Introduction
Environmental pollution is a significant concern in both developing and developed countries. The pollution load in the environment accumulates as a result of anthropogenic activity [1]. Both organic and inorganic contaminants are hazardous to living things, and their incidence and persistence have risen dramatically in current years. Human activities, in certain ways, are straining oceans, rivers, and other inland waterways to the point that their quality is substantially damaged. Toxic chemicals such as heavy metals, pesticides, endocrine disruptors, pharmaceutical chemicals, polyaromatic hydrocarbons, organic and inorganic solvents, pathogens and other pollutants reach water bodies, dissolve in them, drift in the water or sediment on the bed, follow-on water contamination [2]. Various approaches have been used to improve the quality of naturally available water to a quality appropriate for human use [3]. The use of cutting-edge nanotechnology in traditional engineering lays the door for practical advancements in innovative decontamination technology in this context.
Nanotechnology refers to the use of particles with sizes ranging from a few to hundreds of nanometers in various instruments, methods, and applications. This particle size offers unique physicochemical and surface properties that make it ideal for novel applications. Indeed, proponents of nanotechnology say that this field of research might aid in discovering answers to some of the world’s most urgent problems, such as ensuring a continuous supply of safe drinking water for a growing population. “Compared to conventional micrometresized adsorbents, nanomaterials have a much higher areato- volume ratio, resulting in enhanced photocatalytic activity, high adsorbent capacity, high removal efficiency, and rapid removal dynamics” [4].
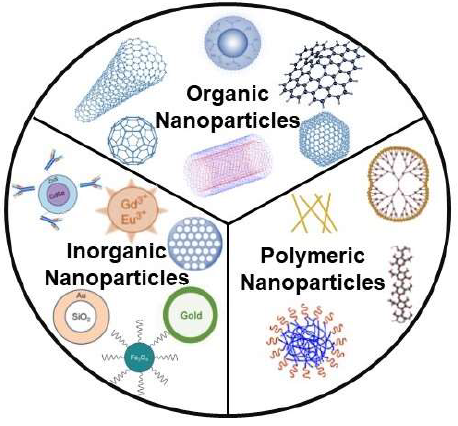
Sensing and detection, treatment and remediation, and pollution prevention are the three main aspects of nanoparticle-related water remediation. It’s also being looked at new novel ways that combine standard cleanup methods with a nanobiotechnological approach. This enables us to boost effectiveness. According to advanced research, nanotechnology has a promising future, either directly or indirectly, by incorporating nanotechnology into different fields [4]. In treatment and remediation, the primary processes used are photocatalysis, adsorption, nanomembranes and antimicrobial activities. A wide array of nanoparticles is used in water remediations, broadly classified into organic, inorganic, and polymeric nanoparticles [5]. The classification is represented in Figure 1.
Figure 1. Classification of nanoparticle in water purification [2].
Organic nanoparticles in water purification
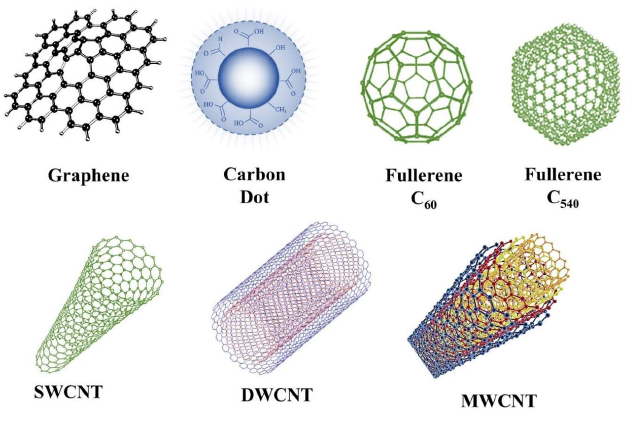
Organic nanomaterials have unique electrical, chemical, and physical properties due to elemental carbon’s structural and changeable hybridization state. “The major nano-structural forms of carbon include singlewalled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs), graphene, carbon dots, fullerene C60, and C540”[6]. Figure 2 schematically represents organic nanoparticles. Carbon dots (CDs) are trendy amongst nanomaterials based on carbon due to their biocompatibility, plenty of raw materials, low toxicity, and low cost. CDs are quasi-spherical carbon nanoparticles (NPs) with diameters less than 10 nm. CDs have attained considerable piety due to their excellent surface properties, chemical stability, excellent aqueous solubility, and fluorescence [4]. Carbon nanomaterials must first be surface modified and activated before they can be used in decontamination. For these uses, SWNT, MWNT, and graphene are usually utilized. These nanomaterials have exceptional adsorption characteristics, making them one of the best for the adsorption of organic and inorganic pollutants in contaminated water [7]. Based on the Surface modification and fabrication process, both physisorption and chemisorption are seen. Apart from adsorption, photocatalysis is another effective process for water cleanup. “Compared to Titanium oxide nanoparticles alone, Titanium oxide nanoparticles conjugated with graphene have demonstrated an increase in photocatalysis” [8].
