Abstract
Background: Infective endocarditis (IE) refers to inflammation of the endocardium, which is the inner layer of the heart. One of the most significant risk factors for developing IE is bioprosthetic valves within the heart. IE can lead to formation of vegetations on the surfaces of heart valves, making treatment difficult with antibiotics alone, often requiring surgery. Although IE can be treated with surgery or antibiotics, the presence of bioprosthetic valves can lead to recurrence of infection. Recurrence can cause several different complications including pseudoaneurysms of the mitral-aortic intervalvular fibrosa (MAIVF).
Case presentation: A 73-year-old male with a history of bicuspid aortic valve requiring several aortic valve replacements for IE presented to a university hospital with altered mental status and hypotension to 79/50. The patient developed severe sepsis with blood cultures growing Streptococcus dysgalactiae. Given the patient’s extensive history of IE, transesophageal echocardiogram (TEE) was done to rule out recurrence of infection. TEE showed pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF). Cardiothoracic surgery was consulted for possible surgical intervention given this finding in the setting of recurrent prosthetic valve endocarditis (PVE) but ultimately decided that the patient was a poor surgical candidate given his extensive history of cardiac surgeries. The patient was stabilized with antibiotics and fluid resuscitation and was discharged with a course of antibiotics followed by lifelong prophylactic antibiotic therapy.
Conclusion: This case highlights the life-threatening sequelae of PVE. Pseudoaneurysm of the MAIVF is a rare complication of PVE that occurs from PVE itself or surgery to the aortic or mitral valves. Providers should be aware of the importance of early detection of these findings with close follow up and imaging with modalities like TEE or cardiac computed tomography angiography (CTA) to see if surgical interventions can be pursued before further complications arise.
Keywords
Cardiac Surgery, Endocarditis, Pseudoaneurysm
Introduction
Infective endocarditis (IE) is a condition involving inflammation of the endocardium, or inner layer of the heart. It has classically been associated with Rheumatic heart disease, especially amongst individuals living in less developed countries [1]. In more modern times, this disease has commonly been found to affect individuals at an average age of 65 years with risk factors such as having prosthetic valves, indwelling cardiac devices, history of intravenous drug use, acquired valvular disease, chronic kidney disease requiring hemodialysis, and diabetes mellitus [2].
Among these risk factors, bioprosthetic valve placement is a common contributor to IE, often referred to as prosthetic valve endocarditis (PVE). In comparison to native heart valves, bioprosthetic valves provide a more suitable environment for bacterial growth and the formation of biofilms [3]. Typically, PVE is treated with antibiotic therapy, and the most common culprit organisms include Staphylococcus, Streptococcus, and Enterococcus [4]. However, antibiotics are not easily able to penetrate biofilms, which makes treating these infections more difficult. One of the most common manifestations of IE is the formation of vegetations, which are masses composed of microorganisms, along with platelets and fibrin, that accumulate on the surfaces of heart valves. These vegetations can cause many complications, such as embolization, abscess formation, recurrence of infection, as well as destruction to the native valve [5]. Evaluating the extent of vegetation formation is generally accomplished by using transesophageal echocardiography (TEE). Depending on the degree of disease progression, surgical removal of vegetations can be performed in circumstances in which antibiotic therapy alone does not suffice.
In some cases, a pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF) can be seen as a rare sequela of IE. The MAIVF is a fibrous structure that connects the anterior leaflet of the mitral valve to the aortic valve [6]. Without surgical intervention, this complication has the potential to cause tamponade, heart failure, or even an acute myocardial infarction [7].
We present a case of a patient with a history of multiple bioprosthetic aortic valve replacements who developed infective endocarditis and was found to have a pseudoaneurysm of the MAIVF on TEE.
Case
A 73-year-old male with a history of heart failure with reduced ejection fraction (30–35%), biventricular implantable cardioverter-defibrillator (ICD) placement, and bicuspid aortic valve status post four aortic valve replacements (AVRs) presented to a university medical center with altered mental status and hypotension after being found unresponsive at home by his husband.
