Abstract
Titanium dioxide (TiO2 ) based nano-sized photocatalysts (NPCs) were synthesized following a green method from the extract of Peepal (Ficus religiosa) leaves and titanium tetrachloride as precursors. Doping of TiO2 with Magnesium (Mg) was done using magnesium chloride through the method of chemical precipitation. Size of the NPC samples were estimated and characterized by CPS Disc Centrifuge, Diffracted Light Scattering (DLS) and Field Emission Gun Scanning Electron Microscopy (FEG-SEM) techniques. UV-visible spectroscopy was employed in the study of photocatalytic properties of the NPCs. A low-cost, simple photo-reactor was fabricated and photocatalytic degradation of Methyl Orange (MO) and Methylene Blue (MB) dye solutions was investigated. Reasonable degradation of the dyes was observed in the presence of the NPCs. It was therefore concluded that the NPCs studied in this work can be promising nanomaterials for the degradation of MO and MB dyes under visible light irradiation. Nevertheless, a detailed investigation of various factors influencing the processes involved will be required to optimize the conditions in order to achieve the best degradation efficiency.
Keywords
Titanium dioxide nanoparticles, Ficus religiosa, Photocatalytic degradation, Organic dyes
Introduction
Titanium oxide (TiO2), or titania, is a very important semiconducting material that has attracted great interests due to its unique physical and chemical properties. TiO2 exists in three major crystalline phases, namely, anatase, rutile and brookite. Out of these three, the brookite phase is not practically beneficial because it is stable only at low temperatures. On the other hand, relatively high chemical inertness of the rutile phase makes it a suitable choice in applications where stability is critical.
Chemically the anatase phase of TiO2 is the one with best response, especially in catalytic applications. Under appropriate excitation, it is generally capable of degrading common pollutants of water, such as, carboxylic acids and alcohols completely to carbon dioxide, water and simpler minerals. However, for it to be used more efficiently and effectively, certain properties, such as, particle size and shape, crystallinity, etc. can be modified. Moreover, incorporation (or, doping) of metal ions into host TiO2 has also been investigated extensively as a method to improve the photocatalytic activity [1-5].
Out of several applications associated with photocatalysis, the treatment of wastewater is perhaps the most notable one. Numerous verities of pollutants emitted as industrial wastewater can possibly be degraded through photocatalysis making it reusable [4-9]. Water being one of the most vital commodities, there is no wonder so many materials scientists around the globe are investigating photocatalytic remediation of polluted water mediated by TiO2 based materials.
Nanomaterials have a larger surface-to-volume ratio compared to their bulk counterparts. This has important consequences for all those processes that occur at the surface of a material, such as, catalysis and detection. Therefore, nano-sized TiO2 particles usually show improved photocatalytic activity. However, pure TiO2 is a wide bandgap semiconductor that absorbs in the UV region of spectrum. As a result, the photocatalysis with TiO2 can occur only under UV irradiation making it an expensive affair. A possible solution to this could be doping the host TiO2 with a material that reduces the bandgap in such a way that photocatalysis can take place with inexpensive sources of visible light [6].
As discussed above, the photocatalysis of TiO2 is a complex function of its physicochemical properties, including size, shape, surface characteristics and inner structure. Therefore, in order to enhance the photocatalytic efficiency, the TiO2 may be doped with suitable ions to reduce its band gap. At the same time, appropriate methods can be employed to synthesize nano-sized particles of the catalyst [3-5,10].
Many existing physical and chemical synthesis approaches require high radiation, highly toxic reluctant, and stabilizing agents, which can cause pernicious effects to the ecosystem. In contrast, green synthesis of metallic and metal-oxide nanoparticles is an eco-friendly method that involves the simple process of bio-reduction and therefore, requires relatively low energy to initiate the reaction. Consequently, it is also cost effective also [10-13].
Recently, environment friendly, green, biosynthesis techniques employing plant extracts have gained added consideration as a simple, efficient, cost effective and feasible method, and an excellent alternative mean to conventional methods of nanoparticles production. Many researchers have employed green synthesis process for preparation of metal/metal oxide nanoparticles via plant leaf extracts [10-16].
For plant leaf extract-based synthesis of nanoparticle, the extract is mixed with metal precursor solutions at different reaction conditions. The parameters determining the conditions of the plant leaf extract (such as types and concentration of phytochemicals, metal salt concentration, pH, and temperature) can be adjusted and tuned to optimize the rate of nanoparticle formation, their yield and stability. The leaf-extract based phytochemicals are known to usually reduce the precursor solutions into metal ions in short time, and they act also as a stabilizer for the nanoparticles produced[10-17]. For example, Fe-, Zn- and rare earth doped TiO2 [4,5], Mg-doped ZnO [18] and TiO2 [19] and many other pure, doped and modified nanomaterials are being investigated by a number of groups.
