Abstract
Although pneumonia and acute respiratory failure are the most frequent and severe complications of patients with SARS-CoV-2 infection, many of them also develop neurological signs and symptoms. From sickness behavior to coma, neurological disorders are associated with impairment of consciousness and dysautonomia, resulting from brainstem dysfunction. We propose here that SARS-CoV-2 invasion via the olfactory and trigeminal nerves generates a local neuro-inflammatory process aggravated by systemic inflammatory responses and micro-circulatory ischemic process. In addition to its short and long-term neurological consequences, the COVID-19-related brainstem dysfunction compromises the adaptive response to stress and contributes to organ failure and death. The detection of brainstem dysfunction mainly relies on neurological examination and non-invasive methods, such as pupillometer and spectral analyses of heart rate and blood pressure. We review the existing evidence of brainstem involvement in the course of Covid-19, its main mechanisms, diagnostic strategies, and potential outcomes.
Keywords
Brainstem; Neurotropism; COVID-19; Nucleus Tractus Solitarius; Olfaction, Neuroinflammation
Abbreviations
ACE2: Angiotensin Converting Enzyme-2; ADEM: Acute Disseminated Encephalomyelitis; ANS: Autonomic Nervous System; ARAS: Activating Reticular Ascending System; ARDS: Acute Respiratory Distress Syndrome; BBB: Blood-Brain Barrier; BP: Blood Pressure; CNS: Central Nervous System; COVID-19: Coronavirus Disease 2019; CSF: Cerebrospinal Fluid; DWI: Diffusion-Weighted Imaging; ECG: Electrocardiogram; EGG: Encephalogram ; GBS: Guillain-Barré Syndrome; HF: High Frequency; HR: Heart Rhythm; ICU: Intensive Care Unit; LF: Low Frequency; MRI: Magnetic Resonance Imaging; MSSNA: Muscle Skin Sympathetic Nerve Activity; NTS: Nucleus Tractus Solitarius; OSNs: Olfactory Sensory Neurons; PD: Pupillary Diameter; PLR: Pupillary Light Reflex; QSTART: Quantitative Axonal Sweat Reflex Test; RR: Respiratory Rhythm; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2; SSNA: Skin Sympathetic Nerve Activity; SSR: Sympathetic Skin Response; TMPRS2: Transmembrane Serine Protease 2
Background
COVID-19 patients can develop various central neurological disorders, including loss of smell and taste (anosmia and ageusia, respectively), ischemic injury (stroke), encephalopathy (delirium), and encephalitis [1,2], but also peripheral damages such as Guillain-Barré Syndrome (GBS) [3]. If the severity of respiratory failure remains the major determinant of the COVID-19 patient outcome, neurological disorders are also associated with increased mortality and morbidity [4]. In addition, to the role of COVID-19 severity markers, neurological disorders are also determinants of the COVID-19 course, as they can compromise the adaptive response to the systemic inflammation and may account for long-term psychological and cognitive impairments in those who survive COVID infections (i.e., the Long-COVID). In this context, the brainstem is likely the most vulnerable structure from the circuits potentially involved with both short- and longterm consequences. The brainstem controls arousal via the activating reticular ascending system (ARAS) and vital functions and immune responses integrity via autonomic centers. COVID-19 has severely challenged health care systems worldwide, with the urgent need for medical countermeasures, including the development of efficacious therapeutics. Here, we present the clinical spectrum, the diagnostic methods, and the potential pathogenesis of the COVID-19 that result from brainstem dysfunctions.
Neurological Manifestations and ICUrelated Complications
The neurological manifestations are considered relevant components of the clinical spectrum of the COVID-19. They can be classified from acute neurological manifestations as anosmia, ageusia or sickness behavior, to long-term consequences such as cognitive decline or mood disorders. In retrospective cohort studies, neurological symptoms were reported in one-third to half of the patients [5,6]. The incidence of neurological disorders increases with the severity of the COVID-19, from 45% in severe pneumonia to 85% in acute respiratory distress syndrome (ARDS) [6]. Moreover, the severity of COVID-19-related neurological disorders is associated with the presence of encephalopathy, encephalitis, stroke, or myopathy as reported in Intensive Care Unit (ICU) [4-6]. However, some of these dysfunctions can be complications of critical illness, rather than COVID-19 itself. Nevertheless, all these neurological disorders are associated with increased mortality and morbidity, notably by inducing long-term functional, psychological, and cognitive impairments.
Non-specific neurological symptoms include dizziness, headache, and malaise are reported especially at the onset of the infection [3]. Hypo/anosmia and hypo/ dysgeusia have been rapidly identified as early specific neurological symptoms, which occur in 80% of the cases [7]. Importantly, these symptoms are considered as clinical evidence for a potential SARS-CoV-2 neurotropism [8].
Any infection can induce stereotyped behavioral changes, the so-called sickness behavior [9] that includes among others, loss of appetite, asthenia, lethargy, social withdrawal, apathy, and impaired attention and concentration. Sickness behavior has been shown to be associated with long-term psychological disorders, notably depression [9]. It is likely that many neurological and psychological symptoms that COVID-19 patients complain about belong to the sickness behavior syndrome (that can mimic hypoactive delirium). Conversely, encephalopathy is a pathophysiological condition that results from a global brain dysfunction. It is characterized by impairment of consciousness, ranging from delirium to coma, with electroencephalographic changes from slowness activity of electrical discharge to status epilepticus. It can be associated with seizure, extrapyramidal and pyramidal signs, but also neuropsychiatric manifestations including psychosis in 8% of the cases [10]. Brain imaging is usually normal. The rate of encephalopathy is higher with severity of COVID-19, as 13% to 38% of ICU COVID-19 patients will develop a disorder of consciousness [11]. Some risk factors include patient’s age and pre-existing neurological disease [12]. One of the main specificities of COVID-19- related encephalopathy is the EEG pattern characterized by periodic discharges located in the frontal region (Table 1). MRI reveals hypersignals of white matter in T2/FLAIR sequence of cortical or subcortical localization [13,14]. Encephalopathy results also from critical illness and the adverse effects of ICU management, including hypoxemia, metabolic electrolyte disturbances, drug toxicity or withdrawal hemodynamic, and renal or liver failures. However, SARS-CoV-2-induced vasculopathy, endothelial dysfunction, hypercoagulability/antifibrinolysis, and systemic inflammation might contribute to the pathogenesis. Disorders of consciousness can be secondary to encephalitis reported in 10% to 30% of COVID-19 patients according to the study design, diagnosis methods, and severity of COVID-19 [11]. They can be revealed by impaired consciousness, seizure, or focal neurological signs. According to clinical and MRI findings, encephalitis is classified as acute disseminated encephalomyelitis (ADEM)-like or auto-immune like disorders [15-17] (Table 1).