Figure 2. Types of organic nanoparticles [2].
Inorganic nanoparticles in water purification
The major inorganic nanoparticle used in water remediation includes metal and metal oxide NPs (M&MO NPs) such as TiO2 NPs, Ag NPs, Fe and FeO NPs, CuO NPs and silica nanoparticles [9]. M&MNO NPs have excellent adsorption effectiveness for heavy metals and chlorinated organic water pollutants. M&MO NPs are unavoidable in remediation, particularly in aqueous environments, because of their rapid kinetics, adsorption capacity, and elasticity to adjust in both in-situ and ex-situ applications [9]. Mesoporous silica nanoparticles (SiO2 NPs) with pore diameters ranging from 2 to 50 nm have received much interest in the decontamination field. The adjustable pore diameter and ease of surface functionalization make SiO2 NPs perfect for an extensive range of uses. [10]-OH groups located on SiO2 NPs aid in surface modification and facilitate the decontamination of a wide variety of pollutants [11].
Polymeric nanoparticles in water purification
Even while nanoparticles offer numerous advantages, as we have shown, one of their limits is particle stability. Many studies indicate that following synthesis, nanoparticles can aggregate based on a variety of circumstances. Particle aggregation harms adsorption capacity. Polymeric NPs might be used as an alternative. In this case, we utilize a matrix or backing material to retain the NPs, which thwarts agglutination and improves the steadiness of pure nanoparticles. Stabilizing agents, surface-modification ligands, emulsifiers, and surfactants are all components of the polymeric host. Polymeric NPs are particles within the dimension of 1 to 1000 nm. Polymeric NPs are used in the detection and removal of a variety of contaminants, including heavy metals such as Fe, Hg, Mn, As, organic pollutants such as pesticides, pharmaceuticals, volatile organic compounds, other aliphatic and aromatic compounds, gases such as SO2, CO2, NO2, and microbes such as bacteria, viruses and other pathogens [12]. “Among the numerous concepts proposed, densely packed nanoparticle and nanofiber membranes, aligned nanotube membranes, self-assembled two-dimensional layer materials, and composites are among the most promising membrane technologies” [13].
Challenges and prospects
Water treatment research utilizing nanotechnology is now confined to laboratory and pilot-scale investigations, owing to the current economic distress, technical difficulties, high operational costs, and possible ecological and human harm [14]. A critical problem is the dearth of mechanistic investigation of nanomaterials employed in wastewater decontamination. Nanomaterials deposited on waterbodies might persist for a long time, potentially leading to biomagnification and entry into the ecosystem’s higher trophic levels. As a result, at various trophic levels, NPs can influence both species and interspecies interactions [15]. The processes causing these changes and their consequences for the wider food chain or entire ecosystems are still basically unknown. The lack of methods to transfer colloidal particles via the cell wall makes a protective barrier in prokaryotic cells surface, preventing many NPs from being absorbed. On the other hand, Eukaryotes have routes for nanoparticle and microparticle entry, such as endocytosis and phagocytosis [16]. As a result, it is critical to undertake environmental risk assessments of NPs during their lives. It’s vital to investigate various techniques to prevent NPs deposition on waterbodies and balance the environment abstaining toxicity. The fabrication of biogenic nanomaterials with features such as sustainability, low cost, and ecological dependability can significantly contribute to the purification of contaminated water in the future.
Even while commercial nanomaterial manufacturing is easy, ecologically sound, and can produce ultra-purity, it has the potential to modify physio-chemical characteristics of NPs, such as shape, stability, and pollutants remediation effectiveness. Although nanotechnology methods such as adsorption, photocatalysis, and membrane filtrations are highly successful in wastewater purification, their widespread use is restricted. As a result, further study is needed to solve these challenges, emphasizing largescale particle production and sustainability. Combining nanotechnology with other treatment approaches can help solve these issues and speed up the process.
