [139]. One approach to nanotechnology for cardiovascular diseases involves the use of nanoparticles for drug delivery. Nanoparticles can be loaded with drugs or other therapeutic agents and designed to release these agents at the site of disease, such as atherosclerotic plaques or damaged heart tissue. This can improve the efficacy of the therapy while reducing the risk of side effects [140]. Nanotechnology is also being investigated as a potential way to non-invasively diagnose and monitor cardiovascular diseases. Nanoparticles can be designed to bind to specific molecules or cells associated with cardiovascular diseases, allowing for the detection of these diseases with greater sensitivity and specificity than current diagnostic approaches [141].
Examples of nanoparticles for the treatment and diagnosis of cardiovascular diseases
1. Liposomes: Liposomes are spherical nanoparticles composed of a lipid bilayer that can encapsulate drugs or other therapeutic agents [142]. Liposomes have been used to deliver drugs to atherosclerotic plaques and to improve the efficacy of heart failure therapies [143].
2. Gold nanoparticles: Gold nanoparticles have unique optical and electronic properties that make them useful for both imaging and therapy. For example, gold nanoparticles have been used for non-invasive imaging of atherosclerotic plaques and for targeted drug delivery [144].
3. Iron oxide nanoparticles: Iron oxide nanoparticles have magnetic properties that make them useful for MRI imaging. Iron oxide nanoparticles have been used for non-invasive imaging of atherosclerotic plaques and for targeted drug delivery [145].
4. Nanoparticle-based vaccines: Nanoparticles can be used as carriers for vaccines targeting cardiovascular diseases, such as atherosclerosis. Nanoparticle-based vaccines have shown promising results in preclinical studies for inducing immune responses against atherosclerosis [146].
5. Carbon nanotubes: Carbon nanotubes have unique mechanical and electrical properties that make them useful for both imaging and therapy [147]. Carbon nanotubes have been used for non-invasive imaging of atherosclerotic plaques and for targeted drug delivery [148].
While nanotechnology offers many potential benefits for the treatment and diagnosis of cardiovascular diseases, there are also challenges and limitations associated with these approaches. These include issues related to safety, efficacy, and regulatory approval. Ongoing research and development are needed to fully understand the safety and efficacy of these approaches and to address the challenges and limitations associated with nanotechnology for cardiovascular diseases.
Wearable technology: Wearable technology refers to electronic devices that can be worn on the body, either as accessories or as clothing, and that are designed to collect and transmit data about the user's health and physical activity. Wearable technology has shown promise in the management of cardiovascular diseases by providing real-time data about the user's health and physical activity levels [149]. Wearable technology can provide continuous monitoring of the user's heart rate, blood pressure, and physical activity levels. This can lead to earlier detection of cardiovascular diseases and better management of existing conditions. Wearable technology can provide feedback and motivation to the user, encouraging them to engage in healthy behaviors, such as regular exercise and healthy eating [150]. This can lead to improved cardiovascular health outcomes. Moreover, wearable technology can facilitate communication between the user and their healthcare provider, allowing for more frequent and timely updates on the user's health status. This can lead to more personalized and effective treatment plans [151]. Additionally, several studies have shown that the use of wearable technology can lead to improved outcomes for patients with cardiovascular diseases. For example, a study of patients with heart failure found that the use of a wearable device led to a significant reduction in hospital readmissions. However, there are limitations associated with the use of wearable technology for cardiovascular diseases. These include issues related to accuracy and reliability of the data collected by the devices, as well as concerns about privacy and security of the data [152]. Table 3 outlines some of the most common types of wearable technology used for CVD management along with their capabilities and limitations.
|
Device |
Measures |
Capabilities |
Limitations |
|
Smartwatch |
Heart rate, heart rhythm, activity, sleep |
Real-time ECG monitoring; irregular rhythm detection; fitness tracking |
Battery life; skin irritation |
|
Fitness tracker |
Steps, activity, sleep |
Activity and sleep tracking; goal setting |
Limited accuracy; no vital sign monitoring |
|
Chest strap monitor |
Continuous ECG, heart rate |
Detailed heart rhythm assessment; remote monitoring |
Can be uncomfortable with long-term wear |
|
Smart clothing |
Vital signs, posture |
Integrated sensors provide continuous monitoring without wearable |
Still in early development; cost |
|
Blood pressure monitor |
Blood pressure |
Frequent home blood pressure monitoring |
Cuff can be cumbersome |
|
Implantable loop recorder |
Continuous ECG |
Precise arrhythmia diagnosis; long-term monitoring |
Requires surgical implantation |
Examples of wearable technology for cardiovascular diseases:
1. Smartwatches: Smartwatches can track the user's heart rate, blood pressure, and physical activity levels. Some smartwatches can also perform electrocardiogram (ECG) tests to detect irregular heart rhythms, such as atrial fibrillation [153].
2. Fitness trackers: Fitness trackers are wearable devices that track the user's physical activity levels, such as steps taken and calories burned. These devices can also track heart rate and sleep quality [154].
3. Blood pressure monitors: Wearable blood pressure monitors can track the user's blood pressure throughout the day, providing a more comprehensive picture of the user's cardiovascular health [155].
4. Smart clothing: Smart clothing can incorporate sensors that track the user's heart rate, breathing rate, and physical activity levels. Smart clothing can also monitor the user's posture and provide alerts if the user is sitting or standing in a way that could be harmful to their cardiovascular health [156].
5. Implantable devices: Implantable devices, such as pacemakers and defibrillators, are wearable devices that can monitor and regulate the user's heart rate and rhythm. These devices can also provide alerts if the user experiences a cardiac event [157].
Telemedicine: Telemedicine refers to the use of technology, such as video conferencing and remote monitoring, to provide healthcare services remotely. Telemedicine has the potential to revolutionize the way we manage cardiovascular diseases by improving access to care, reducing healthcare costs, and increasing patient engagement [158]. Table 4 provides a high-level summary of some of the key evidence supporting the use of telemedicine interventions through remote monitoring, virtual care, and teleconsultations to improve outcomes in a range of cardiovascular diseases.
|
Intervention |
Key Findings |
|
Remote patient monitoring |
Reduced hospital readmissions, improved medication adherence in heart failure patients |
|
Virtual cardiac rehabilitation |
Improved fitness, reduced cardiovascular risk factors compared to standard care |
|
Telephonic coaching |
Improved diet, physical activity, and medication adherence in high-risk patients |
|
Remote hypertension management |
Equivalent lowering of BP compared to in-person care |
|
Telestroke evaluation |
Similar neurological outcomes vs in-person evaluation; faster time to treatment |
|
Teleconsultation for MI |
Reduced time to PCI; lower 1-year mortality compared to standard transfer |
Examples of telemedicine in the management of cardiovascular diseases
Remote monitoring: Telemedicine can be used to remotely monitor patients with cardiovascular diseases, such as heart failure or hypertension. Patients can use wearable devices to track their vital signs, such as blood pressure and heart rate, and transmit the data to their healthcare provider for review. This allows healthcare providers to monitor patients' health status and make timely adjustments to their treatment plans [158].
Virtual consultations: Telemedicine can be used to provide virtual consultations between patients and healthcare providers. Patients can use video conferencing technology to meet with their healthcare provider from the comfort of their own home, reducing the need for in-person visits. This can improve access to care for patients who live in remote areas or who have mobility issues [159].