Twenty-five years earlier, he had undergone mechanical AVR for bicuspid aortic stenosis, followed by bioprosthetic AVR two years later due to prosthetic valve endocarditis (PVE). He later required two additional bioprosthetic AVRs for recurrent endocarditis. He denied intravenous drug use, and prior episodes of endocarditis were associated with bloodstream infections without a clear alternate source of sepsis. Previously identified organisms included Streptococcus agalactiae and Staphylococcus aureus.
On presentation, he was febrile to 39.1°C, hypotensive to 79/50 mmHg, and oriented only to name. Lab work revealed a leukocytosis of 16.64, and blood cultures grew Streptococcus dysgalactiae. He was admitted to the cardiology service for severe sepsis with concern for recurrent PVE.
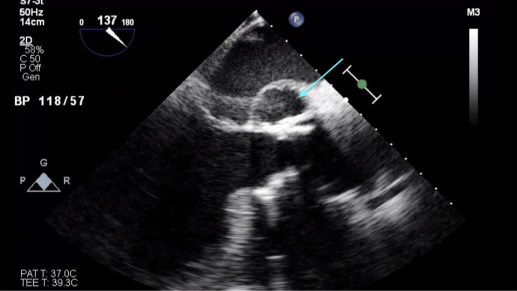
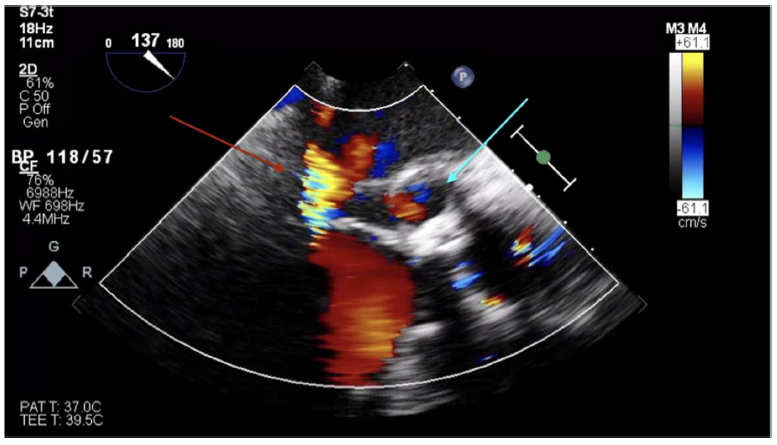
Transthoracic echocardiogram (TTE) showed a left ventricular ejection fraction of 30–35%. Transesophageal echocardiogram (TEE) revealed a mild-to-moderate anterior paravalvular leak of the bioprosthetic AVR and a pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF) with communication to the left ventricle (Figures 1 and 2). No paravalvular leak had been identified on echocardiograms following his most recent AVR, and there was no evidence of pseudoaneurysm or paravalvular leak on prior TTEs or TEEs. The exact dimensions of the pseudoaneurysm were not documented in the report.
Cardiothoracic surgery was consulted for possible intervention. Given the patient’s history of four prior AVR procedures, he was deemed a poor surgical candidate. During his fourth AVR, an aortic root abscess extending to the mitral leaflet was identified and surgically debrided. There was no documentation of MAIVF pseudoaneurysm or significant annular calcifications at that time.
Percutaneous closure of the paravalvular leak was considered, but interventional cardiology recommended conservative management due to the patient’s stability. Electrophysiology was consulted to evaluate potential ICD extraction; TEE and chest radiography showed no lead vegetations or abnormalities, and the device was left in place due to high procedural risk.
Antibiotic therapy was initiated with intravenous gentamicin for 10 days and intravenous ceftriaxone for 4 weeks via a peripherally inserted central catheter. This regimen was selected based on organism susceptibility and guidance from infectious disease specialists. He was discharged on lifelong oral amoxicillin 500 mg twice daily for endocarditis prophylaxis, in light of his surgical ineligibility and high recurrence risk. His condition stabilized over the following days, as his mental status and blood pressure improved. At outpatient cardiothoracic follow-up, he remained asymptomatic, and the decision was made to defer surgical intervention. Repeat TEE in one year was planned to monitor the MAIVF pseudoaneurysm.