In the present study, we discuss the green-synthesis of pure, and Mg-ions doped, TiO2 nano-sized photo catalyst (NPC) particles obtained from reduction of titanium tetrachloride precursor solution using Peepal (Ficus religiosa) leaf-extract. Even though the magnesium doped titanium-oxide based nanomaterials have been studied by some groups, the method of synthesis of this material through simple, inexpensive green route, particularly using the Peeple leaf-extract is being reported for the first time. Moreover, as a part of potential applications, we also report the results of photocatalytic degradation of Methylene Blue (MB) and Methyl Orange (MO) dyes using these nano-photo-catalysts, under visible light illumination of a tungsten lamp.
Figure 1. Peepal tree leaves (Ficus religiosa) and Scientific Classification.
Materials and Methods
AR grade (99.5% pure) Titanium Tetrachloride (TiCl4) wasobtained from Loba Chemicals and AR grade Sodium Hydroxide (NaOH) pellets were obtained from SDFCL Fine-Chem Limited. LR grade Magnesium Chloride hexahydrate (MgCl2.6H2O) was obtained from Qualigens Company. All the chemicals were used without further purification. Solutions of 1 M NaOH and 1 M MgCl2 were also prepared separately using distilled water.
Preparation of Peepal leaf-extract
The Peepal leaf extract was prepared using distilled water. Fresh leaves were taken from the campus of B. K. Birla College of Arts, Science and Commerce (Autonomous) Kalyan. Leaves were thoroughly washed with distilled water and 10 g of these leaves was crushed in a Mortar-Pestle. Then 100 ml of distilled water was added to this and the solution was boiled for 45 min. Then the leaf extract solution was cooled, double filtered and centrifuged (REMI) at 5000 rpm for 5 min to remove all the debris and to obtain a clear solution.
Green synthesis of pure titanium dioxide nanoparticles
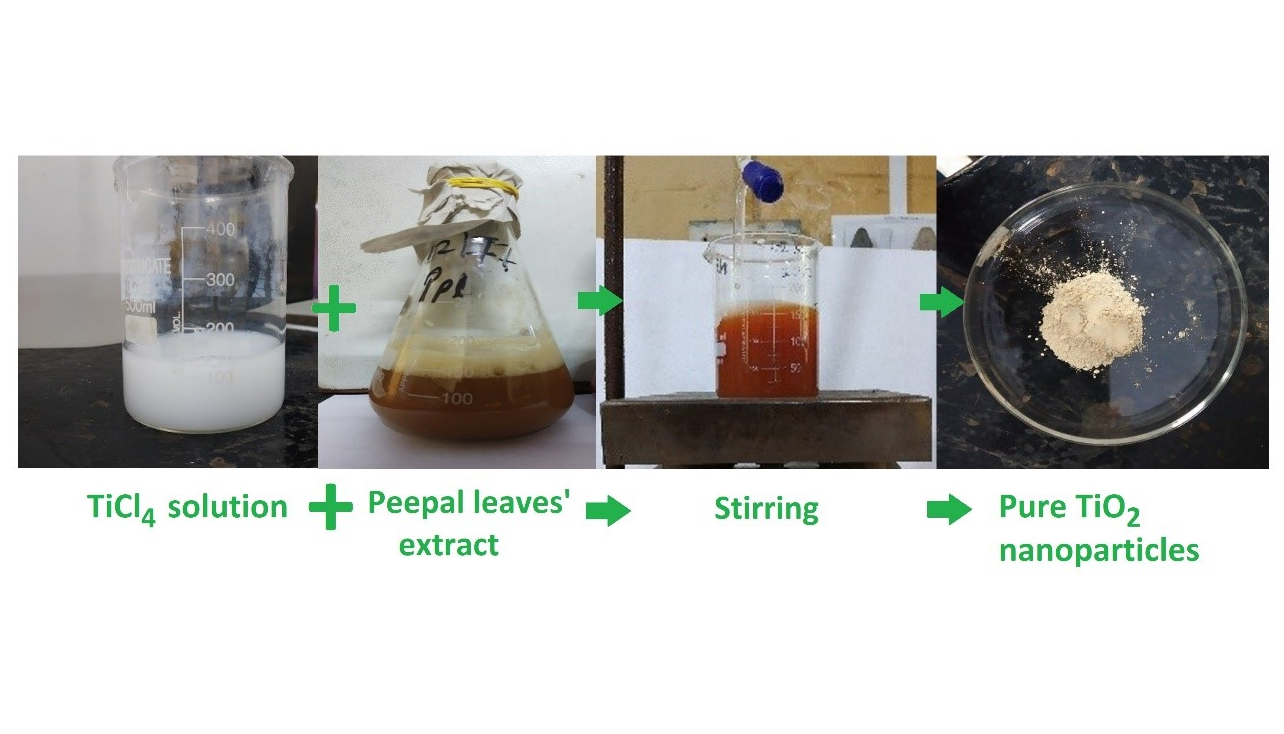
TiCl4 solution was prepared by adding 10 ml TiCl4 slowly into 100 ml of distilled water at 5 – 6°C. 100 ml of TiCl4 solution was slowly (drop wise) added to 100 ml of leaf extract on a magnetic stirrer (REMI 2MLH) at 500 – 600 rpm. The solution was magnetically stirred for 3 h and then sonicated for 2 h using an ultrasonic sonicator. The solution was kept overnight for settling. Next day, the solution was centrifuged at 5000 rpm for 10 min and supernatant was collected. The supernatant was calcinated (GRAVITY LAB INSTRUMENTS CO.) at 200°C for 6 h. White colored powder of TiO2 was obtained which was collected and stored (Figure 2). The off-white-colored product obtained was collected and stored for characterization. It is a simple, eco-friendly way and economical method for synthesis of titanium dioxide nanoparticles.