| Main Clinical neurological syndromes | Neurological symptoms and manifestations | Finded biological data | Electroencephalogram | Neuroimaging (Brain MRI) | References |
|---|---|---|---|---|---|
| Encephalopathy |
Fever, coma, myoclonus, impairment of brainstem reflexes, absent withdrawal to pain and diminished deep tendon reflexes. |
Increased levels of IL-6, IL-8, IL-10, IP-10 and TNF- alpha in CSF. RT-PCR for viral analysis in CSF negative. Increased levels of plasma anti-S1 IgG Increased levels of anti-S1 and anti-E IgM in CSF. |
Diffuse slowing. |
Cerebral edema with hypoxic ischemic changes and abnormalities with restricted diffusion concerning right hemisphere, grey matter, deep grey nuclei, splenium and temporal lobe, carotid artery thrombus. |
(Benameur et al., 2020) |
| Encephalitis/ Encephalomyelitis | Asthenia, disorientation with attention deficit, verbal and motor perseverations with grasping, myalgia, tonico- clonic seizure and psychotic symptoms, hemianopsia, hemineglect. Delirium and altered consciousness. Headache, fever, lethargy, stiff neck, photophobia and new onset seizure. Headache, generalized fatigue and fever, consciousness disturbances, neck stiffness and multiple generalized seizure. |
Biochemical arguments for meningoencephalitis with lymphocytic pleiocytosis. Negative RT PCR SARS- CoV-2 in CSF. high CSF protein levels without pleocytosis and PCR negative for viruses. Elevated albumin quotient without oligoclonal bands. CSF with lymphocytic pleiocytosis, proteinase. RT PCR for SARS-CoV-2 in CSF non available. CSF cell count was 12/ mL–10 mononuclear and 2 polymorphonuclear cells without red blood cells. Positive RT PCR SARS- CoV-2 in CSF. |
non-convulsive, focal status epilepticus (abundant bursts of anterior low- to medium- voltage irregular spike and waves super- imposed on an irregularly slowed theta background) then moderate theta background slowing without epileptiform features. Non available. Generalized slowing with no epileptic discharges. Epileptic discharges. |
Normal Cortical or white matter hyperintensities, contrast enhancement, and sulcal he- morrhagic features. Normal Hyperintensity along the wall of inferior horn of right lateral ventricle in DWI. FLAIR hyperintense signals changes in the right mesial temporal lobe and hippocampus with slight hippocampal atrophy. Right lateral ventriculitis and encephalitis. |
(Bernard- Valnet et al., 2020) (Dogan et al., 2020) (Duong et al., 2020) (Moriguchi et al., 2020) |
| Sickness Behavior |
Sadness, fatigue, depression with passive suicidal statements, fever, sleep disturbances, poor appetite, anxiety and panic attacks. |
Elevated IL-6 levels (> 25 pg/ml) |
Seizure like activity with brain dysfunction on EEG consistent with metabolic encephalopathy. |
Only head CT performed (negative). |
(Alpert et al., 2020) |
| Acute Ischemic stroke | Dysarthria, left hemiplegia, and alteration of consciousness Nonfluent aphasia. Word-finding difficulties, bilateral incoordination, homonymous hemianopia, confusion and altered consciousness, incoordination. Dysarthria or aphasia and facial droop and weakness. |
Non available. Elevated D-dimer levels (> 7000 µg/L) with positive lupus anticoagulant. |
Non available. Non available. |
Hyperintensive lesions of bilateral cerebellar hemispheres, right occipital cortex, bilateral centrum semiovale and bilateral parietal cortex in DWI with hyperintensities in FLAIR. Multiple small ischemic infarctions with hyperintensive lesions (arrows) in bilateral cerebellar hemispheres. Vertebral artery thrombus and acute cerebellar artery territory infarction with petechial haemorrhagic transformation. acute infarction in the right corpus striatum and an established infarct in the same region with moderate background cerebral small vessel disease acute infarction in the right thalamus, left pons, right occipital lobe and right cerebellar hemisphere with thrombotic material in the basilar artery and bilateral mild-to-moderate P2 segment stenosis. Bilateral subcortical and deep nuclei lesions Multifocal patchy areas of abnormal signs, diffusion weighted lesions and ADC changes in bilateral frontoparietal, anterior temporal lobes, ganglia, external capsules and thalami. |
(Zayet et al., 2020) (Beyrouti et al., 2020) |
| ADEM |
Dysarthria, Dysphagia (bulbar impairment), expressive aphasia, headache and myalgia. Coma, impaired oculocephalic response and left hemiparesis, reflex impairment. Fever and altered mental status. |
CSF analysis with normal cell counts, protein and glucose RT-PCR for SARS-CoV-2 in CSF non available. RT PCR SARS-CoV-2 in CSF non available. |
No electrical arguments for seizure. Non available. |
Hyperintense lesions on T2 FLAIR sequence in deep hemispheric and juxtacortical white matter Signs of Acute Necrotizing encephalopathy i.e hemorrhagic rim- enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions. |
(Zhang et al., 2020) (Poyiadji et al., 2020) |
Acute ischemic stroke, or transient ischemic attack, are diagnosed in 26% of non-ICU COVID-19 patients [11]. Severe COVID-19 patients are at high-risk of ischemic and hemorrhagic strokes, and the potential mechanisms includes hypercoagulabilty, antifibrinolysis and endothelial activation/dysfunction, potentially leading to vasculitis and thrombo-embolism. Among the peripheral nervous system disorders, so far, GBS has been the most reported one [3]. GBS must be differentiated from ICUacquired paresis, which spare cranial nerves, and is related to a myopathy and/or axonal polyneuropathy [18].
Skeletal striated muscles are also a privileged target of SARS-CoV-2 which can be explained by the surface expression of the TMPRSS2 receptor [19] or by the consequences of the actors of systemic inflammation on the tissues (IFN-gamma, TNF-alpha, IL-1ß, IL-6, IL-17) inducing an increase in protein catabolism, a decrease in synthesis capacities and inhibiting repair mechanisms (decrease in differentiation and pro- fibrosing and atrophying) on a clinical and experimental point of view [20,21]. The prevalence of myalgia, if it does not appear to be constantly associated with severe clinical pictures, nevertheless represents up to 50% of cases and is predictive of a pejorative evolution [22,23]. Muscle involvement may either be related to direct muscle injury, characterized by an increase in creatine kinase proportional to severity with arrays of rhabdomyolysis [24], or to motor neuron involvement [21]. This muscle dysfunction can persist 3 to 6 months after hospital discharge representing up to 63% of cases, manifested by reduced muscle strength of the distal muscles, adaptation to effort, and functional capacity [25].
A brainstem dysfunction can be a component of this COVID-19 neurological spectrum and may negatively impact the COVID-19 course. Together, these clinical observations strongly support the neurotropism of SARSCoV- 2 and, in particular, the brainstem hypothesis that we now describe in more detail.
Can the Brain be a Key Target of SARSCoV2?
The viral brain invasion
Because COVID-19 is not a restricted respiratory infection but rather a multisystem disease resulting from a complex interplay of viral, immunological, inflammatory and coagulative cascades, a variety of potential biomarkers have been identified (reviewed in [26]). These biological markers are manifold, including biochemical (e.g., albumin, LDH), immunological (e.g., CD4+ cells, CD8+ cells, B cells and natural killers (NK) cells), inflammatory (e.g., CRP, cytokines), coagulation or hematological (e.g., ferritin/transferrin ratio, Procalcitonin).
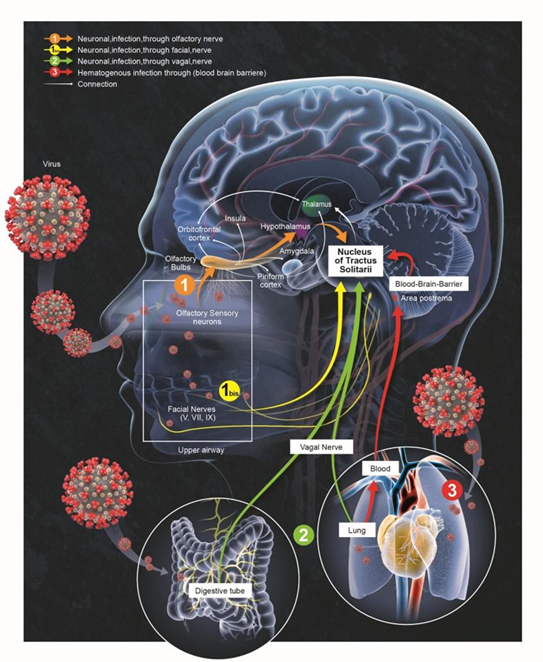
Regarding brain dysfunctions, increased concentration of Neurofilament light chain protein (NfL) and glial fibrillary acidic protein (GFAp) in COVID-19 patients on admission correlates with increased mortality risk [27]. A study on 47 mild, moderate and severe COVID-19 revealed that patients with severe COVID-19 exhibited higher plasma concentrations of GFAp and NfL than controls, while GFAp was also increased in patients with moderate disease [28]. In patients with severe disease, an early peak in plasma GFAp decreased on follow-up, while NfL showed a sustained increase from first to last follow-up [28]. Thus monitoring blood biomarkers for nervous system injury is useful in early suspicion, diagnosis, monitoring, and recognition of complications, management and disposition of COVID-19 patients with CNS injury. During the former SARS epidemic outburst, studies have reported the presence of SARS-CoV particles in brain samples from infected individuals [29-31]. Yet, the mechanism by which neuroinvasion occurs remains unclear, even if the neurotropism of SARS-CoV-2 is not surprising, bearing in mind its structural homology with SARS-CoV. The humoral and the neuronal retrograde routes have been proposed for respiratory viruses to reach the CNS [32,33]. Once in the brain, SARS-CoV-2 might reach the brainstem and compromise autonomous functions (Figure 1).