Lifestyle coaching: Telemedicine can be used to provide lifestyle coaching to patients with cardiovascular diseases, such as advice on healthy eating and exercise. Patients can use video conferencing technology to meet with a lifestyle coach or dietician, who can provide personalized advice and support.
Education and support: Telemedicine can be used to provide education and support to patients with cardiovascular diseases, such as information about their condition and tips for managing their symptoms. Patients can access educational materials and support groups through online platforms [160].
Remote cardiac rehabilitation: Telemedicine can be used to provide remote cardiac rehabilitation to patients with cardiovascular diseases, such as after a heart attack or heart surgery. Patients can use video conferencing technology to meet with a cardiac rehabilitation specialist who can guide them through exercises and provide education and support. This can improve outcomes for patients by promoting recovery and reducing the risk of complications.
Telecardiology: Telemedicine can be used to provide remote cardiology consultations, such as for the interpretation of electrocardiograms (ECGs). Patients can send their ECGs to a cardiologist for review, who can provide a diagnosis and treatment plan remotely. This can improve access to care for patients who live in remote areas or who have difficulty accessing cardiology services [161].
Medication management: Telemedicine can be used to provide medication management services to patients with cardiovascular diseases, such as reviewing medication regimens and adjusting dosages. Patients can meet with a pharmacist or healthcare provider through video conferencing technology to discuss their medications and receive advice on how to manage their symptoms. This can improve medication adherence and reduce the risk of adverse drug events [162].
Data analytics and population health management: Telemedicine can be used to collect and analyze large amounts of health data from patients with cardiovascular diseases, which can be used to identify trends and patterns in disease management. This can inform population health management strategies and improve outcomes for patients with cardiovascular diseases at a population level [163].
limitations and challenges of telemedicine for cardiovascular diseases
While telemedicine has the potential to transform the way we manage cardiovascular diseases, there are also limitations and challenges that need to be addressed.
Limited physical examination: One of the primary limitations of telemedicine is the limited ability to perform a physical examination. This can be a challenge for cardiovascular diseases, which often require a physical examination, such as listening to the heart and lungs or palpating the peripheral pulses. While wearable technology can provide some information on vital signs and physical activity, it cannot replace a comprehensive physical examination [164].
Technical challenges: Telemedicine relies on technology, such as video conferencing and remote monitoring devices, which can be subject to technical challenges, such as poor internet connectivity or device malfunctions. Technical challenges can lead to delays in care, miscommunication, or inaccurate data, which can negatively affect patient outcomes [165].
Privacy and security concerns: Telemedicine involves the transmission of sensitive health information over the internet, which can raise privacy and security concerns. Ensuring that patient data is protected and secure is essential to maintaining patient trust and promoting the adoption of telemedicine.
Reimbursement and licensure: Telemedicine reimbursement and licensure policies vary by state and payer, which can create confusion and barriers to adoption. Healthcare providers may be hesitant to provide telemedicine services if they are not reimbursed or licensed to do so.
Limited reimbursement for remote monitoring: While remote monitoring has the potential to improve outcomes and reduce healthcare costs, reimbursement for remote monitoring is limited and varies by payer. This can create financial challenges for healthcare providers and limit the widespread adoption of remote monitoring.
Inability to perform certain procedures: Telemedicine is not suitable for all procedures, such as invasive procedures or surgeries. Patients may still need to visit a healthcare facility for certain procedures [166].
Limited access to technology: Not all patients have access to the technology required for telemedicine, such as a smartphone, computer, or internet connection. This can create disparities in access to care and limit the potential benefits of telemedicine.
Difficulty building rapport: Telemedicine can make it more difficult to build rapport between patients and healthcare providers, which can negatively affect the patient-provider relationship and patient outcomes [167].
Legal and regulatory issues: Telemedicine is subject to legal and regulatory issues, such as licensure, liability, and malpractice. Healthcare providers need to be aware of the legal and regulatory requirements for telemedicine in their state and ensure that they comply with these requirements.
Patient acceptance: Some patients may be hesitant to use telemedicine due to a lack of familiarity or concerns about the quality of care. Ensuring that patients are comfortable with telemedicine and providing education and support can help to promote patient acceptance and engagement [168].
General challenges in implementing new advances
Cost-effectiveness and access to care: One of the biggest challenges in implementing new advances in the management of cardiovascular diseases is ensuring that they are cost-effective and accessible to all patients. While some new advances, such as wearable technology and telemedicine, have the potential to reduce healthcare costs and increase access to care, other advances, such as gene editing and immunotherapy, may be expensive and may not be accessible to all patients. Ensuring that new advances are cost-effective and accessible to all patients is essential to improving outcomes for patients with cardiovascular diseases [169].
Resistance to change in clinical practice: Another challenge in implementing new advances is resistance to change in clinical practice. Healthcare providers may be hesitant to adopt new approaches or technologies if they are not familiar with them or if they are concerned about the potential risks and benefits. Addressing concerns and providing education and training to healthcare providers can help to overcome resistance to change and promote the adoption of new advances in clinical practice [170].
Ethical considerations in personalized medicine: Personalized medicine, which involves tailoring treatments to an individual's unique genetic or physiological profile, raises ethical considerations related to privacy, informed consent, and access to care. For example, some patients may not be able to afford personalized treatments or may not have access to genetic testing. Ensuring that personalized medicine is implemented in an ethical and equitable manner is essential to improving outcomes for patients with cardiovascular diseases [171].
Conclusion
In conclusion, the management and treatment of cardiovascular diseases require a multidisciplinary approach, incorporating conventional treatments with emerging therapies and technologies. The use of AI, biomarkers, cardiovascular imaging, wearable technology, and point-of-care testing can improve the accuracy and speed of diagnosis, and ultimately lead to better patient outcomes. Additionally, precision medicine, gene therapy, regenerative medicine, traditional Chinese medicine and nanotechnology offer promising approaches to the treatment of cardiovascular diseases. However, addressing challenges related to safety, efficacy, and cost-effectiveness will be essential to ensure that these innovative approaches are used effectively and safely in clinical practice.
Furthermore, promoting healthy lifestyles, including a healthy diet, regular exercise, and smoking cessation, remains a critical component of preventing cardiovascular diseases. Targeted prevention strategies, genetic testing, and risk stratification can also improve the accuracy of risk assessment and guide individualized prevention strategies. As the field continues to evolve, collaboration between researchers, clinicians, industry partners, and policymakers will be essential to address challenges and limitations and ensure that new advances are implemented in an equitable and effective manner. By investing in research and innovation and promoting healthy lifestyles, we can reduce the global burden of cardiovascular diseases and improve outcomes for patients.