Figure 2. Various stages of green synthesis of TiO2 nanoparticles.
Chemical synthesis of Mg-TiO2 samples
Hundred ml of TiCl4 solution and 100 ml 1 M MgCl2 solution were mixed together, and 1 M NaOH solution (precipitate precursor) was added drop by drop to it until the pH value reached to 7, under continuous stirring on a magnetic stirrer (REMI 2MLH). The solution was kept stirring for 6 hours continuously. The solution was kept overnight for settling. Afterwards, the brown colored precipitate was filtered and dried at 100°C in hot air oven for 6 h. The precipitate was then calcinated (GRAVITY LAB INSTRUMENTS CO.) at 200°C for 6 h. The powder obtained was stored carefully for further analysis. The resulting brown powder obtained was collected and stored for further studies. It is a chemical way for synthesis of titanium dioxide based nanoparticles (Figure 3).
Figure 3. Various stages of synthesis of Mg-TiO2 nanoparticles.
Prior to investigation of degradation of dyes, the Mg-TiO2 samples (Mg ions loaded/doped TiO2 NPCs) were pre-treated in two different ways. NPC sample called D1 was stirred continuously for 6 h, and the one called D2 was stirred for 8 h, followed by a 4 h sonication.
Characterization of samples
Spectroscopic analysis was performed using an UV-Vis spectrophotometer (SYSTRONICS). The samples were analyzed in Quartz cuvettes of 1 cm path length, scanning from 200 to 700 nm wavelength. Distilled water was used as reference for the baseline correction.
Determination of particle size of samples was done using CPS Disc Centrifuge UHR (Model DC24000). The CPS Disc Centrifuge separated the particles by size using centrifugal sedimentation in a liquid medium. Prior to measurements, the unit was calibrated using 8% and 24% of standard sucrose solution. Then titanium dioxide-based NPC samples were injected one at a time using a syringe, and analysis was carried out.
- Field Emission Gun-Scanning Electron Microscopy (FEG-SEM) was employed to determine the size, shape and morphology of the NPCs. These analyses were done at Sophisticated Analytical Instrument Facility (SAIF), IIT Bombay on JEOL JSM-7600F FEG-SEM unit.
DLS sample analysis was done at Mumbai University using MALVERN PANANALYTICAL Zetasizer Nano range. The NPC particles were dispersed in distilled water and diluted. Prior to analysis, the solution was sonicated for 15 min in order to make the dispersion uniform.
Fabrication of photo-reactor
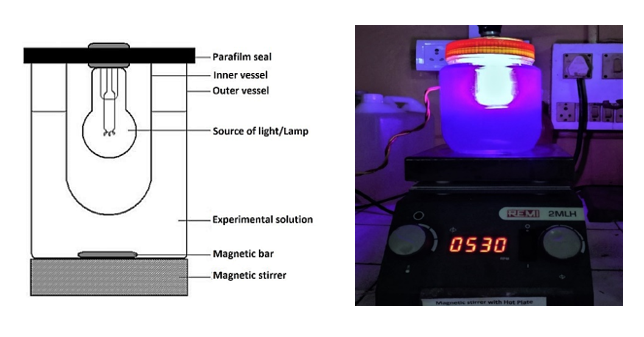
The visible–light photocatalytic activity was carried out in a locally fabricated, simple and inexpensive photo reactor [4,5,20] consisting of a 1 liter glass jar into which was placed a magnetic stirring bar and a smaller glass vessel that housed the light source dipped into the solution without touching directly (Figure 4). A ring attached to a stand was fitted on top of the inner glass vessel to hold it in place inside the beaker, which was placed on a magnetic stirrer-table. A 100 W Tungsten lamp (Phillips) was placed in the middle of this vessel so that it could not touch the sides and its end was at least 2 cm from the bottom of the inner vessel. Finally, the inner vessel was sealed with parafilm where it touched the top of the beaker.
Figure 4. Schematic diagram of photocatalytic reactor (a) and the photocatalytic rector (fabricated by Jaybhaye et al.) used in the present work (b).