The influence of NPs on industrial and non-industrial workrooms must also be investigated. NPs are far from equilibrium on account of their high surface energy. Thereby, NPs are extremely unstable, and they can easily change or interact with other chemicals to create a more stable state in specific conditions. NPs undergo advantageous or unfavorable changes in this fashion, follow-on in high reactivity and poor stability. This double nature of NPs has both constructive and destructive implications for NP processing. On the other hand, the current study has concentrated on NPs’ high reactivity, but their low stability has been neglected or rejected. As a result, future research should focus on the consequences of low stability NPs in wastewater purification and solutions for improving NPs stability.
So far, limited methods for recycling and reusing NPs have been discovered. These approaches are challenging to develop since they must be accessible, affordable, fast, and energy-efficient. The most common recovery method involves using magnets to remove Fe-containing NPs from contaminated water. And, while an insufficient strategy for removing, sorting, and reusing NPs from water media have been used, little study has been done on the subject. Future research should focus on the recyclability of NPs. [1] This might open the way for present NPs applications in a complex environmental setting.
Conclusion
Water purification is essential for sustainable development. Nanotechnology, among other sophisticated technologies, may be utilized to achieve this aim and cure previously untreatable and hazardous contaminants. Nanotechnology-based solutions have tremendous potential for increasing the competence and costeffectiveness of water purification, but more large-scale studies are needed. Low fabrication costs are critical for nanomaterials’ broad usage in water purification. Reducing the toxicity and enhancing stability is other import aspect for the applicability of nanoparticle. Therefore, future studies should improve their economic efficiency, enhance stability, reduce toxicity and assess the associated water treatment interaction processes.
Acknowledgement
Thankfully financial assistance is acknowledged for the INSPIRE fellowship of the Department of Science and Technology, New Delhi, India to AMP.
References
2. Ajith MP, Aswathi M, Priyadarshini E, Rajamani P. Recent Innovations of Nanotechnology in Water Treatment: A Comprehensive Review. Bioresource Technology. 2021 Sep 22:126000.
3. El-Sayed ME. Nanoadsorbents for water and wastewater remediation. Science of the Total Environment. 2020 Oct 15;739:139903.
4. Ajith MP, Priyadarshini E, Rajamani P. Effective and selective removal of heavy metals from industrial effluents using sustainable Si–CD conjugate based column chromatography. Bioresource Technology. 2020 Oct 1;314:123786.
5. Ajith MP, Aswathi M, Priyadarshini E, Rajamani P. Recent Innovations of Nanotechnology in Water Treatment: A Comprehensive Review. Bioresource Technology. 2021 Sep 22:126000.
6. Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environmental science & technology. 2008 Aug 15;42(16):5843-59.
7. Kharisov BI, Dias HR, Kharissova OV. Nanotechnologybased remediation of petroleum impurities from water. Journal of Petroleum Science and Engineering. 2014 Oct 1;122:705-18.
8. Zhang Y, Tang ZR, Fu X, Xu YJ. TiO2- graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: is TiO2- graphene truly different from other TiO2- carbon composite materials?. ACS nano. 2010 Dec 28;4(12):7303-14.
9. Bashambu L, Singh R, Verma J. Metal/metal oxide nanocomposite membranes for water purification. Materials Today: Proceedings. 2021 Jan 1;44:538-45.
10. Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, Bhatnagar A. Role of nanomaterials in water treatment applications: a review. Chemical Engineering Journal. 2016 Dec 15;306:1116-37.
11. Das SK, Khan MM, Parandhaman T, Laffir F, Guha AK, Sekaran G, et al. Nano-silica fabricated with silver nanoparticles: antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale. 2013;5(12):5549-60.
12. Tsekhmistrenko OS, Bityutskyy VS, Tsekhmistrenko SI, Kharchishin VM, Melnichenko OM, Rozputnyy OI, et al. Nanotechnologies and environment: A review of pros and cons. Ukrainian Journal of Ecology. 2020;10(3):162– 72.
13. Ying Y, Ying W, Li Q, Meng D, Ren G, Yan R, et al. Recent advances of nanomaterial-based membrane for water purification. Applied Materials Today. 2017 Jun 1;7:144-58.
14. Khan NA, Khan SU, Ahmed S, Farooqi IH, Dhingra A, Hussain A, et al. Applications of nanotechnology in water and wastewater treatment: A review. Asian Journal of Water, Environment and Pollution. 2019 Jan 1;16(4):81-6.
15. Nisticò R. Magnetic materials and water treatments for a sustainable future. Research on Chemical Intermediates. 2017 Dec;43(12):6911-49.
16. Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, et al. Cellular uptake of nanoparticles: journey inside the cell. Chemical Society Reviews. 2017;46(14):4218-44.