Abbreviations List
CVDs: Cardiovascular Diseases; AI: Artificial Intelligence; MI: Myocardial Infarction; AHF: Acute Heart Failure; mHealth: Mobile Health; ECG: Electrocardiogram; FDA: Food and Drug Administration; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; ACE: Angiotensin-Converting Enzyme; ARB: Angiotensin Receptor Blocker; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Grafting; HF: Heart Failure; ECMO: Extracorporeal Membrane Oxygenation; CRT: Cardiac Resynchronization Therapy; ICD: Implantable Cardioverter-Defibrillator; AF: Atrial Fibrillation; TAVR: Transcatheter Aortic Valve Replacement; SCD: Sudden Cardiac Death; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; SPECT: Single-Photon Emission Computed Tomography; ACS: Acute Coronary Syndrome; NSTEMI: Non-ST Segment Elevation Myocardial Infarction; STEMI: ST Segment Elevation Myocardial Infarction; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; VLDL: Very Low-Density Lipoprotein; Lp(a): Lipoprotein(a); Lp-PLA2: Lipoprotein-Associated Phospholipase A2; TG – Triglycerides; CKD: Chronic Kidney Disease; HFrEF: Heart Failure with Reduced Ejection Fraction; HFpEF: Heart Failure with Preserved Ejection Fraction; NOAC: Non-Vitamin K Oral Anticoagulant; TIA: Transient Ischemic Attack; NIHSS: National Institutes of Health Stroke Scale, AS: Aortic Stenosis, DAPT: Dual Antiplatelet Therapy, OAC: Oral Anticoagulation, P2Y12: Platelet ADP Receptor, LAA: Left Atrial Appendage, RVOT: Right Ventricular Outflow Tract, LV: Left Ventricular, RV: Right Ventricular, LVAD: Left Ventricular Assist Device, RVAD: Right Ventricular Assist Device, ECM: Extracellular Matrix
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Availability of data and materials
All data are available, and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors reviewed and confirmed the final version of the manuscript.
Acknowledgements
Authors thank all the researchers who have made great efforts in their studies. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Getahun GK, Goshu BY, Goshu DY, Mekuria ZN. Cardiovascular disease risk among hypertensive patients and associated determinants in Addis Ababa, Ethiopia: an institutional-based cross-sectional study. BMJ open. 2023;13(6).
3. Al-Ghamdi S, Aldosari KH, Hussain AB. Predictors of Cardiovascular Morbidity Among Adult Hypertensive Patients: A Cross-Sectional Study from the Kingdom of Saudi Arabia. Patient preference and adherence. 2023 Dec 31:1361-9.
4. Mir A, Badi Y, Bugazia S, Nourelden AZ, Fathallah AH, Ragab KM, et al. Efficacy and safety of cardioprotective drugs in chemotherapy-induced cardiotoxicity: an updated systematic review & network meta-analysis. Cardio-Oncology. 2023 Dec;9(1):1-34.
5. Hsiao LC, Lin YN, Shyu WC, Ho M, Lu CR, Chang SS, et al. First-in-human pilot trial of combined intracoronary and intravenous mesenchymal stem cell therapy in acute myocardial infarction. Frontiers in Cardiovascular Medicine. 2022 Aug 9;9:961920.
6. Wang Y, Cui H, Li L, Cao Y, Qu H, Ailina H, et al. Digitalization of prevention and treatment and the combination of western and Chinese medicine in management of acute heart failure. Frontiers in Cardiovascular Medicine. 2023 May 25;10:1146941.
7. Öner A, Dittrich H, Arslan F, Hintz S, Ortak J, Brandewiede B, et al. Comparison of telemonitoring combined with intensive patient support with standard care in patients with chronic cardiovascular disease-a randomized clinical trial. European Journal of Medical Research. 2023 Jan 11;28(1):22.
8. Wilson N, Cleghorn C, Nghiem N, Blakely T. Prioritization of intervention domains to prevent cardiovascular disease: a country-level case study using global burden of disease and local data. Population Health Metrics. 2023 Jan 26;21(1):1.
9. Dale CE, Takhar R, Carragher R, Katsoulis M, Torabi F, Duffield S, et al. The impact of the COVID-19 pandemic on cardiovascular disease prevention and management. Nature Medicine. 2023 Jan;29(1):219-25.
10. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. Journal of the American College of Cardiology. 2022 Dec 20;80(25):2361-71.
11. Cofer LB, Barrett TJ, Berger JS. Aspirin for the primary prevention of cardiovascular disease: time for a platelet-guided approach. Arteriosclerosis, Thrombosis, and Vascular Biology. 2022 Oct;42(10):1207-16.
12. Kodeboina M, Piayda K, Jenniskens I, Vyas P, Chen S, Pesigan RJ, et al. Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review. International Journal of Environmental Research and Public Health. 2023 Apr 25;20(9):5633.
13. Hammond MM, Everitt IK, Khan SS. New strategies and therapies for the prevention of heart failure in high-risk patients. Clinical Cardiology. 2022 Jun;45:S13-25.
14. Keshavarz-Motamed Z, Del Alamo JC, Bluestein D, Edelman ER, Wentzel JJ. Novel methods to advance diagnostic and treatment value of medical imaging for cardiovascular disease. Frontiers in Bioengineering and Biotechnology. 2022 Aug 31;10:1501.
15. Subramani S, Varshney N, Anand MV, Soudagar ME, Al-Keridis LA, Upadhyay TK, et al. Cardiovascular diseases prediction by machine learning incorporation with deep learning. Frontiers in Medicine. 2023 Apr 17;10:1150933.
16. Khalil H. Traditional and novel diagnostic biomarkers for acute myocardial infarction. The Egyptian Journal of Internal Medicine. 2022 Dec;34(1):87.
17. Thupakula S, Nimmala SS, Ravula H, Chekuri S, Padiya R. Emerging biomarkers for the detection of cardiovascular diseases. The Egyptian Heart Journal. 2022 Oct 20;74(1):77.
18. de Oliveira Laterza Ribeiro M, Correia VM, Herling de Oliveira LL, Soares PR, Scudeler TL. Evolving Diagnostic and Management Advances in Coronary Heart Disease. Life. 2023 Apr 5;13(4):951.
19. Hasnie A, Clarkson S, Hage FG. A novel cardiovascular risk assessment tool for the prediction of myocardial ischemia on imaging. Journal of Nuclear Cardiology. 2023 Feb;30(1):335-42.
20. Tenorio-Mucha J, Busta-Flores P, Lazo-Porras M, Vetter B, Safary E, Moran AE, et al. Facilitators and barriers of the implementation of point-of-care devices for cardiometabolic diseases: a scoping review. BMC Health Services Research. 2023 Dec;23(1):1-1.
21. Choudhary S, Altintas Z. Development of a point-of-care SPR sensor for the diagnosis of acute myocardial infarction. Biosensors. 2023 Feb 5;13(2):229.
22. Chung CT, Lee S, King E, Liu T, Armoundas AA, Bazoukis G, et al. Clinical significance, challenges and limitations in using artificial intelligence for electrocardiography-based diagnosis. International Journal of Arrhythmia. 2022 Oct 1;23(1):24.
23. Mavrogeni SI, Kallifatidis A, Kourtidou T, Lama N, Christidi A, Detorakis E, et al. Cardiovascular Magnetic Resonance for evaluation of patients with cardiovascular disease. An overview of current indications, limitations and procedures. Hellenic Journal of Cardiology. 2023 Jan 24;70:53-64.
24. Saikumar K, Rajesh V, Srivastava G, Lin JC. Heart disease detection based on internet of things data using linear quadratic discriminant analysis and a deep graph convolutional neural network. Frontiers in Computational Neuroscience. 2022 Oct 7;16:964686.