Is the Olfactory Route a Potential Path for SARS-CoV-2?
Previous studies have reported smell and taste disturbances in the course of SARS-CoV-2 infection [5,34]. Anosmia and dysgeusia were more frequent in less severe cases, and dysautonomia had been noticed in 3% of patients [34]. Though less frequent, it has been reported some cases of oculomotricity anomalies [35] and trigeminal nerve damage [36]. The peripheral nervous system may thus constitute one of the entries for SARSCoV- 2 to infect the CNS.
A meta-analysis from case series, cohort studies and case control studies, reports that predominant involvement of the olfactory system with disruptions across olfactory structures, including the olfactory bulb/tract and the primary olfactory cortex [37]. Interestingly, these abnormalities also extended to the corpus callosum, cingulate cortex, and insula, jointly enabling propagation of SARS-CoV-2 toward secondary olfactory areas. In particular, some related cytotoxic lesions of the corpus callosum have been reported from presumed COVID-19 [38].
SARS-CoV-2 is detected in the brain of 53% of COVID-19 patients, with SARS-CoV-2 viral proteins found in cranial nerves originating from the lower brainstem and in isolated cells of the brainstem [39]. The nerves V, VII, and IX, which ultimately lead to the nucleus tractus solitarius (NTS) are prime suspects since gustatory dysfunction have been reported from COVID-19 patients [34]. The virus may also use the olfactory nerve as an alternative retrograde route to access the brain [40]. The olfactory sensory neurons (OSNs) of the olfactory epithelium, including immature neurons, project to the olfactory bulb [41]. From there, the virus can broadly spread within different brain areas as the primary projections of the olfactory bulb are the olfactory nucleus, olfactory tubercle, piriform cortex, the anterior part of the parahippocampal gyrus (entorhinal cortex) and the peri-amygdaloid cortex of the amygdala [42]. Human brain tissues express SARS-CoV-2 cell receptor ACE2 (angiotensin converting enzyme-2) [43], including the ventricles or the substantia nigra, but also brain areas directly or indirectly associated with the olfactory pathway, including the amygdala, the hippocampus, hypothalamic nuclei and the frontal cortex [44]. Furthermore, it is likely that ACE2 is not the sole host factor associated with neuroinvasion of SARS-CoV-2 and Neuropilin-1 represents a strong alternative candidate [45].
The hypothesis of a viral invasion through the olfactory route is supported by RNA-seq analyses reporting expression of ACE2 and TMPRSS2 (Transmembrane Serine Protease-2) [46] in the olfactory sustentacular cells, microvillar cells, Bowman’s glands, and horizontal basal cells [47,48], and at lower level in the OSNs [49]. Also, SARS-CoV-2 has been shown to invade olfactory nerves in hamsters [50,51] and brains of COVID-19 patients [52,53]. SARS-CoV-2-induced acute anosmia and ageusia in golden Syrian hamsters, are notably reported to last as long as the virus remained in the olfactory epithelium and the olfactory bulb [54]. Moreover, olfactory mucosa sampling from patients showing long-term persistence of COVID- 19-associated anosmia 196 day after infection still revealed the presence of virus transcripts and of SARS-CoV-2- infected cells, together with protracted inflammation [54]. SARS-CoV-2 persistence and associated inflammation in the olfactory neuroepithelium may account for prolonged or relapsing symptoms of COVID-19 such as loss of smell. The way OSNs are infected by SARS-CoV-2 remains still unknown, but as horizontal basal cells are progenitors that continually divide to replace OSNs [55], it is possible that infected horizontal basal cells could produce newly formed OSNs infected by SARS-CoV-2, and by axonal transportation allow the virus to migrate from the sensory epithelium to the olfactory bulb [41], and from there spreads into further central targets [40] (Figure 1).
The vagus nerve: a genuine pathway for neuroinvasion or an effector of brainstem dysfunction?
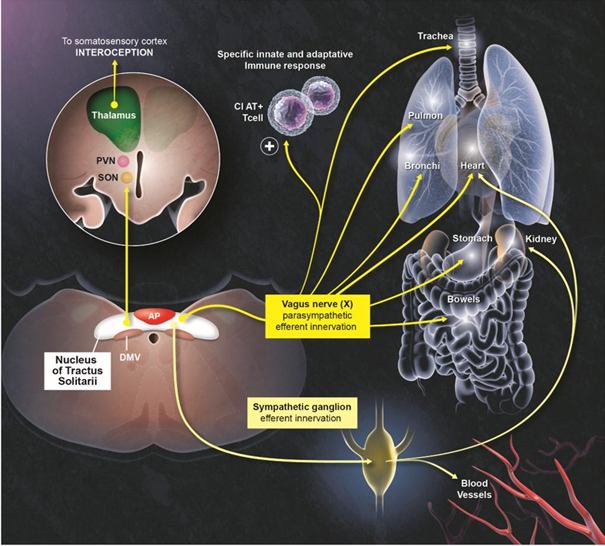
One important common point between the pulmonary and digestive systems concerns their innervation by the vagus nerve (Figure 1). It has bidirectional connections to both the neuroepithelial and smooth muscle cells of both respiratory and gastrointestinal tracts [56,57], and exhibits motor, sensory, and immuno-modulating functions [58,59]. To exert such pleiotropic effects, the vagus nerve exhibits both afferences and efferences with actions mediated by neurotransmitters on several subtypes of tissue immune cells [60,61]. As such, a stimulation of the vagus nerve can reduce the neuroinflammation both experimentally by the lipopolysaccharide as well as in more naturalistic conditions [59,62]. The vagus nerve is also involved in the reflex mechanisms regulating physiological respiratory adaptation to stress, heart rate, and the digestion processes. Most of the afferents from the vagus nerve project onto the NTS in the medulla of the brainstem. The vagus nerve appears therefore as a gateway from the respiratory or digestive systems to the CNS. In turn, it could be a target on its own leading to deleterious consequences, particularly respiratory, when the NTS is damaged (Figure 2). A possible alternative option embraced by our ‘vagal hypothesis would be that the vagus nerve constitutes the way by which the brainstem dysfunction can be transmitted to organs innervated by this nerve (lungs, heart, etc.). Along these lines, it should be mentioned that the vagus nerve indeed exerts an important function in regulating inflammation by controlling innate immune responses during pathogen invasion or tissue injury. The nature of the efferent vagus nerve-mediated immunoregulatory output is cholinergic. Via the so-called inflammatory reflex, the vagus nerve controls immune activation and suppresses proinflammatory cytokine release. Further preclinical or clinical investigations should clarify whether the vagus nerve allows the viral neuroinvasion or rather it constitutes an effector of brainstem dysfunction responsible for inflammatory dysregulation in COVID-19 patients.
The hematogenous route
The virus might also enter the brainstem via the humoral route, through endotheliitis [63]. Indeed, SARS-CoV-2 could gain access to the CNS by infecting endothelial cells of the blood–brain barrier (BBB), epithelial cells of the bloodcerebrospinal fluid barrier in the choroid plexus, or from a “Trojan Horse” strategy by hacking inflammatory cells [64]. A special entrance in the humoral route is embodied by features of the area postrema: i.e., a highly vascular paired structure in the medulla oblongata of the brainstem. While the detection of the virus in the cerebrospinal fluid (CSF) is still controversial [1,17,65], antibodies against SARSCoV- 2 were however detected in the CSF of COVID-19 patient [66]. Similarly, a transmission electron microscopy study has evidenced the presence of viral-like proteins inside brain microvascular endothelial cells in the frontal lobe of a COVID-19 patient and confirmed the presence of SARS-CoV-2 within the tissue using RT-PCR assays [67]. This observation provided the evidence that SARS-CoV-2 can infect the brain microvascular endothelial cells of the BBB. Nonetheless, whether the infection originated from the brain, blood, or CSF remains still unclear. As CSF communicates with nasal lymph through the cribriform plate [68,69], inoculation of the CSF through this channel seems pertinent with the olfactory impairments reported in COVID-19 patients.