Study of photocatalytic activity
Photocatalytic activity of the prepared NPC samples was examined in terms of the degradation of Methylene Blue (MB) and Methyl Orange (MO) dyes in solution under illumination with a tungsten incandescent lamp. Chemical structures of the two dyes are shown in (Figure 5).
Figure 5. The MB [7-(dimethylamino) phenothiazin-3-ylidene]-dimethylazanium; chloride; and MO Sodium; 4-[[4-(dimethylamino) phenyl]diazenyl]benzenesulfonate has formula C16H18ClN3S and C14H14N3NaO3S, respectively.
Experimental solutions with 10 ppm of both dyes were prepared. In the reactor 500 ml of dye solution, a magnetic stirring bar and 150 mg of nanoparticles were placed. For uniform distribution of nanoparticles throughout the solution, after addition of NPC sample the solution was kept stirring for 15 min, without switching on of bulb. Then the light was switched on to begin the processes. Stirring and lamp were both kept on continuously through the experiment.
Every 10 minutes, 5 ml of the experimental mixture was taken out, centrifuged, and the resulting clear solution was taken for recording its absorbance spectra. on a UV-Vis double beam spectrophotometer (SYSTRONICS). The baseline correction of UV-Vis double beam spectrophotometer was done using distilled water prior to the experiment.
For MB the wavelength range of recorded spectrum was 500 to 700 nm, and for MO it was 300 to 500 nm because the respective absorption peaks (660 nm for MB and 460 nm for MO) were expected within these ranges. The peak values of absorption data were used as a direct indicator of the dye concentration and therefore used to plot the degradation curves.
The percentage of degradation was estimated in terms of the relative concentration (C/C0) in percent fraction, of the dye left in solution after a given time from the beginning. Here, C0 is the initial concentration and C is the concentration at time t.
The experimental procedure was carried out for all NPC samples placing the reactor in dark conditions to minimize the effects of various external sources of light.
Results and Discussion
X-ray diffraction analysis
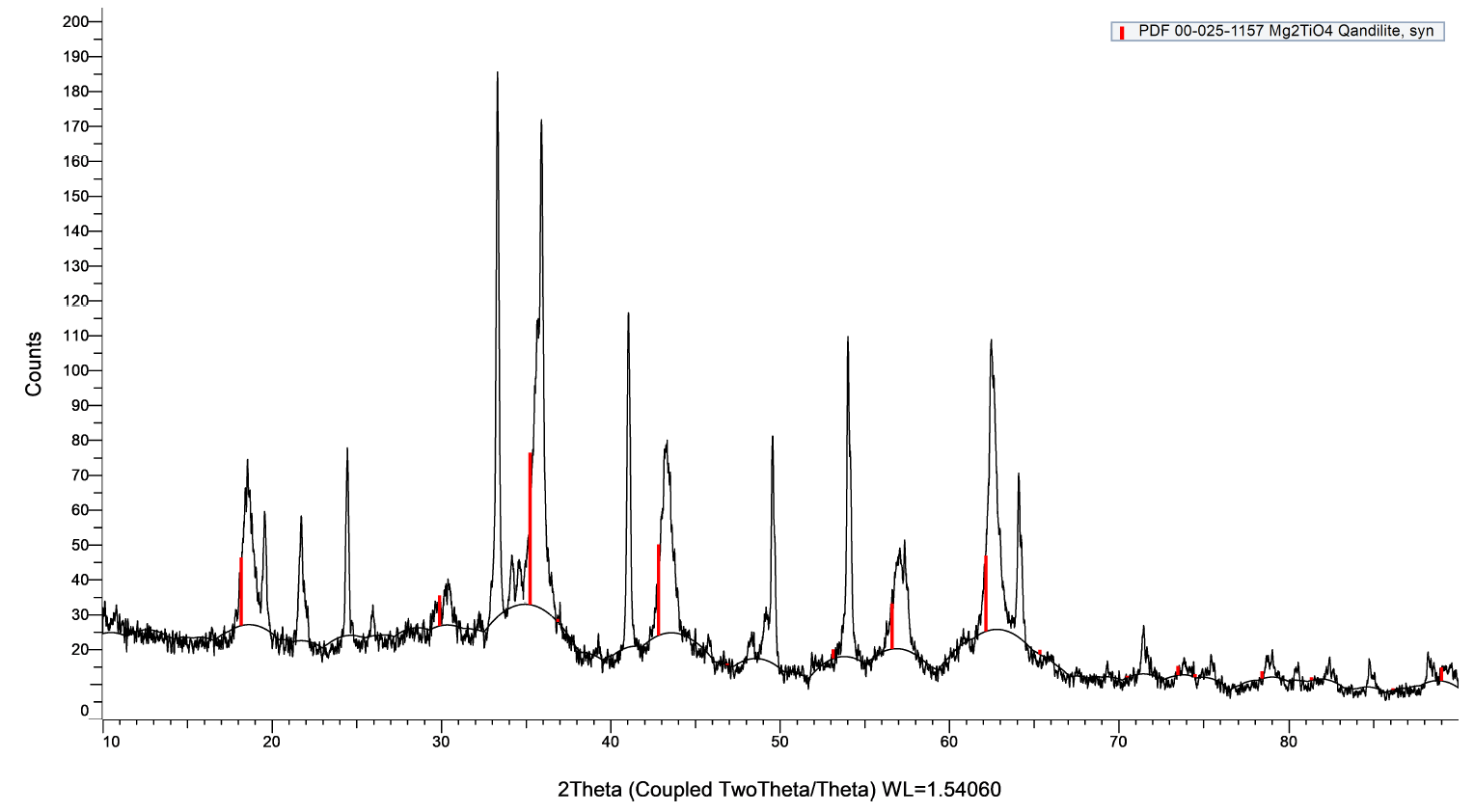
(Figure 6) shows the XRD patterns for the synthesized nanoparticles. A careful observation shows that rutile phase, with (110) peak around 27.7°, is more dominant phase of TiO2 in the synthesized NPCs. Formation of a magnesium titanate phase of Qandilite, (or, Mg2TiO4) is reasonably evident from the plot. The average particle size from the XRD data using Debye-Scherrer formula was estimated to be around 46 nm for a Gaussian peak fitting.