25. Lönn A, Kallings LV, Andersson G, Paulsson S, Wallin P, Eriksson JS, et al. Lifestyle-related habits and factors before and after cardiovascular diagnosis: a case control study among 2,548 Swedish individuals. International Journal of Behavioral Nutrition and Physical Activity. 2023 Dec;20(1):41.
26. Limbachia J, Ajmeri M, Keating BJ, de Souza RJ, Anand SS. Effects of lifestyle interventions on cardiovascular risk factors in South Asians: a systematic review and meta-analysis. BMJ open. 2022 Dec 1;12(12):e059666.
27. Tyrovola D, Soulaidopoulos S, Tsioufis C, Lazaros G. The Role of Nutrition in Cardiovascular Disease: Current Concepts and Trends. Nutrients. 2023 Feb 21;15(5):1064.
28. Szczepańska E, Białek-Dratwa A, Janota B, Kowalski O. Dietary therapy in prevention of cardiovascular disease (CVD)—Tradition or modernity? A review of the latest approaches to nutrition in CVD. Nutrients. 2022 Jun 27;14(13):2649.
29. Diab A, Dastmalchi LN, Gulati M, Michos ED. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now?. Vascular health and risk management. 2023 Dec 31:237-53.
30. Tamer AA, Fayed AK, Ayman EE, Ibrahim EE. Efficiency of mixture of olives oil and figs as an antiviral agent: a review and perspective. International Journal of Medical Science and Health Research. 2020;4:107-11.
31. Kazemi A, Sasani N, Mokhtari Z, Keshtkar A, Babajafari S, Poustchi H, et al. Comparing the risk of cardiovascular diseases and all-cause mortality in four lifestyles with a combination of high/low physical activity and healthy/unhealthy diet: a prospective cohort study. International Journal of Behavioral Nutrition and Physical Activity. 2022 Dec;19(1):138.
32. Desjardins LC, Vohl MC. Precision Nutrition for Cardiovascular Disease Prevention. Lifestyle Genomics. 2023 Jan 30;16(1):73-82.
33. Perry AS, Dooley EE, Master H, Spartano NL, Brittain EL, Pettee Gabriel K. Physical activity over the lifecourse and cardiovascular disease. Circulation Research. 2023 Jun 9;132(12):1725-40.
34. Wang B, Gan L, Deng Y, Zhu S, Li G, Nasser MI, et al. Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications. Journal of Clinical Medicine. 2022 Dec 19;11(24):7511.
35. Choi H, Kim SH, Han K, Park TS, Park DW, Moon JY, et al. Association between exercise and risk of cardiovascular diseases in patients with non-cystic fibrosis bronchiectasis. Respiratory Research. 2022 Dec;23(1):288.
36. Länsitie M, Kangas M, Jokelainen J, Venojärvi M, Timonen M, Keinänen-Kiukaanniemi S, et al. Cardiovascular disease risk and all-cause mortality associated with accelerometer-measured physical activity and sedentary time‒a prospective population-based study in older adults. BMC geriatrics. 2022 Dec;22(1):729.
37. Isath A, Koziol KJ, Martinez MW, Garber CE, Martinez MN, Emery MS, et al. Exercise and cardiovascular health: A state-of-the-art review. Progress in Cardiovascular Diseases. 2023 Apr 28.
38. Chen H, Chen C, Spanos M, Li G, Lu R, Bei Y, et al. Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics. Signal Transduction and Targeted Therapy. 2022 Sep 1;7(1):306.
39. Getz V, Munkhaugen J, Lie HC, Dammen T. Barriers and facilitators for smoking cessation in chronic smokers with atherosclerotic cardiovascular disease enrolled in a randomized intervention trial: A qualitative study. Frontiers in Psychology. 2023 Mar 22;14:1060701.
40. Klonizakis M, Gumber A, McIntosh E, Brose LS. Medium-and longer-term cardiovascular effects of e-cigarettes in adults making a stop-smoking attempt: a randomized controlled trial. BMC Medicine. 2022 Aug 16;20(1):276.
41. Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers in Pharmacology. 2021 Sep 7;12:725268.
42. Okorare O, Evbayekha EO, Adabale OK, Daniel E, Ubokudum D, Olusiji SA, et al. Smoking Cessation and Benefits to Cardiovascular Health: A Review of Literature. Cureus. 2023 Mar 9;15(3).
43. Young JB, Raizner AE. Cardiovascular Pharmacology: Contemporary Physiologic and Scientific Approaches to Cardiovascular Disease Prevention and Treatment. Methodist DeBakey Cardiovascular Journal. 2022;18(5):1-4.
44. Matei D, Buculei I, Luca C, Corciova CP, Andritoi D, Fuior R, et al. Impact of Non-Pharmacological Interventions on the Mechanisms of Atherosclerosis. International Journal of Molecular Sciences. 2022 Aug 13;23(16):9097.
45. Colombijn JM, Idema DL, van der Braak K, Spijker R, Meijvis SC, Bots ML, et al. Evidence for pharmacological interventions to reduce cardiovascular risk for patients with chronic kidney disease: a study protocol of an evidence map. Systematic Reviews. 2022 Dec;11(1):238.
46. Ibdah R, Alrawashdeh A, Rawashdeh S, Melhem NY, Hammoudeh AJ, Jarrah MI. Statin Eligibility according to 2013 ACC/AHA and USPSTF Guidelines among Jordanian Patients with Acute Myocardial Infarction: The Impact of Gender. Cardiovascular Therapeutics. 2023 Jun 5;2023.
47. He WB, Ko HT, Curtis AJ, Zoungas S, Woods RL, Tonkin A, et al. The Effects of Statins on Cardiovascular and Inflammatory Biomarkers in Primary Prevention: A Systematic Review and Meta-Analysis. Heart, Lung and Circulation. 2023 Jun 7.
48. Steenhuis D, de Vos S, Bos JH, Hak E. Risk factors for drug-treated major adverse cardio-cerebrovascular events in patients on primary preventive statin therapy: A retrospective cohort study. Preventive Medicine Reports. 2023 May 29:102258.
49. Capodanno D, Mehran R, Krucoff MW, Baber U, Bhatt DL, Capranzano P, et al. Defining strategies of modulation of antiplatelet therapy in patients with coronary artery disease: a consensus document from the Academic Research Consortium. Circulation. 2023 Jun 20;147(25):1933-44.
50. Capodanno D, Angiolillo DJ. Personalised antiplatelet therapies for coronary artery disease: what the future holds. European Heart Journal. 2023 Jun 22:ehad362.
51. Halegoua-De Marzio D, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In: Drug-induced liver disease. Academic Press; 2013 Jan 1. pp. 519-40.
52. Yang HG, Kim DK. Concomitant Use of Antiplatelet Agents and Proton-Pump Inhibitors Increases the Risk of Adverse Cardiovascular Events: A Nationwide Population-Based Cohort Study Using Balanced Operational Definitions. Journal of Cardiovascular Development and Disease. 2023 Jun 20;10(6):264.
53. Hommels TM, Hermanides RS, Fabris E, Kedhi E. Exploring new insights in coronary lesion assessment and treatment in patients with diabetes mellitus: the impact of optical coherence tomography. Cardiovascular Diabetology. 2023 May 24;22(1):123.