Biomarkers and Dysregulated Inflammatory Response in COVID-19
A neuro-inflammation is mainly the consequence of BBB impairment and endothelial activation induced by circulating pro-inflammatory cytokines and chemokines, whose levels correlate with severity of COVID-19 [70]. It is characterized mostly by an activation of microglial cells or the presence of T-lymphocytes that acquire a neurotoxic immune profile. The neuro-inflammatory process is common in various infectious conditions, such as sepsis, however the overproduction of IL-17 by T-helper 17 (Th17) cells would be particularly involved in coronavirus infections [71]. This up-regulation is also responsible for chemokines induction of expression, such as CCL2 (MCP-1) and CXCL1, by the cerebral vascular endothelium with increase in the migration of activated Th17 cells within the brain parenchyma [72]. The presence of IL-17 in abundance in the brain is responsible for triggering numerous pro-inflammatory cytokines, chemokines, and protein mediators (e.g., COX-2, PGE-2) pathways [73]. The activation of these various inflammatory pathways during SARS-CoV-2 infection leads to higher production of cytotoxic T-lymphocytes and activation of the NFκB pathway with consequences for neuronal toxicity [74,75].
A recent review established and classified an up-to date list of biomarkers in COVID-19 according to organ/system involved, their temporal trends, and their association with clinical phenotype and therapeutic responses [26]. Abers et al. analyzed levels of 66 soluble biomarkers in 175 Italian patients with COVID-19 ranging from mild/moderate to critical severity and assessed type I IFN–, type II IFN–, and NF-κB–dependent whole-blood transcriptional signatures [76]. They identified 14 biomarkers (CCL2, IL-15, soluble ST2 [sST2], NGAL, sTNFRSF1A, ferritin, IL-6, S100A9, MMP-9, IL-2, sVEGFR1, IL-10, lactoferrin, CXCL9) that when increased were independently associated with mortality, while IL-1α was associated with mortality when decreased. Among these, sST2, sTNFRSF1A, IL- 10, and IL-15 were consistently higher throughout the hospitalization in patients who died versus those who recovered, suggesting that these biomarkers may provide an early warning of eventual disease outcome [76].
By investigating the brain tissue of patients who died from COVID-19, Matschke et al. detected SARS-CoV-2 in the brains of 21 (53%) out of 40 examined patients [39], with SARS-CoV-2 viral proteins found in cranial nerves originating from the lower brainstem and in isolated cells of the brainstem. Neuroimmune activation was observed in all examined brains, with prominent involvement of the brainstem and neuroimmune reaction, in line with our ‘brainstem hypothesis’. The presence of SARSCoV- 2 did not seem to be associated with the severity of neuroimmune activation. Remarkably, diffuse activation of microglia, with occasional microglial nodules, and presence of cytotoxic T lymphocytes were pronounced in the brainstem.
The Brainstem Hypothesis
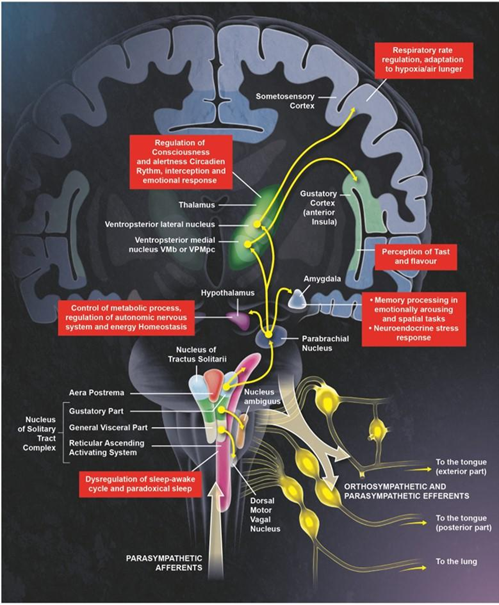
Previous coronaviruses have been proven to invade the brainstem in humans [77,78]. As discussed above, SARS-CoV-2 could propagate within infected patients through the humoral route or might spread by the mean of trans-synaptic and exo-synaptic propagation [79-81], along the vagus nerve or the olfactory system, eventually reaching respiratory centers in the brainstem, adding a neurogenic component to the respiratory failure [81,82]. Indeed, the SARS-CoV-2 neurotropism and expression of ACE2 receptors (or Neuropilin-1) in the brainstem make this structure a well-qualified target for SARS-CoV-2 [80,83,84]. By connecting the diencephalon to the spinal cord and the cerebellum, the brainstem is located at the interface between the limbic system, and the efferent signaling pathways to peripheral organs [85,86]. It controls oculomotricity, facial sensory and motor functions, hearing, balance, and oropharyngeal function via cranial nerves. The brainstem regulates the sleep/wake cycles via the ARAS, thereby it is involved in alertness and awareness [87]. It plays a major role in hemostasis via the autonomic nervous system (ANS) [88,89]. Schematically, the ANS controls the pupillary size and light reflex, heart rate (HR), blood pressure, the tidal volume and respiratory rate (RR), gastrointestinal motility, and bladder tone as well as the immune response [90]. The brainstem autonomic centers integrate bottom-up body signals and efferent top-down signals from the cortex (Figure 3).
Located at the level of the dorso-medial medulla, the NTS is a key center in the autonomic control as it processes: 1) taste information from the facial, glossopharyngeals, and vagal nerves (the latter is the main nerve of the parasympathetic division of the autonomic nervous system); 2) mechano- and chemo-receptors inputs from the carotid, aorta, and sinoatrial node, and also from the respiratory tract and lungs, via the glossopharyngeal and vagal nerves; 3) gastrointestinal and liver inputs via the vagal nerve. Through its direct projection to the vagal efferent neurons in the dorsal motor nucleus of the vagus nerve, the NTS mediates several reflexes including the gag reflex, the carotid sinus reflex, the aortic reflex, the cough reflex, the baroreceptor and chemoreceptor reflexes, several respiratory reflexes, and reflexes within the gastrointestinal system regulating motility and secretion. The rostral (rNTS) and caudal (cNTS) parts participate in cough reflex mechanisms, modulation of inspiratory frequency and amplitude, and heart rate and blood pressure [91]. The NTS also innervates the sympathetic neurons of the ventrolateral region of medulla oblongata, a major site controlling sympathetic preganglionic neurons and involved in the control of cardiovascular functions [92,93].
Because of its connections to various brain regions, the NTS is a hub connecting reciprocal signaling between visceral organs and the neuroendocrine and limbic centers [94,95]. Its caudo-medial part (cmNTS) receives signals from the vagus nerve and regulates food intake by negative feedback [96]. It also integrates hormonal and non-hormonal signals (circulating nutrients) related to the energy status of the organism [97,98] (Figure 3). The NTS plays an indirect yet essential role in the individual’s ability to adapt to emotionally stressful situations and set-up avoidance behaviors when facing a danger [99,100]. Due to its connections with the amygdala’s central and basolateral nuclei [101,102], the NTS contributes to behavioral responses to stress. The NTS can influence vasopressin release and activate the hypothalamic-pituitary-adrenal axis by its direct connections with the hypothalamus’s paraventricular nucleus. The NTS is a central relay of the interoceptive network that allows the perception and integration of signals originating from the inner body and it is essential to trigger responses to stress, emotional behavior, attention, alertness, and self-consciousness.