Figure 6. X-ray diffraction pattern the NPCs with PDF 00-025-1157 Mg2TiO4 peak positions shown in red.
Particle size analysis
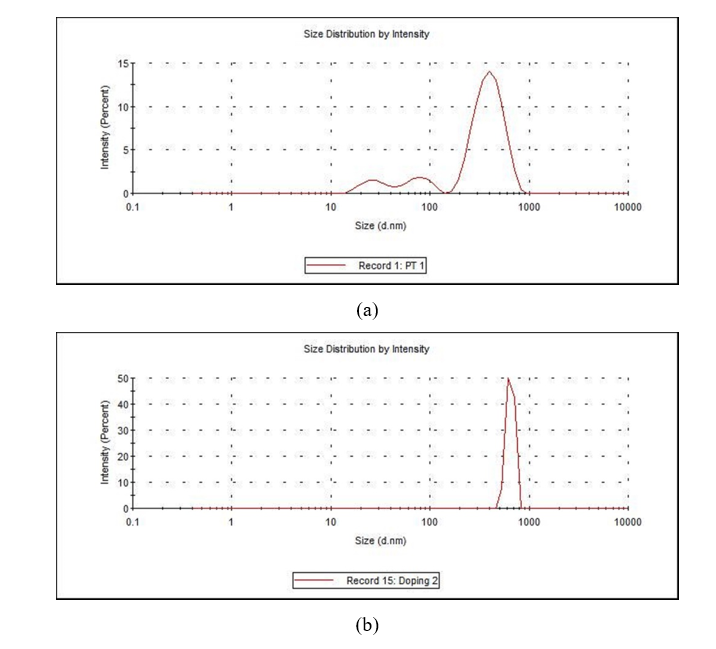
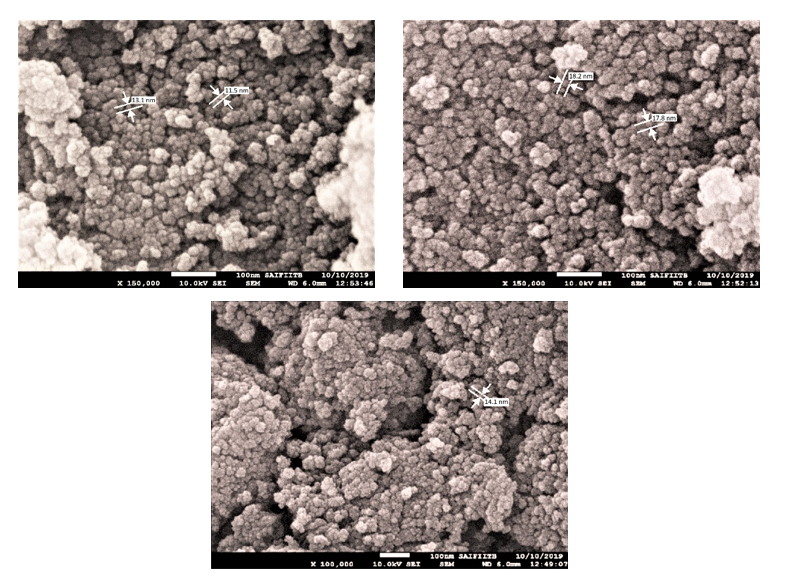
The average particle size of TiO2 nanoparticles was determined using CPS Disc Centrifuge particle size analyzer. The procedure for analysis was set in the software and the sample was processed. The particle of sample was found to be in range of 50 to 100 nm, with more than 90% particles having approximately 91 nm particle size. Another estimation of particle size was done through the DLS method, which gives an average mean value of size of sample. The (Figure 7 a) represents the average size distribution for TiO2 nanoparticles. It shows that the sample is polydisperse in nature. The size of TiO2 nanoparticles is >20 nm. The (Figure 7 b) represents the size distribution data for Mg-TiO2 nanoparticles. Due to agglomeration of the smaller grains of Mg-TiO2 particles, the average size of was estimated to be >500 nm. In order to further consolidate the observations, FEG-SEM was carried out, which not only estimated the size but also furnished visual information about the shape and morphology of the NPCs. The FEG-SEM image of TiO2 nanoparticle is shown in (Figure 8). The morphology of TiO2 nanoparticle shows that smaller particles of TiO2 NPCs have agglomerated into bigger grains. The agglomeration occurs because the TiO2 seems to be unstable in the nanoparticles form and it tends to join other nearby nanoparticles until their size becomes relatively stable. As evident from the pictures, the particle size of TiO2 NPCs is in the range from 11.5 nm to 20 nm. Clearly, the SEM images show the particles size whereas the DLS method estimates the agglomerated grains size.