54. Giron N, Lim C, Vallini J, Hallar K. Moving toward improved access to medicines and health technologies for cardiovascular disease. Rev Panam Salud Publica. Rev Panam Salud Publica; 2023 Jun 12:47:e93.
55. Nawata K. Risk factors for heart, cerebrovascular, and kidney diseases: evaluation of potential side effects of medications to control hypertension, hyperglycemia, and hypercholesterolemia. Frontiers in Cardiovascular Medicine. 2023 Jun 2;10:1103250.
56. Rivasi G, Matteucci G, Ungar A. Antihypertensive treatment targets in older adults: an unsolved dilemma. European Journal of Preventive Cardiology. 2023;182:2-3.
57. Smith L, Gossell‐Williams M, Standard‐Goldson A, Jackson MD. Antihypertensive medication class and diastolic orthostatic hypertension in older Caribbean adult patients. The Journal of Clinical Hypertension. 2023 Jul;25(7):618-27.
58. Li C, Pan Y, Zhang R, Huang Z, Li D, Han Y, et al. Genomic innovation in early life cardiovascular disease prevention and treatment. Circulation Research. 2023 Jun 9;132(12):1628-47.
59. Jia C, Zeng Y, Huang X, Yang H, Qu Y, Hu Y, et al. Lifestyle patterns, genetic susceptibility, and risk of valvular heart disease: a prospective cohort study based on the UK Biobank. European Journal of Preventive Cardiology. 2023 Jun 1:zwad177.
60. Liu F, Liu Y, Xu S, Wang Q, Xu F, Liu Y. Mendelian randomization study reveals a causal relationship between serum iron status and coronary heart disease and related cardiovascular diseases. Frontiers in Cardiovascular Medicine. 2023 Jun 13;10:1152201.
61. Krishnamurthy HK, Balaguru UM, Pereira M, Jayaraman V, Song Q, Krishna K, et al. Influence of genetic polymorphisms on serum biomarkers of cardiac health. Medicine. 2023 Jun 6;102(23):e33953.
62. Lo Faro V, Johansson T, Höglund J, Hadizadeh F, Johansson Å. Polygenic risk scores and risk stratification in deep vein thrombosis. Thrombosis Research. 2023 Aug:228:151-62.
63. Amin RJ, Morris-Rosendahl D, Edwards M, Tayal U, Buchan R, Hammersley DJ, et al. The addition of genetic testing and cardiovascular magnetic resonance to routine clinical data for stratification of etiology in dilated cardiomyopathy. Frontiers in Cardiovascular Medicine. 2022 Oct 6;9:1017119.
64. Wilson N, Cleghorn C, Nghiem N, Blakely T. Prioritization of intervention domains to prevent cardiovascular disease: a country-level case study using global burden of disease and local data. Population Health Metrics. 2023 Jan 26;21(1):1.
65. Minja NW, Nakagaayi D, Aliku T, Zhang W, Ssinabulya I, Nabaale J, et al. Cardiovascular diseases in Africa in the twenty-first century: Gaps and priorities going forward. Frontiers in Cardiovascular Medicine. 2022 Nov 10;9:1008335.
66. Sigamani A, Gupta R. Revisiting secondary prevention in coronary heart disease. Indian Heart Journal. 2022 Nov-Dec;74(6):431-40.
67. Kulbayeva S, Tazhibayeva K, Seiduanova L, Smagulova I, Mussina A, Tanabayeva S, et al. The Recent Advances of Mobile Healthcare in Cardiology Practice. Acta Informatica Medica. 2022 Sep;30(3):236-50.
68. Gray R, Indraratna P, Lovell N, Ooi SY. Digital health technology in the prevention of heart failure and coronary artery disease. Cardiovascular Digital Health Journal. 2022 Dec 1;3(6):S9-16.
69. Zwack CC, Haghani M, Hollings M, Zhang L, Gauci S, Gallagher R, et al. The evolution of digital health technologies in cardiovascular disease research. npj Digital Medicine. 2023 Jan 3;6(1):1-11.
70. Maddula R, MacLeod J, McLeish T, Painter S, Steward A, Berman G, et al. The role of digital health in the cardiovascular learning healthcare system. Front Cardiovasc Med. 2022 Nov 3;9:1008575.
71. Ashton RE, Philips BE, Faghy M. The acute and chronic implications of the COVID-19 virus on the cardiovascular system in adults: A systematic review. Prog Cardiovasc Dis. 2023 Jan-Feb;76:31-37.
72. Chang Y, Guo H, Yu C, Sun X, Yang K, Qian X. A novel classification, management and long-term outcomes of coronary artery involvement in acute aortic dissection. BMC Cardiovasc Disord. 2023 Jun 21;23(1):313.
73. Haxhija Z, Seder DB, May TL, Hassager C, Friberg H, Lilja G, et al. External validation of the CREST model to predict early circulatory-etiology death after out-of-hospital cardiac arrest without initial ST-segment elevation myocardial infarction. BMC Cardiovascular Disorders. 2023 Dec;23(1):1-8.
74. Ahmad SM, Ahmed NM. Classification based on event in survival machine learning analysis of cardiovascular disease cohort. BMC Cardiovasc Disord. 2023 Jun 20;23(1):310.
75. Mwakyandile TM, Shayo GA, Sasi PG, Mugusi FM, Barabona G, Ueno T, et al. Hypertension and traditional risk factors for cardiovascular diseases among treatment naïve HIV-infected adults initiating antiretroviral therapy in Urban Tanzania. BMC Cardiovascular Disorders. 2023 Dec;23(1):309.
76. Kamal A, Zaki A, Abdelaaty A, Madkour M. Management of ST-segment elevation myocardial infarction in comparison to European society of cardiology guidelines in Alexandria University Hospitals, Egypt. The Egyptian Heart Journal. 2023 Dec;75(1):1-8.
77. Bai M, Zhang J, Chen D, Lu M, Li J, Zhang Z, et al. Insights into research on myocardial ischemia/reperfusion injury from 2012 to 2021: a bibliometric analysis. European Journal of Medical Research. 2023 Dec;28(1):1-6.
78. Motovska Z, Hlinomaz O, Aschermann M, Jarkovsky J, Želízko M, Kala P, et al. Trends in outcomes of women with myocardial infarction undergoing primary angioplasty—Analysis of randomized trials. Frontiers in Cardiovascular Medicine. 2023 Jan 4;9:953567.
79. Wang Z, Peng J. The predictive value of the nomogram model of clinical risk factors for ischemia–reperfusion injury after primary percutaneous coronary intervention. Scientific Reports. 2023 Mar 28;13(1):5084.
80. He J, Bellenger NG, Ludman AJ, Shore AC, Strain WD. Treatment of myocardial ischaemia-reperfusion injury in patients with ST-segment elevation myocardial infarction: promise, disappointment, and hope. Reviews in Cardiovascular Medicine. 2022 Jan 17;23(1):23.
81. Mauro C, Chianese S, Cocchia R, Arcopinto M, Auciello S, Capone V, et al. Acute Heart Failure: Diagnostic–Therapeutic Pathways and Preventive Strategies—A Real-World Clinician’s Guide. Journal of Clinical Medicine. 2023 Jan 20;12(3):846.