Discussion
Infective endocarditis is a life-threatening condition that is associated with several risk factors. Of these, prosthetic valve implantation poses a major risk, as it accounts for about 10-30% of all cases, and approximately 1-6% of patients with prosthetic valves will develop endocarditis [8].
Individuals who are born with congenital anomalies like a bicuspid aortic valve or develop conditions such as valvular stenosis/regurgitation commonly require placement of an artificial heart valve. Generally, these artificial valves are classified as mechanical or bioprosthetic. Mechanical valves are typically more durable than bioprosthetic valves, but come with an increased risk of thrombosis, resulting in the need for lifelong anticoagulation [9]. While bioprosthetic valves do not have the same risk of thrombosis, there is a higher chance of developing PVE. This risk comes from the fact that bioprosthetic valves provide a surface that bacteria can more easily adhere to, which facilitates the formation of biofilms. Typically, the causative agents of infective endocarditis are Gram positive organisms such as Staphylococcus and Streptococcus species, but in some cases, IE can be caused by Gram negative species and certain types of fungi. Antibiotics and even surgical intervention can be used to treat infective endocarditis, but the remnants of these biofilms can lead to recurrent infections.
The overall mortality rate of patients with prosthetic valve endocarditis is about 25% [10]. However, this value has been shown to be affected by the types of organisms causing the infection. The literature has shown that PVE caused by Staphylococcus aureus had higher mortality rates in comparison to other organisms at 47.5%. In the same study, it was found that early surgical intervention in patients with this kind of PVE saw a decreased mortality rate at 28.6%, specifically in those experiencing cardiac complications, as well as in patients with embolic strokes. In the presented case, the patient had PVE that was culture positive for Streptococcus dysgalactiae, which is a pathogen that is less commonly connected to infective endocarditis, but has been associated with IE that presents with acute endophthalmitis, which was not seen in this patient [11]. Although the patient had recurrent infections requiring AVR on four occasions, he made a full recovery during this most recent episode with medical therapy alone.
Recurrence of infective endocarditis puts patients at increased risk for complications such as embolization of vegetations, formation of abscesses, dehiscence of prosthetic valves, or conduction abnormalities like high-degree atrioventricular block [12]. In this case, the patient developed a pseudoaneurysm of the MAIVF, a rare but severe sequela of IE. The MAIVF is a fibrous, avascular structure that connects the non-coronary cusp and adjacent portion of the left coronary cusp of the aortic valve to the anterior mitral leaflet [13]. Its avascular nature makes it vulnerable to infection and trauma, predisposing it to pseudoaneurysm formation. The two most common causes of this complication are infection from endocarditis and surgical trauma during mitral or aortic valve surgery [14].
Pseudoaneurysms of the MAIVF typically require surgical intervention due to the risk of rupture into the pericardium (causing tamponade), internal communication with the left ventricle (causing heart failure), or external compression of the coronary arteries (leading to myocardial infarction). However, in this patient, it was determined that surgical risk outweighed potential benefits. The case demonstrates that, in select patients, medical management with targeted antibiotics can lead to resolution of symptoms and clearance of infection, even in the presence of structural complications such as pseudoaneurysm. This adds to the growing body of literature supporting individualized management strategies in high-risk surgical patients.
Untreated pseudoaneurysm carries potential risks, including rupture, fistula formation, and embolization, but given the patient’s surgical risk and clinical stability, a conservative approach was chosen. He was discharged on lifelong antibiotic suppression, and close outpatient follow-up with serial imaging was arranged to monitor for future complications.
TEE remains the imaging modality of choice for detecting vegetations, abscesses, and pseudoaneurysms in patients with suspected IE, especially those with prosthetic valves [15]. Cardiac CTA is emerging as a useful adjunct, particularly when TEE is contraindicated. While TEE is superior for identifying vegetations under 10 mm and leaflet perforations, CTA may offer better detection of pseudoaneurysms and abscesses [16].