Figure 7. (a) DLS of TiO2 Nanopar ticles; (b) DLS of Mg-TiO2 nanoparticles.
Figure 8. FEG-SEM images of TiO2 nanoparticles.
UV-Visible Spectroscopy
UV-Vis Spectroscopy was carried out as priliminary test to determine the absorption properties and characteristics maxima of the samples. The absorption spectrum of pure TiO2 nanoparticles showed a prominent peak at 377 nm and Mg-TiO2 nanoparticles exhibited a peak around 390 nm. Throughout the study, the formation of TiO2 nanoparticles and Mg-TiO2 nanoparticles was confirmed by this method. The absorption maximum was noted in the range 300-430 nm for pure TiO2 nanoparticles and for Mg-TiO2 nanoparticles the range was 250-450 nm.
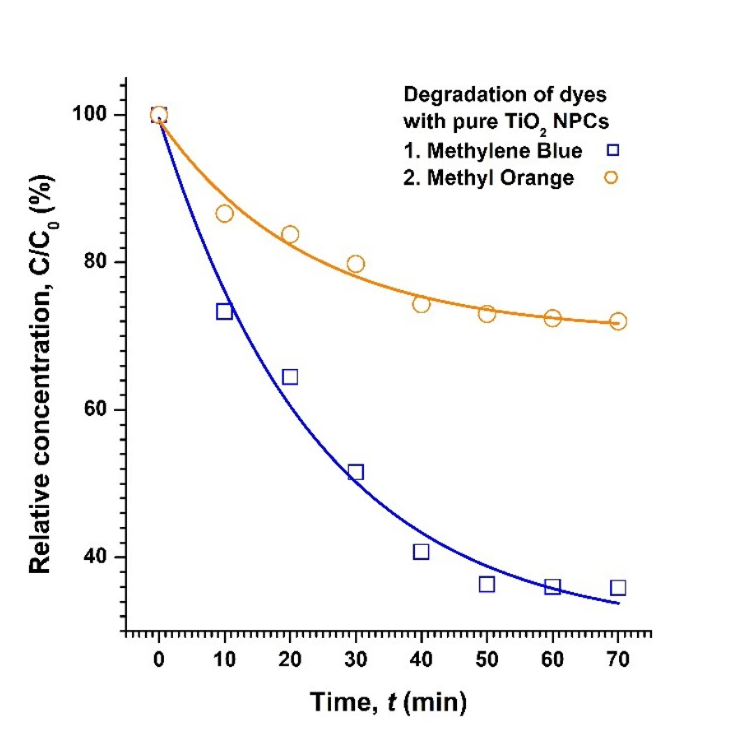
The graph depicted in (Figure 9) shows the comparative photocatalytic degradation of the dyes MB and MO in the presence of pure TiO2 NPCs. It is evident that the degradation of MB dye by pure TiO2 is better than that of MO dye as the NPC degrades MB by 64 %, but MO only by 28 % in approximately an hour time. These values also seem to be the maximum achievable degradation as the curve flattened out after 70 min.
Figure 9. Degradation of both MB and MO dyes in the presence of TiO2 NPC particles. The solid lines are just a guide to eyes.
From the data obtained, it was determined that the rate of degradation follows a first-order pattern for all NPCs. The observed rate-constant (kobs) was estimated from the slope of a linear regression analysis of ln (C/C0) versus time, and was found to be 1.51 × 10-2 min-1 and 0.44 × 10-2 min-1, for degradation of MB and MO dyes, respectively. It was also noted that before flattening-out after 50 minutes, the rate of degradation was marginally faster with (kobs) being 2.01 × 10-2 min-1 and 0.59 × 10-2 min-1, respectively.