Dysautonomia could be defined as a failure or, in some cases, an overactivity of the sympathetic and/or parasympathetic nervous systems [90,103]. Dysautonomia has been described in various central and peripheral neurological diseases, either acute or chronic. NTS dysfunction can lead to major dysfunction of the cardiorespiratory centers and the metabolic homeostasis [104]. NTS dysfunction can be a cause of neurogenic hypertension, obstructive sleep apnea, and metabolic disorders [105,106]. Experimental studies have shown that it induces severe disturbances of eating behavior and energy balance (Figure 3). NTS dysfunction impairs the interoceptive-dependent functions, such as the perception of danger, emotional behavior, attention, and self-consciousness.
Numerous clinical manifestations result from brainstem dysfunction in COVID-19 patients. First, anosmia and dysgeusia can be related to NTS dysfunction. From a direct viral invasion to the olfactory bulb or by inflammation at the lamina propria, an NTS dysfunction might interrupt the link between the insular and temporal taste and the afferent pathways conveying signals from specialized chemoreceptors [107] via facial (cranial nerve (CN) VII), glossopharyngeal (CN IX) and vagus (CN X) nerves (Figure 3). Second, impairment of consciousness, ranging from delirium to coma, could result from the brainstem dysfunction at the ARAS or NTS level, which controls the sleep-wake cycle and contributes to interoceptivedependent attention and self-consciousness. Thus, brainstem dysfunction could contribute to the COVID-19- associated encephalopathy and sleep disturbances, which are reported in up to 15% of hospitalized COVID-19 patients [6], and up to 40% in recovered COVID-19 patients [108]. Impairment of interoception is illustrated by the low level, or even the absence, of anxiety and fear responses often observed in COVID-19 patients who will shortly require mechanical ventilation [109]. This phenomenon is called “happy hypoxia” since very low blood oxygenation does not trigger dyspnea [110]. In a retrospective cohort of COVID-19 patients in Wuhan, only 20% complained of shortness of breath. Moreover, 62% of patients with severe COVID-19 and 46% of those who required intubation did not complain of any dyspnea [111].
The NTS sends efferences with synapses of the noradrenergic type to the amygdala’s central and basolateral nuclei [101,102]. Adrenergic stimulation of NTS contributes to increasing the retention of spatial or emotional memories [112]. Beyond the phenomenon described in COVID-19 patients as a dysregulated response to hypoxia, a lesion of the NTS could play a role in modulating this stress response. In addition, it seems that certain efferences from the tonsils are, in turn, involved in reaction mechanisms to hypoxia and acute exacerbation of respiratory diseases such as asthma involves the paraventricular nucleus [113]. The abnormal response of COVID-19 patients to hypoxia supports our brainstem hypothesis since this region plays a vital role in respiratory regulation.
Frequent clinical manifestations present in COVID-19 patients, as cardiac arrhythmias, maladaptive response to hypoxia, digestive disorders, or, more generally, lability in blood pressure, could also be linked with brainstem autonomic dysfunctions [81]. Finally, the brainstem dysfunctions can be associated with a pre-existing neurological disease, such as neurodegeneration in elderly patients and critical illness by itself [87]. A brainstem autonomic dysfunction can contribute to COVID-19 related mortality and morbidity by compromising the perception and integration of homeostatic disturbances, impairing the adaptive neuroendocrine, autonomic and behavioral response to stress, thus jeopardizing immune responses. More importantly, brainstem dysfunction in critically ill patients infected with SARS-CoV-2 could contribute to symptoms associated with long-term psycho-cognitive disorders [114, 115].
The nervous system regulates immune responses and inflammation through sensory neurons capable of detecting pathogen fragments, cytokines, and other immune signals. This detection generates immunoregulatory responses through efferent autonomic neuron signaling in a manner of reflex regulation that involves the vagus nerve, among others [116]. Along this line, the brain can integrate neuroimmune communication, and its functions could be altered by peripheral immune dysregulation and inflammation in the context of COVID-19, as described during other infectious diseases [89].
It is noteworthy that the NTS is a potential target of SARSCoV- 2, because it is connected to the olfactory tract, the trigeminal nerve, and the vagus nerve, thereby facilitating axonal propagation of the virus [117,118]. The NTS is also located at the vicinity of the area postrema, thus favoring the inflammatory response through the humoral pathway [87,119]. Figure 2 illustrates the brainstem’s potential role in the pathophysiology of SARS-CoV-2 infection, which is also suggested during other viral infections, notably by retrovirus (HIV and human T-lymphotropic virus), herpes viruses, flavivirus, enterovirus 71 and lyssavirus [87].
Assessing Brainstem and Autonomic Nervous System Dysfunctions in Critically Ill patients
As we previously described [87], assessment of brainstem dysfunctions relies essentially on non-invasive neurophysiological tests, including evoked potentials, blink-reflex and EEG. enables to assess brainstem conduction time, of which increase can result from structural or functional impairment, the somatosensory and brainstem auditory evoked potentials (i.e., SSEP and BAEP) could be recorded [120, 121]. Interestingly, it has been shown that SSEP P14-N20 inter-latency and BAEP III-Waves inter-latency are associated respectively with increased mortality and post-sedation delayed awakening or delirium in deeply sedated critically ill patients [122]. The electrophysiological testing of the blink reflex explores the trigeminal facial loop but its prognosis value in comatose patients needs to be confirmed. Interestingly, it has been recently shown that the response R2 of the blink reflex is impaired in mechanically ventilated COVID-19 patients [123], suggesting a dysfunction at the level of the pontomedullary circuitry involving the reticular formation. The EEG reactivity to auditory, visual or nociceptive stimuli involves also, but not exclusively, the ascending reticular activating system [124]. Its absence is associated with adverse outcomes, in various causes of coma, including deeply sedated critically ill patients [125].
The diagnosis of brainstem autonomic dysfunction relies on various methods, more or less invasive and feasible at bedside in ICU patients [33,126]. These methods and their application in COVID-19 patients have been recently addressed by the American Autonomic Society [127]. The pupillary diameter (PD) and pupillary light reflex (PLR) are regulated by both sympathetic and parasympathetic synergistic control in response to light intensity. The measurement of PD and dynamic changes of the PLR (latency and velocity of the response) with pupillometry enables to non-invasively assess the sympatho-vagal balance [128]. The analysis of the respiratory rate (RR), the tidal-volume variability, the diaphragm functions and patient-ventilator asynchronies [129,130] are useful for detecting impaired central control of the respiratory system.
Regarding the cardiovascular system, the assessments of the hemodynamic changes during a Valsalva test [131], a deep inspiration [132], anisometric grip, a cold test [133] or during orthostatism [134,135] are not easily performable in critically ill patients. Conversely, the measurement of heart rate variability (HRV) enables to non-invasively and at bedside assess the autonomic control of the cardiovascular system. This method is based on the analysis of the fluctuations of the RR intervals in the different frequency domains of ECG using, for instance, a Fast Fourier Transform. Each frequency domain is finely regulated and influenced by physiological functions such as respiratory rate, circadian rate, blood pressure or neuroendocrine system [136]. The HRV analysis is usually performed in the time or frequency domains, most often over a period of 24 hours or sometimes over a shorter period of 5 minutes to assess the influence of various external factors. For instance, the analysis in the frequency domains allows to identify the low frequencies (LF: 0.04- 0.15 Hz) that depends on the activity of baroreceptors and high frequencies (HF: 0.15-0.4 Hz) that depends on the variations of breathing and blood pressure. The LF/ HF ratio is therefore a marker of the sympathetic and parasympathetic activity balance [137,138].
Thermoregulatory activity can also be assessed by measuring sweating and sudomotor function, mediated by cholinergic postganglionic fibers of the sympathetic system. Several evaluation methods are available, including the sweating test that evaluates the change in color of powders (most often quinizarin) during sweating [139], the quantitative axonal sweat reflex test (QSART) [133], the sympathetic skin response (SSR) and the Sudoscan, the latter being the least invasive method based on measuring the cutaneous conductance [140]. Sudoscan is a recently developed technique using an applied electric current that induces a shift of chloride ions from the sweat glands to the skin surface. The conductance measured between the anode and a reference electrode is proportional to the chloride concentration, itself a reflection of the sweat capacity [141].