82. Chioncel O, Adamo M, Nikolaou M, Parissis J, Mebazaa A, Yilmaz MB, et al. Acute heart failure and valvular heart disease: a scientific statement of the Heart Failure Association, the Association for Acute CardioVascular Care and the European Association of Percutaneous Cardiovascular Interventions of the European Society of Cardiology. European Journal of Heart Failure. 2023 Jul;25(7):1025-48.
83. Robbins BT, Howington GT, Swafford K, Zummer J, Woolum JA. Advancements in the management of acute ischemic stroke: A narrative review. Journal of the American College of Emergency Physicians Open. 2023 Feb;4(1):e12896.
84. Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers in Pharmacology. 2021 Sep 7;12:725268.
85. Jiang L, Zhou Y, Zhang L, Wu L, Shi H, He B, et al. Stroke health management: Novel strategies for the prevention of recurrent ischemic stroke. Frontiers in Neurology. 2022 Oct 26;13:1018794.
86. Kuan PX, Chan WK, Ying DK, Rahman MA, Peariasamy KM, Lai NM, et al. Efficacy of telemedicine for the management of cardiovascular disease: a systematic review and meta-analysis. The Lancet Digital Health. 2022 Sep 1;4(9):e676-91.
87. Zhu J, Wang W, Wang J, Zhu L. Change in coronary heart disease hospitalization after chronic disease management: a programme policy in China. Health Policy and Planning. 2023 Mar 1;38(2):161-9.
88. Yang Y, Gao J, Qin Z, Lu Y, Xu Y, Guo J, et al. The Present Clinical Treatment and Future Emerging Interdisciplinary for Heart Failure: Where We Are and What We Can Do. Intensive Care Research. 2023 Mar;3(1):3-11.
89. DeVore AD, Bosworth HB, Granger BB. Improving implementation of evidence-based therapies for heart failure. Clinical Cardiology. 2022 Jun;45:S52-9.
90. Moore JP, Marelli A, Burchill LJ, Chubb H, Roche SL, Cedars AM, et al. Management of Heart Failure With Arrhythmia in Adults With Congenital Heart Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2022 Dec 6;80(23):2224-38.
91. Chen S, Schmidt B, Chun JK. Pulmonary veins isolation using cryoballoon and pulsed field ablation for atrial fibrillation: practical techniques in variable scenarios. International Journal of Arrhythmia. 2023 Dec;24(1):1-9.
92. Fudim M, Abraham WT, von Bardeleben RS, Lindenfeld J, Ponikowski PP, Salah HM, et al. Device therapy in chronic heart failure: JACC state-of-the-art review. Journal of the American College of Cardiology. 2021 Aug 31;78(9):931-56.
93. Guzik M, Urban S, Iwanek G, Biegus J, Ponikowski P, Zymliński R. Novel therapeutic devices in heart failure. Journal of Clinical Medicine. 2022 Jul 25;11(15):4303.
94. de Oliveira Cardoso C, Elgalad A, Li K, Perin EC. Device-based therapy for decompensated heart failure: An updated review of devices in development based on the DRI2P2S classification. Frontiers in Cardiovascular Medicine. 2022 Sep 21;9:962839.
95. Alkhatib D, Isa S, Pour-Ghaz I, Butt A, Al-Taweel O, Ugonabo I, et al. Novel Device Therapies for Heart Failure. Journal of Cardiovascular Development and Disease. 2023 Apr 11;10(4):165.
96. Sadler D, Okwuosa T, Teske AJ, Guha A, Collier P, Moudgil R, et al. Cardio oncology: Digital innovations, precision medicine and health equity. Frontiers in Cardiovascular Medicine. 2022 Nov 3;9:951551.
97. Sethi Y, Patel N, Kaka N, Kaiwan O, Kar J, Moinuddin A, et al. Precision Medicine and the future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. Journal of Clinical Medicine. 2023 Feb 23;12(5):1799.
98. Johansson Å, Andreassen OA, Brunak S, Franks PW, Hedman H, Loos RJ, et al. Precision medicine in complex diseases—Molecular subgrouping for improved prediction and treatment stratification. Journal of Internal Medicine. 2023 Oct;294(4):378-396.
99. Franklin BA, Eijsvogels TM, Pandey A, Quindry J, Toth PP. Physical activity, cardiorespiratory fitness, and cardiovascular health: A clinical practice statement of the American Society for Preventive Cardiology Part II: Physical activity, cardiorespiratory fitness, minimum and goal intensities for exercise training, prescriptive methods, and special patient populations. American Journal of Preventive Cardiology. 2022 Oct 13:100425.
100. Lee MS, Flammer AJ, Lerman LO, Lerman A. Personalized medicine in cardiovascular diseases. Korean Circulation Journal. 2012 Sep 1;42(9):583-91.
101. Cruz-Correia R, Ferreira D, Bacelar G, Marques P, Maranhão P. Personalised medicine challenges: quality of data. International Journal of Data Science and Analytics. 2018 Nov;6(3):251-9.
102. Johnson KB, Wei WQ, Weeraratne D, Frisse ME, Misulis K, Rhee K, et al. Precision medicine, AI, and the future of personalized health care. Clinical and Translational Science. 2021 Jan;14(1):86-93.
103. Al Ahdal A, Rakhra M, Rajendran RR, Arslan F, Khder MA, Patel B, et al. Monitoring Cardiovascular Problems in Heart Patients Using Machine Learning. Journal of Healthcare Engineering. 2023 Feb 8;2023.
104. Karatzia L, Aung N, Aksentijevic D. Artificial intelligence in cardiology: Hope for the future and power for the present. Frontiers in Cardiovascular Medicine. 2022 Oct 13;9:945726.
105. Kagiyama N, Shrestha S, Farjo PD, Sengupta PP. Artificial intelligence: practical primer for clinical research in cardiovascular disease. Journal of the American Heart Association. 2019 Sep 3;8(17):e012788.
106. Gill SK, Karwath A, Uh HW, Cardoso VR, Gu Z, Barsky A, et al. Artificial intelligence to enhance clinical value across the spectrum of cardiovascular healthcare. European Heart Journal. 2023 Mar 1;44(9):713-25.
107. Kitsuka T, Takahashi F, Reinhardt J, Watanabe T, Ulziibayar A, Yimit A, et al. Advances in cardiac tissue engineering. Bioengineering. 2022 Nov 16;9(11):696.
108. Martin M, Gähwiler EK, Generali M, Hoerstrup SP, Emmert MY. Advances in 3D Organoid Models for Stem Cell-Based Cardiac Regeneration. International Journal of Molecular Sciences. 2023 Mar 8;24(6):5188.
109. Moradi SZ, Jalili F, Hoseinkhani Z, Mansouri K. Regenerative Medicine and Angiogenesis; Focused on Cardiovascular Disease. Advanced Pharmaceutical Bulletin. 2022 Aug;12(4):686.
110. Altyar AE, El-Sayed A, Abdeen A, Piscopo M, Mousa SA, Najda A, et al. Future regenerative medicine developments and their therapeutic applications. Biomedicine & Pharmacotherapy. 2023 Feb 1;158:114131.
111. Zhuo D, Lei I, Li W, Liu L, Li L, Ni J, et al. The origin, progress, and application of cell-based cardiac regeneration therapy. Journal of Cellular Physiology. 2023 Aug;238(8):1732-55.