Conclusion
This case illustrates the potential for successful conservative management of prosthetic valve endocarditis (PVE) in high-risk surgical patients, including rare complications such as pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF). While surgery remains the standard of care for such complications, this case demonstrates that with proper imaging and prolonged antibiotic therapy, medical management can be a reasonable alternative in select patients. Nonetheless, close follow-up and re-evaluation of surgical candidacy are crucial given the risk of life-threatening sequelae.
References
2. Yallowitz AW, Decker LC. Infectious Endocarditis. 2023 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
3. Rodriguez-Nava G, Mohamed A, Yanez-Bello MA, Trelles-Garcia DP. Advances in medicine and positive natural selection: Prosthetic valve endocarditis due to biofilm producer Micrococcus luteus. IDCases. 2020 Mar 9;20:e00743.
4. Barrau K, Boulamery A, Imbert G, Casalta JP, Habib G, Messana T, et al. Causative organisms of infective endocarditis according to host status. Clin Microbiol Infect. 2004 Apr;10(4):302–8.
5. Liu-An Z, Joseph V, Damito S, Stoupakis G. Multiple Recurrent Infective Endocarditis Secondary to Streptococcus mitis Bacteremia Despite Proper Antibiotic and Surgical Treatment. Cureus. 2023 May 13;15(5):e38981.
6. Apostolidou E, Beale C, Poppas A, Stockwell P. Pseudoaneurysm of the Mitral-Aortic Intervalvular Fibrosa: A Case Series with Literature Review. CASE (Phila). 2017 Oct 19;1(6):221–6.
7. Nakata K, Moriyama S, Takaki J, Takeo M, Doi H, Matsumura T, et al. Pseudoaneurysm of mitral-aortic intervalvular fibrosa with rupture: a case report. Surg Case Rep. 2023 Dec 4;9(1):210.
8. Bezak B, Artemiou P, Hulman M. Valve-related factors and incidence of prosthetic valve endocarditis. Kardiochir Torakochirurgia Pol. 2020 Dec;17(4):178–82.
9. Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart. 2007 Jan;93(1):137–42.
10. Chen H, Zhan Y, Zhang K, Gao Y, Chen L, Zhan J, et al. The Global, Regional, and National Burden and Trends of Infective Endocarditis From 1990 to 2019: Results From the Global Burden of Disease Study 2019. Front Med (Lausanne). 2022 Mar 9;9:774224.
11. Yong AS, Lau SY, Woo TH, Li JY, Yong TY. Streptococcus dysgalactiae endocarditis presenting as acute endophthalmitis. Infect Dis Rep. 2012 Feb 22;4(1):e16.
12. Philip M, Hourdain J, Resseguier N, Gouriet F, Casalta JP, Arregle F, et al. Atrioventricular conduction disorders in aortic valve infective endocarditis. Arch Cardiovasc Dis. 2024 May;117(5):304–12.
13. Antonellis J, Kostopoulos K, Routoulas T, Patsilinakos S, Kranidis A, Salahas A, et al. Aneurysm of the mitral-aortic intervalvular fibrosa as a rare cause of angina pectoris: angiographic demonstration. Cathet Cardiovasc Diagn. 1997 Dec;42(4):423–6.
14. Muretti M, Elmahdy W, Ttofi I, Mozalbat D, Murphy M, Asimakopoulos G, et al. Surgical Repair of Mitral-Aortic Intervalvular Fibrosa Pseudoaneurysm in a High-Risk Patient 13 Years after Aortic Root Replacement. Tex Heart Inst J. 2019 Apr 1;46(2):147–50.
15. Tak T. Pseudoaneurysm of mitral-aortic intervalvular fibrosa. Clin Med Res. 2003 Jan;1(1):49–52.
16. Saeedan MB, Wang TKM, Cremer P, Wahadat AR, Budde RPJ, Unai S, et al. Role of Cardiac CT in Infective Endocarditis: Current Evidence, Opportunities, and Challenges. Radiol Cardiothorac Imaging. 2021 Feb 18;3(1):e200378.