The co-monitoring of EEG characteristics in sleep or awake states and HR variability represents the “heart-brain coupling” which is controlled by the ANS afferences and efferences [142]. There are other methods for assessing the ANS, but they are more difficult to perform, to interpret, or they are more invasive. Thus, the measurement of circulating catecholamines [143] is feasible but their physiological significance questionable. Their measurement with radionucleotides like 123-metaiodobenzyl- guanidine [139] is more accurate but not feasible in routine. Microneurography is a quantitative method of the sympathetic activity of the post-ganglionic innervation of the muscle or skin vessels (i.e., MSSNA and SSNA for Muscle Skin Sympathetic Nerve Activity and Skin Sympathetic Nerve Activity, respectively), with the help of two electrodes inserted into the muscles or the skin fascia. The MSSNA reflects the vasoconstrictor signal that depends on baroreflex activity and arterial pressure level, while SSNA depends on skin vasomotor activity.
Conclusions and Perspectives
An increasing number of observations related to neurological manifestations bring compelling evidence about SARS-CoV-2 neurotropism. It is clear that not only SARS-CoV-2 has developed several strategies for neural system invasion, but also that certain key structures of the CNS, particularly the brainstem, are related to the onset, and maintenance, of some of the COVID-19 symptoms. Beyond the clinical consequences related to the CNS disorders, the capacity of SARS-CoV-2 (and its new variants) to affect brainstem might be a cornerstone to decipher their pathogenicity. The brainstem dysfunctions might result from a direct viral invasion and/or non-viral neuro-inflammatory or ischemic processes. Therefore, clinical examination and non-invasive methods such as pupillometer and spectral analyses of cardiovascular signals should be used more routinely at bedside in COVID-19 ICU patients. Speculatively, antiviral and immunomodulatory therapeutics could be proposed for targeting the underlying mechanisms of brain dysfunction. Plasma exchanges and high-doses of steroids should be proposed in COVID-19 patients in a similar way they are frequently used during auto-immune-like encephalitis. If for obvious reasons, pulmonary and vascular manifestations had received a lot of attention at onset of the outbreak, neurological-associated symptoms of the COVID-19, especially those concerning the structural and functional integrity of the autonomous nervous system, should receive further attention because of their direct or indirect impacts on the maintenance of homeostasis of many systems.
Acknowledgments
The authors thank the Per Fumum Endowment Fund for the financial support, and Gabriel Lepousez, Rim Hassouna and Françoise Lazarini for critical reading of the manuscript. The Lledo laboratory is supported by the « URGENCE COVID-19 » fundraising campaign of Institut Pasteur and by the SRA3-SPAIS seed grant call from Institut Pasteur Paris.
Competing Interest
The authors declare having no competing interests.
References
2. Yin R, Feng W, Wang T, Chen G, Wu T, Chen D, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. Journal of Medical Virology. 2020 Oct;92(10):1782-1784.
3. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. The Lancet Neurology. 2020 Sep;19(9):767-783.
4. Metlay JP, Waterer GW. Treatment of communityacquired pneumonia during the coronavirus disease 2019 (COVID-19) pandemic. Annals of Internal Medicine. 2020 Aug 18;173(4):304-305.
5. Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clinical Infectious Diseases. 2020 Jul 28;71(15):889-90.
6. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020 Jun 1;77(6):683-90.
7. Baj J, Karakula-Juchnowicz H, Teresinski G, Buszewicz G, Ciesielka M, Sitarz E, et al. COVID-19: specific and nonspecific clinical manifestations and symptoms: the current state of knowledge. Journal of Clinical Medicine. 2020 Jun;9(6):1753.
8. Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020 Jul 22;107(2):219-233.
9. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiological Reviews. 2018 Jan 1;98(1):477-504.
10. Varatharaj A, Thomas N, Ellul MA, Davies NW, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. The Lancet Psychiatry. 2020 Oct 1;7(10):875-82.
11. Meppiel E, Peiffer-Smadja N, Maury A, Bekri I, Delorme C, Desestret V, et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clinical Microbiology and Infection. 2021 Mar 1;27(3):458-66.
12. Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 Aug 25;95(8):e1060-70.
13. Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. Journal of the Neurological Sciences. 2020 Jul 15;414:116923.
14. Mahammedi A, Saba L, Vagal A, Leali M, Rossi A, Gaskill M, et al. Imaging of neurologic disease in hospitalized patients with COVID-19: an Italian multicenter retrospective observational study. Radiology. 2020 Nov;297(2):E270-3.
15. Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurology-Neuroimmunology Neuroinflammation. 2020 Sep 3;7(5).
16. Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM). Journal of Neurology. 2020 Oct;267:2799-802.
17. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020 Aug;296(2):E119-20.
18. Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Clinical review: critical illness polyneuropathy and myopathy. Critical Care. 2008 Dec;12(6):1-9.
19. Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, et al. Musculoskeletal consequences of COVID-19. Journal of Bone and Joint Surgery. 2020 Jul 15;102(14):1197-204.
20. Tang H, Pang S, Wang M, Xiao X, Rong Y, Wang H, et al. TLR4 activation is required for IL-17–induced multiple tissue inflammation and wasting in mice. The Journal of Immunology. 2010 Aug 15;185(4):2563-9.
21. Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020 Jun 2;94(22):959-69.
22. Hasani H, Mardi S, Shakerian S, Taherzadeh- Ghahfarokhi N, Mardi P. The novel coronavirus disease (COVID-19): a PRISMA systematic review and metaanalysis of clinical and paraclinical characteristics. BioMed Research International. 2020 2020 Aug 14;2020:3149020.
23. Zhang X, Cai H, Hu J, Lian J, Gu J, Zhang S, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. International Journal of Infectious Diseases. 2020 May 1;94:81-7.
24. Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurological Sciences. 2020 Sep 15:1-8.
25. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021 Jan 16;397(10270):220-32.
26. Samprathi M, Jayashree M. Biomarkers in COVID-19: An up-to-date review. Frontiers in Pediatrics. 2021 Mar 30;8:607647
27. Aamodt AH, Høgestøl EA, Popperud TH, Holter JC, Dyrhol-Riise AM, Tonby K, et al. Blood neurofilament light concentration at admittance: a potential prognostic marker in COVID-19. Journal of Neurology. 2021 Mar 20:1.
28. Kanberg N, Ashton NJ, Andersson LM, Yilmaz A, Lindh M, Nilsson S, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020 Sep 22;95(12):e1754-9.
29. Ding Y, He LI, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004 Jun;203(2):622-30.
30. Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. Journal of Experimental Medicine. 2005 Aug 1;202(3):415-24.
31. Li SW, Wang CY, Jou YJ, Yang TC, Huang SH, Wan L, et al. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-ß1 via ROS/p38 MAPK/STAT3 pathway. Scientific Reports. 2016 May 13;6(1):1-3.
32. Andries K, Pensaert MB. Virus isolated and immunofluorescence in different organs of pigs infected with hemagglutinating encephalomyelitis virus. American Journal of Veterinary Research. 1980 Feb 1; 41(2):215-8.
33. Matsuda K, Park CH, Sunden Y, Kimura T, Ochiai K, Kida H, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Veterinary Pathology. 2004 Mar; 41(2):101-7.
34. Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. Journal of Internal Medicine. 2020 Sep; 288(3):335-44.
35. Pascual-Goñi E, Fortea J, Martínez-Domeño A, Rabella N, Tecame M, Gómez-Oliva C, et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurology® Neuroimmunology & Neuroinflammation. 2020 Jun 25;7(5):e823.
36. Ferreira AD, Romão TT, Macedo YS, Pupe C, Nascimento OJ, Fellow of the American Academy of Neurology (FAAN). COVID-19 and herpes zoster coinfection presenting with trigeminal neuropathy. European Journal of Neurology. 2020 Sep;27(9):1748-50.