112. Zhi W, Liu Y, Wang X, Zhang H. Recent advances of traditional Chinese medicine for the prevention and treatment of atherosclerosis. Journal of Ethnopharmacology. 2023 Jan 30;301:115749.
113. Yang Z, Lin S, Liu Y, Ren Q, Ge Z, Wang C, et al. Traditional chinese medicine in coronary microvascular disease. Frontiers in Pharmacology. 2022 Aug 8;13:929159.
114. Lu L, He W, Guan D, Jiang Y, Hu G, Ma F, et al. Acupuncture in treating cardiovascular disease complicated with depression: A systematic review and meta-analysis. Frontiers in Psychiatry. 2022 Dec 1;13:1051324.
115. Shaito A, Thuan DT, Phu HT, Nguyen TH, Hasan H, Halabi S, et al. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Frontiers in Pharmacology. 2020 Apr 7;11:422.
116. Olabiyi AA, de Castro Brás LE. Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions. Biomedicines. 2023 Jun 13;11(6):1697.
117. Park M, Song R, Ju K, Shin JC, Seo J, Fan X, et al. Effects of Tai Chi and Qigong on cognitive and physical functions in older adults: systematic review, meta-analysis, and meta-regression of randomized clinical trials. BMC Geriatrics. 2023 Jun 6;23(1):352.
118. Hui J, Wang Y, Zhao J, Cong W, Xu F. Effects of Tai Chi on health status in adults with chronic heart failure: A systematic review and meta-analysis. Frontiers in Cardiovascular Medicine. 2022 Sep 9;9:953657.
119. Lee SH, Jeon Y, Huang CW, Cheon C, Ko SG. Qigong and tai chi on human health: An overview of systematic reviews. The American Journal of Chinese Medicine. 2022 Oct 20;50(08):1995-2010.
120. Cooke JP, Youker KA. Future Impact of mRNA Therapy on Cardiovascular Diseases. Methodist DeBakey Cardiovascular Journal. 2022;18(5):64-73.
121. Kumric M, Urlic H, Bozic J, Vilovic M, Ticinovic Kurir T, Glavas D, et al. Emerging Therapies for the Treatment of Atherosclerotic Cardiovascular Disease: From Bench to Bedside. International Journal of Molecular Sciences. 2023 Apr 29;24(9):8062.
122. Kharlamov A, Lamberts M. Digital medicine: the next big leap advancing cardiovascular science. BMC Cardiovascular Disorders. 2023 Jan 17;23(1):30.
123. Javaid A, Zghyer F, Kim C, Spaulding EM, Isakadze N, Ding J, et al. Medicine 2032: The future of cardiovascular disease prevention with machine learning and digital health technology. American Journal of Preventive Cardiology. 2022 Aug 29:100379.
124. Zhang H, Zhan Q, Huang B, Wang Y, Wang X. AAV-mediated gene therapy: Advancing cardiovascular disease treatment. Frontiers in Cardiovascular Medicine. 2022 Aug 19;9:952755.
125. Romeo FJ, Mavropoulos SA, Ishikawa K. Progress in clinical gene therapy for cardiac disorders. Molecular Diagnosis & Therapy. 2023 Mar;27(2):179-91.
126. Saleem A, Abbas MK, Wang Y, Lan F. hPSC gene editing for cardiac disease therapy. Pflügers Archiv-European Journal of Physiology. 2022 Nov;474(11):1123-32.
127. Kyriakopoulou E, Monnikhof T, van Rooij E. Gene editing innovations and their applications in cardiomyopathy research. Disease Models & Mechanisms. 2023 May 1;16(5) :dmm050088.
128. Li ZH, Wang J, Xu JP, Wang J, Yang X. Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research. Military Medical Research. 2023 Mar 10;10(1):12.
129. Saeed S, Khan SU, Khan WU, Abdel-Maksoud MA, Mubarak AS, Kiani FA, et al. Genome Editing Technology: A New Frontier for the Treatment and Prevention of Cardiovascular Diseases. Current Problems in Cardiology. 2023 Mar 9:101692.
130. Yousif LI, Screever EM, Versluis D, Aboumsallem JP, Nierkens S, Manintveld OC, et al. Risk Factors for Immune Checkpoint Inhibitor–Mediated Cardiovascular Toxicities. Current Oncology Reports. 2023;25:753-63.
131. Wang C, Zoungas S, Yan M, Wolfe R, Haydon A, Shackleton M, et al. Immune checkpoint inhibitors and the risk of major atherosclerotic cardiovascular events in patients with high-risk or advanced melanoma: a retrospective cohort study. Cardio-Oncology. 2022 Dec;8(1):1-8.
132. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Can Vaccines Stop Cancer Before It Starts? Assessing the Promise of Prophylactic Immunization Against High-Risk Preneoplastic Lesions. Journal of Cellular Immunology. 2023 Nov 29;5(4):127-40.
133. Chan JS, Tang P, Ng K, Dee EC, Lee TT, Chou OH, et al. Cardiovascular risks of chemo-immunotherapy for lung cancer: A population-based cohort study. Lung Cancer. 2022 Dec 1;174:67-70.
134. Kumar V, Prabhu SD, Bansal SS. CD4+ T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Frontiers in Cardiovascular Medicine. 2022 Aug 25;9:992653.
135. Rurik JG, Aghajanian H, Epstein JA. Immune cells and immunotherapy for cardiac injury and repair. Circulation Research. 2021 May 28;128(11):1766-79.
136. Abplanalp WT, John D, Cremer S, Assmus B, Dorsheimer L, Hoffmann J, et al. Single-cell RNA-sequencing reveals profound changes in circulating immune cells in patients with heart failure. Cardiovascular Research. 2021 Feb 1;117(2):484-94.
137. Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 contributes to activation-induced cell death of pathological CD4+ T lymphocytes during ischemic heart failure. Basic to Translational Science. 2022 Oct 1;7(10):1038-49.
138. Smith BR, Edelman ER. Nanomedicines for cardiovascular disease. Nature Cardiovascular Research. 2023 Apr;2(4):351-67.
139. Bocancia-Mateescu LA, Stan D, Mirica AC, Ghita MG, Stan D, Ruta LL. Nanobodies as Diagnostic and Therapeutic Tools for Cardiovascular Diseases (CVDs). Pharmaceuticals. 2023 Jun 9;16(6):863.
140. Hosseinzadeh A, Zamani A, Johari HG, Vaez A, Golchin A, Tayebi L, et al. Moving beyond nanotechnology to uncover a glimmer of hope in diabetes medicine: Effective nanoparticle-based therapeutic strategies for the management and treatment of diabetic foot ulcers. Cell biochemistry and function. 2023 Jun 6.
141. Almas T, Haider R, Malik J, Mehmood A, Alvi A, Naz H, et al. Nanotechnology in interventional cardiology: A state-of-the-art review. IJC Heart & Vasculature. 2022 Dec 1;43:101149.
142. Wan J, Yang J, Lei W, Xiao Z, Zhou P, Zheng S, et al. Anti-Oxidative, Anti-Apoptotic, and M2 Polarized DSPC Liposome Nanoparticles for Selective Treatment of Atherosclerosis. International Journal of Nanomedicine. 2023 Dec 31:579-94.