Degradation of dyes with Mg-TiO2 nanoparticles
As mentioned earlier, prior to degradation experiments, the Mg-TiO2 NPC samples were pre-treated in two different ways: While both D1 and D2 samples were kept stirred for a long time, the latter was sonicated too.
(Figure 10) shows the degradation of MB and MO dyes in the presence of Mg-TiO2 NPC samples. The percent degradation was obtained by taking the difference between initial and final optical density of solution at 660 and 460 nm, respectively, for MB and MO dyes.
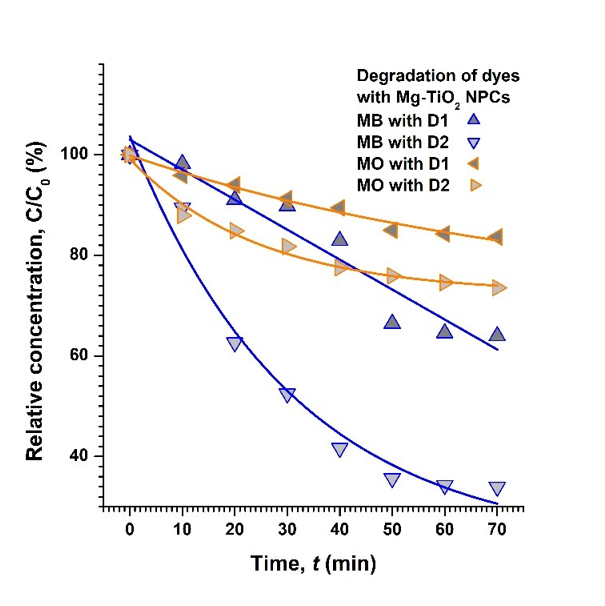
Figure 10. Degradation of both MB and MO dyes in the presence of Mg-TiO2 NPC particles. The solid lines are just guides to eyes.
On the other hand, it is observed that D1 Mg-TiO2 sample produced only about 16%, whereas D2 Mg-TiO2 led to nearly 26 % degradation of MO in approximately 1 h time. It may further be noted that the degradation of MO dye with pure TiO2 NPC is better than both D1 and D2 types of Mg-TiO2 NPC samples. Nevertheless, compared to the un-sonicated D1 sample, the sonicated D2 Mg-TiO2 NPC sample seems to be more active, and is nearly as efficient as pure TiO2 NPCs, as expected. Similar analysis of data gives the values of (kobs) as 0.74 × 10-2 min-1 and 1.70 × 10-2 min-1, respectively, for degradation of MB dye with D1 and D2 Mg-TiO2 NPC samples. On the other hand, for degradation of MO dye with D1 and D2 NPCs, the (kobs) was estimated to be 0.26 × 10-2 min-1 and 0.40 10-2 min-1, respectively.
Stirring of solution ensured that every part of the solution was exposed to the illumination from source of light for enhanced photo-excitation. On the other hand, the process of sonication breaks the agglomerated lumps into their constituents, smaller particles and grains, and also helps them disperse uniformly throughout the solution. Larger specific surface area due to the smaller particles and grains supports more adsorption, which in turn facilitates better photo-sensitization. As a result, the uniformly suspended, smaller NPC particles produce better degradation of both MB and MO dyes, as observed with D2 Mg-TiO2 NPC sample.
The probable mechanism of degradation could be attributed to SPR (Surface Plasmon Resonance) effect where excited surface electrons interact with the dissolved oxygen molecule and produce highly active hydroxyl radicals, while allowing nanoparticles interact with the ionic dyes. In terms of the basic constituents, the chromophores responsible for characteristic colours in dyes, i.e. MB and MO in present case, were broken down and thus the dyes degraded with in the presence of NPCs under visible light illumination. Therefore, it is evident that the synthesized NPCs have potential photocatalytic properties for the degradation of dyes in the presence of visible light.
Conclusion
Titanium dioxide-based nanoparticles can be successfully produced through biosynthesis, which is a simple, eco-friendly, less-toxic and inexpensive method of synthesis. In the available literature, sol-gel technique has been reported as the most popular method of synthesis, of these, and other similar, nanomaterials. In the present study, we employed the wet chemical method of synthesis of nano-sized pure TiO2 sample using the leaf extract of Peepal (Ficus religiosa) along with Mg doped TiO2 nanomaterials. The range of grain-size was estimated to be within 11 to 20 nm for the pure NPCs and up to 91 nm for the agglomerated particles, as confirmed from DLS and FEG-SEM results. For the Mg doped samples, the smaller grains agglomerated up to 500 nm sized particles.