37. Najt P, Richards HL, Fortune DG. Brain Imaging in Patients with COVID-19: A Systematic Review. Brain, Behavior, & Immunity-health. 2021 Jul 2:100290.
38. Gaur P, Dixon L, Jones B, Lyall H, Jan W. COVID- 19-associated cytotoxic lesions of the corpus callosum. American Journal of Neuroradiology. 2020 Oct 1; 41(10):1905-7.
39. Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. The Lancet Neurology. 2020 Nov 1; 19(11):919-29.
40. DosSantos MF, Devalle S, Aran V, Capra D, Roque NR, Coelho-Aguiar JD, et al. Neuromechanisms of SARSCoV- 2: a review. Frontiers in Neuroanatomy. 2020 Jun 16;14:37.
41. Huang JS, Kunkhyen T, Liu B, Muggleton RJ, Avon JT, Cheetham CE. Immature olfactory sensory neurons provide behaviorally useful sensory input to the olfactory bulb. bioRxiv. 2021 Jan 1.
42. Nolte J. The human brain. Mosby/Elsevier. 1993 May.
43. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty. 2020 Dec;9(1):1-7.
44. Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Frontiers in Neurology. 2021 Jan 20;11:1860.
45. Daly JL, Simonetti B, Klein K, Chen KE, Williamson cMK, Antón-Plágaro C, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020 Nov 13;370(6518):861-5.
46. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertension Research. 2020 Jul;43(7):648-54.
47. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS chemical Neuroscience. 2020 May 7;11(11):1555-62.
48. Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, et al. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. BioRxiv. 2020 Jan 1.
49. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science Advances. 2020 Jul 31; 6(31):eabc5801.
50. Sia SF, Yan LM, Chin AW, Fung K, Choy KT, Wong AY, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020 Jul;583(7818):834-8.
51. Zhang AJ, Lee AC, Chu H, Chan JF, Fan Z, Li C, et al. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clinical Infectious Diseases. 2021 Jul 15;73(2):e503-12.
52. Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Gouilh MA, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain, Behavior, and Immunity. 2020 Oct 1;89:579-86.
53. Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020 Jul;583(7816):437-40.
54. de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Science Translational Medicine. 2021 Jun 2;13(596).
55. Durante MA, Kurtenbach S, Sargi ZB, Harbour JW, Choi R, Kurtenbach S, et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nature Neuroscience. 2020 Mar;23(3):323-6.
56. De Virgiliis F, Di Giovanni S. Lung innervation in the eye of a cytokine storm: neuroimmune interactions and COVID-19. Nature Reviews Neurology. 2020 Nov;16(11):645-52.
57. Chuyue DY, Xu QJ, Chang RB. Vagal sensory neurons and gut-brain signaling. Current Opinion in Neurobiology. 2020 Jun 1;62:133-40.
58. Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Frontiers in Immunology. 2017 Nov 2;8:1452.
59. Meneses G, Bautista M, Florentino A, Díaz G, Acero G, Besedovsky H, et al. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. Journal of Inflammation. 2016 Dec;13(1):1-1.
60. Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. American Review of Respiratory Disease. 1976 Feb;113(2):131-9.
61. Koarai A, Traves SL, Fenwick PS, Brown SM, Chana KK, Russell RE, et al. Expression of muscarinic receptors by human macrophages. European Respiratory Journal. 2012 Mar 1;39(3):698-704.
62. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Frontiers in Psychiatry. 2018 Mar 13;9:44.
63. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020 May 2;395(10234):1417-8.
64. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system?. Viruses. 2020 Jan;12(1):14.
65. Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochirurgica. 2020 Jul;162(7):1491-4.
66. Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerging Infectious Diseases. 2020 Sep;26(9):2016.
67. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Journal of Medical Virology. 2020 Jul;92(7):699-702.
68. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002 Jun;5(6):514-6.
69. Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Research. 2004 Dec;1(1):1-3.
70. Lawler NG, Gray N, Kimhofer T, Boughton B, Gay M, Yang R, et al. Systemic Perturbations in Amine and Kynurenine Metabolism Associated with Acute SARSCoV- 2 Infection and Inflammatory Cytokine Responses. Journal of Proteome Research. 2021 Mar 16;20(5):2796- 811.
71. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology and Infection. 2020 Jun 1;53(3):368-70.
72. Wojkowska DW, Szpakowski P, Glabinski A. Interleukin 17A promotes lymphocytes adhesion and induces CCL2 and CXCL1 release from brain endothelial cells. International Journal of Molecular sciences. 2017 May;18(5):1000.
73. Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-a is mediated by CCAAT/enhancer-binding protein family members. Journal of Biological Chemistry. 2004 Jan 23;279(4):2559-67.
74. Cao X. COVID-19: immunopathology and its implications for therapy. Nature Reviews Immunology. 2020 May;20(5):269-70.
75. Moon C. Fighting COVID-19 exhausts T cells. Nature Reviews Immunology. 2020 May;20(5):277-.
76. Abers MS, Delmonte OM, Ricotta EE, Fintzi J, Fink DL, de Jesus AA, et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021 Jan 11;6(1).
77. Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. 2015 Aug;43(4):495-501.
78. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. Journal of Virology. 2000 Oct 1;74(19):8913-21.
79. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chemical Neuroscience. 2020 Mar 13;11(7):995-8.
80. Dubé M, Le Coupanec A, Wong AH, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuronto- neuron propagation of human coronavirus OC43. Journal of Virology. 2018 Sep 1;92(17):e00404-18.
81. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. Journal of Medical Virology. 2020 Jun;92(6):552-5.
82. Tassorelli C, Mojoli F, Baldanti F, Bruno R, Benazzo M. COVID-19: what if the brain had a role in causing the deaths?. European Journal of Neurology. 2020 Sep;27(9):e41-e42.
83. Freund M, Walther T, und Halbach OV. Immunohistochemical localization of the angiotensin-(1–7) receptor Mas in the murine forebrain. Cell and Tissue Research. 2012 Apr;348(1):29-35.
84. Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin-(1–7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Molecular and Cellular Neuroscience. 2005 Jul 1;29(3):427-35.
85. Arrigo A, Mormina E, Calamuneri A, Gaeta M, Marino S, Milardi D, et al. Amygdalar and hippocampal connections with brainstem and spinal cord: A diffusion MRI study in human brain. Neuroscience. 2017 Feb 20;343:346-54.
86. Hurley RA, Flashman LA, Chow TW, Taber KH. The brainstem: anatomy, assessment, and clinical syndromes. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010 Jan;22(1):iv-7.
87. Benghanem S, Mazeraud A, Azabou E, Chhor V, Shinotsuka CR, Claassen J, et al. Brainstem dysfunction in critically ill patients. Critical Care. 2020 Dec;24(1):1-4.
88. Kenney MJ, Barman SM, Kocsis B, Gebber GL. Flexibility of the map of brainstem neurons with sympathetic nerve-related activity. InCentral Neural Mechanisms in Cardiovascular Regulation 1991 (pp. 37-54). Birkhäuser Boston.
89. Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annual Review of Immunology. 2018 Apr 26;36:783-812.
90. Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Comprehensive Physiology. 2011 Jan 17;6(3):1239-78.
91. Kotmanova Z, Simera M, Veternik M, Martvon L, Misek J, Jakus J, et al. GABA-ergic neurotransmission in the nucleus of the solitary tract modulates cough in the cat. Respiratory Physiology & Neurobiology. 2018 Nov 1;257:100-6.
92. Moraes DJ, Bonagamba LG, Zoccal DB, Machado BH. Modulation of respiratory responses to chemoreflex activation by L-glutamate and ATP in the rostral ventrolateral medulla of awake rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011 Jun;300(6):R1476-86.
93. Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. Journal of Comparative Neurology. 1984 Sep 10;228(2):168-85.
94. Gasparini S, Howland JM, Thatcher AJ, Geerling JC. Central afferents to the nucleus of the solitary tract in rats and mice. Journal of Comparative Neurology. 2020 Nov 1;528(16):2708-28.
95. Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. Journal of Comparative Neurology. 1980 Sep 15;193(2):435-65.