143. Yang F, Xue J, Wang G, Diao Q. Nanoparticle-based drug delivery systems for the treatment of cardiovascular diseases. Frontiers in Pharmacology. 2022 Sep 12;13:999404.
144. Rafik ST, Zeitoun TM, Shalaby TI, Barakat MK, Ismail CA. Methotrexate conjugated gold nanoparticles improve rheumatoid vascular dysfunction in rat adjuvant-induced arthritis: gold revival. Inflammopharmacology. 2023 Feb;31(1):321-35.
145. Obidah Abert H, Umaru Aduwamai H, Shehu Adamu S. Effect of Green Synthesized Iron Oxide Nanoparticles Using Spinach Extract on Triton X-100-Induced Atherosclerosis in Rats. Biochemistry Research International. 2022 Oct 7;2022.
146. Bezbaruah R, Chavda VP, Nongrang L, Alom S, Deka K, Kalita T, et al. Nanoparticle-based delivery systems for vaccines. Vaccines. 2022 Nov 17;10(11):1946.
147. Murjani BO, Kadu PS, Bansod M, Vaidya SS, Yadav MD. Carbon nanotubes in biomedical applications: current status, promises, and challenges. Carbon Letters. 2022 Aug;32(5):1207-26.
148. Ul Haq A, Carotenuto F, Trovalusci F, De Matteis F, Di Nardo P. Carbon Nanomaterials-Based Electrically Conductive Scaffolds to Repair the Ischaemic Heart Tissue. C. 2022 Dec 4;8(4):72.
149. Hughes A, Shandhi MM, Master H, Dunn J, Brittain E. Wearable devices in cardiovascular medicine. Circulation Research. 2023 Mar 3;132(5):652-70.
150. Moshawrab M, Adda M, Bouzouane A, Ibrahim H, Raad A. Smart Wearables for the Detection of Cardiovascular Diseases: A Systematic Literature Review. Sensors. 2023 Jan 11;23(2):828.
151. Dhingra LS, Aminorroaya A, Oikonomou EK, Nargesi AA, Wilson FP, Krumholz HM, et al. Use of Wearable Devices in Individuals With or at Risk for Cardiovascular Disease in the US, 2019 to 2020. JAMA Network Open. 2023 Jun 1;6(6):e2316634.
152. Seffah K, Zaman MA, Awais N, Satnarine T, Haq A, Hernandez GN, et al. Exploring the role of wearable electronic medical devices in improving cardiovascular risk factors and outcomes among adults: a systematic review. Cureus. 2023 Mar 27;15(3).
153. Strik M, Ploux S, Weigel D, van der Zande J, Velraeds A, Racine HP, et al. The use of smartwatch electrocardiogram beyond arrhythmia detection. Trends in Cardiovascular Medicine. 2023 Jan 2.
154. Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. The Lancet Digital Health. 2022 Aug 1;4(8):e615-26.
155. Li Y, Jiang Y, Tang Y. Is remote blood pressure monitoring and management a better approach for patients with hypertension? A narrative review. The Journal of Clinical Hypertension. 2023 Feb;25(2):121-6.
156. Rêgo AD, Furtado GE, Bernardes RA, Santos-Costa P, Dias RA, Alves FS, et al. Development of smart clothing to prevent pressure injuries in bedridden persons and/or with severely impaired mobility: 4NoPressure research protocol. Healthcare (Basel). 2023 May 9;11(10):1361.
157. Reinhardt A, Ventura R. Remote Monitoring of Cardiac Implantable Electronic Devices: What is the Evidence?. Current Heart Failure Reports. 2023 Feb;20(1):12-23.
158. Ghilencea LN, Chiru MR, Stolcova M, Spiridon G, Manea LM, Stănescu AM, et al. Telemedicine: benefits for cardiovascular patients in the COVID-19 era. Frontiers in cardiovascular medicine. 2022 Jul 20;9:868635.
159. Mahalwar G, Kumar A, Kalra A. Virtual Cardiology: Past, Present, Future Directions, and Considerations. Current Cardiovascular Risk Reports. 2023 May 29:1-6.
160. Alarabyat IA, Al-Nsair N, Alrimawi I, Al-Yateem N, Shudifat RM, Saifan AR. Perceived barriers to effective use of telehealth in managing the care of patients with cardiovascular diseases: a qualitative study exploring healthcare professionals’ views in Jordan. BMC Health Services Research. 2023 Dec;23(1):1-8.
161. Lane J, David K, Ramarao J, Ward K, Raghuraman S, Waheed M, Lau AY. Translating primary care to telehealth: analysis of in-person consultations on diabetes and cardiovascular disease. BJGP open. 2023 Mar 1;7(1):BJGPO.2022.0123.
162. Shibata S, Hoshide S. Current situation of telemedicine research for cardiovascular risk in Japan. Hypertension Research. 2023 Feb 27;46(5):1171-80.
163. Jackson TN, Sreedhara M, Bostic M, Spafford M, Popat S, Lowe Beasley K, et al. Telehealth Use to Address Cardiovascular Disease and Hypertension in the United States: A Systematic Review and Meta-Analysis, 2011–2021. Telemedicine Reports. 2023 May 1;4(1):67-86.
164. Calò L, Martino A, Bollettino M, Scialla L, Cicogna F, Tota C, et al. Heart failure and telemedicine: where are we and where are we going? Opportunities and critical issues. European Heart Journal Supplements. 2023 May 1;25(Supplement_C):C326-30.
165. Shi W, Green H, Sikhosana N, Fernandez R. Effectiveness of Telehealth Cardiac Rehabilitation Programs on Health Outcomes of Patients With Coronary Heart Diseases: AN UMBRELLA REVIEW. Journal of Cardiopulmonary Rehabilitation and Prevention. 2023 Jun 20:10-97.
166. Jaén-Extremera J, Afanador-Restrepo DF, Rivas-Campo Y, Gómez-Rodas A, Aibar-Almazán A, Hita-Contreras F, et al. Effectiveness of telemedicine for reducing cardiovascular risk: a systematic review and meta-analysis. Journal of Clinical Medicine. 2023 Jan 20;12(3):841.
167. Hisan UK, Irianto I, Ghazali I, Amri MM. Telemedicine and COVID-19 Pandemic: Valuable Lessons for Future Implementations. Journal of Novel Engineering Science and Technology. 2022 Dec 30;1(02):63-8.
168. Zhu L, Li D, Jiang XL, Jia Y, Liu Y, Li F, et al. Effects of telemedicine interventions on essential hypertension: a protocol for a systematic review and meta-analysis. BMJ open. 2022 Sep 1;12(9):e060376.
169. Mahmud S, Alam S, Emon NU, Boby UH, Ahmed F, Monjur-Al-Hossain AS, et al. Opportunities and challenges in stem cell therapy in cardiovascular diseases: Position standing in 2022. Saudi Pharmaceutical Journal. 2022 Sep 1;30(9):1360-71.
170. Wargocka-Matuszewska W, Uhrynowski W, Rozwadowska N, Rogulski Z. Recent advances in cardiovascular diseases research using animal models and PET radioisotope tracers. International Journal of Molecular Sciences. 2022 Dec 26;24(1):353.
171. Hong CC. The grand challenge of discovering new cardiovascular drugs. Frontiers in drug discovery. 2022 Sep 23;2:1027401.