As a potential application, the photocatalytic degradation of MB and MO dyes with the synthesized NPCs was carried out under visible light excitation. Degradation of these dyes has been reported, but under different sets of conditions, which could be a combination of various source of light (UV, visible, or both), differently doped TiO2, etc. In the present study, expected results of degradation were obtained. Pure TiO2 showed better degradation primarily because of the smaller particle size. Other possibilities for marginally inferior degradation shown by the doped NPCs may be attributed to (i) the higher recombination of photogenerated electrons with the holes, and (ii) inappropriate excitation wavelength emitted from tungsten lamp. Nevertheless, the results obtained from this study definitely call for more detailed investigations directed to the understanding of various factors that influence the final degradation efficiency, such as, particle size, doping type, excitation wavelengths, etc.
References
2. Linsebigler AL, Lu G, Yates Jr JT. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chemical Reviews. 1995 May;95(3):735-58.
3. Nyamukamba P, Okoh O, Mungondori H, Taziwa R, Zinya S. Synthetic methods for titanium dioxide nanoparticles: a review. titanium dioxide-material for a sustainable environment. 2018 Nov 5;10.
4. Narayan H, Alemu H, Macheli L, Thakurdesai M, Rao TG. Synthesis and characterization of Y3+-doped TiO2 nanocomposites for photocatalytic applications. Nanotechnology. 2009 Jun 3;20(25):255601.
5. Narayan H, Alemu H, Macheli L, Sekota M, Thakurdesai M, Gundu Rao TK. Role of particle size in visible light photocatalysis of Congo Red using TiO2·[ZnFe2O4] x nanocomposites. Bulletin of Materials Science. 2009 Oct;32(5):499-506.
6. Beydoun D, Amal R, Low G, McEvoy S. Role of nanoparticles in photocatalysis. Journal of Nanoparticle Research. 1999 Dec;1(4):439-58.
7. Barka N, Qourzal S, Assabbane A, Nounah A, Ait-Ichou Y. Photocatalytic degradation of an azo reactive dye, Reactive Yellow 84, in water using an industrial titanium dioxide coated media. Arabian Journal of Chemistry. 2010 Oct 1;3(4):279-83.
8. Salama A, Mohamed A, Aboamera NM, Osman TA, Khattab A. Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Applied Nanoscience. 2018 Feb;8(1):155-61.
9. Viswanathan B. Photocatalytic degradation of dyes: an overview. Current Catalysis. 2018 Aug 1;7(2):99-121.
10. Waghmode MS, Gunjal AB, Mulla JA, Patil NN, Nawani NN. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Applied Sciences. 2019 Apr;1(4):1-9.
11. Rao KG, Ashok CH, Rao KV, Chakra CS, Tambur P. Green synthesis of TiO2 nanoparticles using Aloe vera extract. International Journal of Advanced Research in Physical Science. 2015;2(1A):28-34.
12. Singh A, Gaud B, Narayan H, Kurkure R, Jaybhaye S. Eco-friendly synthesis of zinc oxide nanoparticles for rayon pulp. Proc NULISTICE. 2018:76-80.
13. Eswaran SG, Narayan H, Vasimalai N. Reductive photocatalytic degradation of toxic aniline blue dye using green synthesized banyan aerial root extract derived silver nanoparticles. Biocatalysis and Agricultural Biotechnology. 2021 Sep 1;36:102140.
14. Rahman A, Prasanna A. Characterization of silver nanoparticles biosynthesized using ficus religiosa plant leaf extract. Int Res J Eng Tech. 2018;5(12):272.
15. Kavitha KS, Baker S, Rakshith D, Kavitha HU, Yashwantha Rao HC, Harini BP, et al. Plants as green source towards synthesis of nanoparticles. International Research Journal of Biological Sciences. 2013 Jun;2(6):66-76.
16. Gupta V, Jaybhaye S, Chandra N. Biosorption studies of copper, chromium, lead and zinc using fins of Catla catla fish. International Journal for Research in Applied Science and Engineering Technology. 2017;5(4):902-9.
17. Gupta V, Jaybhaye S, Chandra N. Cauliflower leaves, an agro waste: characterization and its application for the biosorption of copper, chromium, lead and zinc from aqueous solutions. International Journal of Scientific Research in Science, Engineering and Technology 185431. 2018;5(4):169-74.
18. Sadaiyandi K, Kennedy A, Sagadevan S, Chowdhury ZZ, Johan M, Bin R, et al. Influence of Mg doping on ZnO nanoparticles for enhanced photocatalytic evaluation and antibacterial analysis. Nanoscale Research Letters. 2018 Dec;13(1):1-3.
19. Kaviyarasu K, Premanand D, Kennedy J, Manikandan E. Synthesis of Mg doped TiO 2 nanocrystals prepared by wet-chemical method: optical and microscopic studies. International Journal of Nanoscience. 2013 Oct 7;12(05):1350033.
20. Tatarko M, Tricker J, Andrzejewski K, Bumpus JA, Rhoads H. Remediation of water contaminated with an azo dye: an undergraduate laboratory experiment utilizing an inexpensive photocatalytic reactor. Journal of Chemical Education. 1999 Dec;76(12):1680-3.