96. Moran TH, Ladenheim EE, Schwartz GJ. Within-meal gut feedback signaling. International Journal of Obesity. 2001 Dec;25(5):S39-41.
97. Blouet C, Schwartz GJ. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metabolism. 2012 Nov 7;16(5):579-87.
98. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metabolism. 2012 Sep 5;16(3):296- 309.
99. Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacologica Sinica. 2000 Jun 1;21(6):481-93.
100. Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1999 Jul 1;72(1):8-12.
101. Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory. 1999 Mar 1;71(2):232-9.
102. Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behavioral and Neural Biology. 1992 Jul 1;58(1):16-26.
103. Hilz MJ, Liu M, Roy S, Wang R. Autonomic dysfunction in the neurological intensive care unit. Clinical Autonomic Research. 2019 Jun;29(3):301-11.
104. Zoccal DB, Furuya WI, Bassi M, Colombari DS, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Frontiers in Physiology. 2014 Jun 25;5:238.
105. Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Research. 1995 Sep 25;693(1- 2):51-63.
106. Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. Journal of Applied Physiology. 2006 Aug;101(2):618-27.
107. Obiefuna S, Donohoe C. Neuroanatomy, nucleus gustatory. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. 2020 Aug 22.
108. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain, Behavior, and Immunity. 2020 Oct 1;89:594-600.
109. González-Duarte A, Norcliffe-Kaufmann L. Is’ happy hypoxia’in COVID-19 a disorder of autonomic interoception? A hypothesis. Clinical Autonomic Research. 2020 Aug;30(4):331-3.
110. Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation. 2020 Jul 14;142(2):101-4.
111. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020 Apr 30;382(18):1708-20.
112. Clayton EC, Williams CL. Noradrenergic receptor blockade of the NTS attenuates the mnemonic effects of epinephrine in an appetitive light–dark discrimination learning task. Neurobiology of Learning and Memory. 2000 Sep 1;74(2):135-45.
113. Chen Z, Liu NN, Xiao J, Wang YH, Dong R. The amygdala via the paraventricular nucleus regulates asthma attack in rats. CNS Neuroscience & Therapeutics. 2020 Jul;26(7):730-40.
114. Gousseff M, Penot P, Gallay L, Batisse D, Benech N, Bouiller K, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound?. Journal of Infection. 2020 Nov 1;81(5):816-46.
115. Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. Eneurologicalsci. 2020 Dec 1;21:100276.
116. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000 May;405(6785):458-62.
117. Garcia-Diaz DE, Jimenez-Montufar LL, Guevara- Aguilar R, Wayner MJ, Armstrong DL. Olfactory and visceral projections to the nucleus of the solitary tract. Physiology & Behavior. 1988 Jan 1;44(4-5):619-24.
118. Paton JF, Li YW, Deuchars J, Kasparov S. Properties of solitary tract neurons receiving inputs from the subdiaphragmatic vagus nerve. Neuroscience. 1999 Nov 1;95(1):141-53.
119. Hay M, Bishop VS. Interactions of area postrema and solitary tract in the nucleus tractus solitarius. American Journal of Physiology-Heart and Circulatory Physiology. 1991 May 1;260(5):H1466-73.
120. Agarwal S, Morris N, Der-Nigoghossian C, May T, Brodie D. The influence of therapeutics on prognostication after cardiac arrest. Current Treatment Options in Neurology. 2019 Dec;21(12):1-6.
121. André-Obadia N, Zyss J, Gavaret M, Lefaucheur JP, Azabou E, Boulogne Set al. Recommendations for the use of electroencephalography and evoked potentials in comatose patients. Neurophysiologie Clinique. 2018 Jun 1;48(3):143-69.
122. Azabou E, Rohaut B, Heming N, Magalhaes E, Morizot-Koutlidis R, Kandelman S, et al. Early impairment of intracranial conduction time predicts mortality in deeply sedated critically ill patients: a prospective observational pilot study. Annals of Intensive Care. 2017 Dec;7(1):1-2.
123. Bocci T, Bulfamante G, Campiglio L, Coppola S, Falleni M, Chiumello D, et al. Brainstem clinical and neurophysiological involvement in COVID-19. Journal of Neurology. 2021 Mar 18:1-3.
124. Azabou E, Navarro V, Kubis N, Gavaret M, Heming N, Cariou A, et al. Value and mechanisms of EEG reactivity in the prognosis of patients with impaired consciousness: a systematic review. Critical Care. 2018 Dec;22(1):1-5.
125. Benghanem S, Paul M, Charpentier J, Rouhani S, Salem OB, Guillemet L, et al. Value of EEG reactivity for prediction of neurologic outcome after cardiac arrest: Insights from the Parisian registry. Resuscitation. 2019 Sep 1;142:168-74.
126. Low PA. Testing the autonomic nervous system. InSeminars in neurology 2003 (Vol. 23, No. 04, pp. 407-422).
127. Figueroa JJ, Cheshire WP, Claydon VE, Norcliffe- Kaufmann L, Peltier A, Singer W, et al. Autonomic function testing in the COVID-19 pandemic: an American Autonomic Society position statement. Clinical Autonomic Research. 2020 Aug;30(4):295-7.
128. Troiani V. The future of quantitative pupillometry in health and disease. Clin Auton Res 30, 11-12.
129. Benarroch EE. Brainstem integration of arousal, sleep, cardiovascular, and respiratory control. Neurology. 2018 Nov 20;91(21):958-66.
130. Sampath V, Gowda MR, Vinay HR, Preethi S. Persistent hiccups (singultus) as the presenting symptom of lateral medullary syndrome. Indian Journal of Psychological Medicine. 2014 Jul;36(3):341-3.
131. Jaradeh SS, Prieto TE. Evaluation of the autonomic nervous system. Physical Medicine and Rehabilitation Clinics. 2003 May 1;14(2):287-305.
132. VAN DEN BERG MP, SMIT AJ. Bedside autonomic function testing in patients with vasovagal syncope. Pacing and Clinical Electrophysiology. 1997 Aug;20(8):2039-42.
133. Pierzchala K, Labuz-Roszak B. Selected methods for evaluating the autonomic nervous system. Wiadomosci Lekarskie (Warsaw, Poland: 1960). 2002 Jan 1;55(5- 6):325-31.
134. Benditt DG, Ferguson DW, Grubb BP, Kapoor WN, Kugler J, Lerman BB, et al. Tilt table testing for assessing syncope. Journal of the American College of Cardiology.1996 Jul 1;28(1):263-75.
135. Grubb BP. Pathophysiology and differential diagnosis of neurocardiogenic syncope. The American Journal of Cardiology. 1999 Oct 21;84(8):3-9.
136. Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. Journal of Physiological Anthropology. 2019 Dec;38(1):1-8.
137. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043-65.
138. Elghozi JL, Julien C. Sympathetic control of shortterm heart rate variability and its pharmacological modulation. Fundamental & Clinical Pharmacology. 2007 Aug;21(4):337-47.
139. Mathias CJ. Autonomic diseases: clinical features and laboratory evaluation. Journal of Neurology, Neurosurgery & Psychiatry. 2003 Sep 1;74(suppl 3):iii31-41.
140. Ziemssen T, Siepmann T. The investigation of the cardiovascular and sudomotor autonomic nervous system—a review. Frontiers in Neurology. 2019 Feb 12;10:53.
141. Krieger SM, Reimann M, Haase R, Henkel E, Hanefeld M, Ziemssen T. Sudomotor testing of diabetes polyneuropathy. Frontiers in Neurology. 2018 Sep 26;9:803.
142. Huang J, Ulke C, Sander C, Jawinski P, Spada J, Hegerl U, Hensch T. Impact of brain arousal and time-ontask on autonomic nervous system activity in the wakesleep transition. BMC Neuroscience. 2018 Dec;19(1):1-1.
143. Sinski M, Lewandowski J, Abramczyk P, Narkiewicz K, Gaciong Z. Why study sympathetic nervous system. J Physiol Pharmacol. 2006 Nov 1;57(Suppl 11):79-92.