Abstract
Cancer immunotherapies using plant virus nanoparticles (PVNPs) have achieved considerable success in preclinical studies. PVNP based nanoplatforms can be endogenous immune adjuvants and act as nanocarriers that stabilize and deliver cancer antigens and exogenous immune adjuvants. Although they do not infect mammalian cells, PVNPs are viruses and they are variably recognized by pathogen pattern recognition receptors (PRR), activate innate immune cells including antigen-presenting cells (APCs), and increase the expression of costimulatory molecules. Novel immunotherapy strategies use them as in situ vaccines (ISV) that can effectively inhibit tumor growth after intratumoral administration and generate expanded systemic antitumor immunity. PVNPs combined with other tumor immunotherapeutic options and other modalities of oncotherapy can improve both local and systemic anti-tumor immune responses. While not yet in clinical trials in humans, there is accelerating interest and research of the potential of PVNPs for ISV immune therapy for cancer. Thus, antitumor efficacy of PVNPs by themselves, or loaded with soluble toll-like receptor (TLR) agonists and/or cancer antigens, will likely enter human trials over the next few years and potentially contribute to next-generation antitumor immune-based therapies.
Keywords
Immunostimulatory reagent, Nanoparticles, Neoantigens, Pathogen-associated molecular patterns, Virus-like particle
Introduction
Plant virus nanoparticles (PVNPs) are increasingly recognized and studied for use in biomedical applications. PVNPs include plant virions with self-assembled capsid protein coats (PC) that encapsulate the virus genome, and virus-like particles (VLPs), a capsid without the viral genome. Both virions and VLPs are noninfectious for mammals and do not replicate within tumor cells or other mammalian cells [1]. Virions can infect appropriate plant hosts while VLPs cannot. PVNPs are considered nanoparticles because of their nanoscale size, which are generally in the range of 20-300 nm. PVNPs are useful in cancer therapy because they not only can act as vehicle for delivery of anticancer agents but some have strong immunostimulatory properties and support antitumor immunity [2]. The hollow structure and external protein surface of PVNP capsids allows loading of cancer therapy agents by genetic and/or physico-chemical engineering. In particular, PVNPs can be engineered to display antigen/epitopes, and/or encapsulate immune agents that further modulate immune cells and enable anti-tumor immunization [2,3]. These properties have been exploited for the generation of vaccines against chronic inflammatory conditions, neurodegeneration, allergies, cancer, bacteria, viruses (including COVID-19) and treatment of autoimmune diseases [4-6].
PVNPs are promising candidates for the development of next generation anticancer immunotherapies. When applied as nanocarriers, the PVNP formulations can go beyond the natural immune stimulatory properties of many PVNPs and deliver cancer antigens or exogenous adjuvants. When applied as immunostimulatory reagents, PVNPs can reprogram the tumor microenvironment from immunosuppressive to more immunostimulatory, which generates local and systemic antitumor immunity [7].
Delivery Strategies of PVNPs for Cancer Immunotherapy
PVNPs have been explored as a unique class of nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications [8]. PVNPs hold promise for cancer immunotherapies via delivery of cancer antigens, adjuvants, and modulation of the immune-suppressive tumor microenvironment (TME) [7]. Delivery strategies of PVNPs for cancer immunotherapy have focused on systemic administration, e.g. intraperitoneal (I.P.), intravenous (I.V.), or intratracheal routes [9-11] that often have challenging pharmacokinetics with insufficient delivery to tumors. Systemic delivery of PVNP cancer therapies has struggled with the reality that phagocytes avidly ingest PVNPs with which they interact [12-15]. Most systemically IV delivered nanoparticles are sequestered in phagocytes in liver or spleen and do not get to the tumor [13-16]. This show that routes of administration can influence the therapeutic efficacy of delivery technologies. A relevant strategy for PVNPs and other nanoparticles is direct intratumoral delivery, as is approved by the FDA for the oncolytic virus T-VEC [17]. One concept relevant to intratumoral immunotherapy is “in situ vaccination” (ISV). This simple strategy relies on three basic concepts in all cancer immunotherapy: 1) tumors are recognized by the immune system; 2) clinically identified tumors are protected from the immune system by local tumor-generated immune suppression; 3) any clinically effective immunotherapy must overcome this immune suppression.
Every vaccine has two components, antigen to be recognized by the immune system and immune adjuvant to stimulate response against microorganisms or cells that carry the antigen [7]. Tumor antigens are highly variable and generally quite specific for each patient, particularly patientspecific are “neoantigens” that are generated by mutations that are randomly generated in many tumors. ISV directly applies an immune stimulating adjuvant to the tumor, and depends on the antigens that are naturally within the tumor as the antigen source for the vaccine [18]. The goal is to disrupt the local immunosuppression, activate the myeloid cells within the tumor and thus stimulate an effective innate immune response against the treated tumor, and activate tumor-recognizing effector T cells that then circulate and increase the immune pressure on other metastatic tumors that were not directly treated [11]. When optimally done, this improved systemic antitumor immunity by itself or with other systemic immunotherapies, like checkpoint blockade, reduces or eliminates the metastatic disease that causes the preponderance of patient morbidity and mortality from most types of solid tumors.
In situ vaccination is not focused on local control of treated tumors, although it can accomplish that. Local control with surgery and/or radiation or other energy delivery like heat or high intensity focused ultrasound is generally quite successful at local tumor elimination. The true value of ISV is in generating expanded systemic antitumor immune responses. While PVNPs can be utilized as systemically-delivered therapy, and some examples are noted below, in situ vaccination using intratumoral delivery of PVNPs is the focus of most current interest.
As immunotherapy, ISV has advantages: rapid delivery since it is not patient specific and causes rapid reprogramming of the TME, so could be done between pathologic diagnosis and surgery; less expense since the amount of reagents used are much lower than what is needed for systemic immunotherapy; generally safe because again, while the reagents are immunostimulatory they are administered at low levels systemically, so the inflammatory response tends to be local. While it is true that different tumor locations vary in their ease of ISV delivery, it is also true that surgeons and interventional radiologists can safely inject tumors found in almost any anatomical location.
The importance of uniform intratumoral distribution of PVNP during treatment application is assumed but not tested, and may not be important for optimal efficacy. It is difficult to obtain uniform distribution with needle injections due to intratumoral heterogenicity and the generally high interstitial fluid pressure in tumors [19-21]. New administration options for localized immunotherapy are being developed, including passive and active microneedle patches and implantable scaffolds that degrade and release reagents [21,22]. Immunotherapeutic vaccines are generally administered multiple times; however, as with any treatment, each required treatment increases expense and has reduced compliance from patients. PVNPs of CPMV have also been applied as a slow-release formulation by forming aggregates with polyamidoamine generation 4 dendrimers (CPMV-G4) [8,39]. Comparing administration techniques with associated results should enable a better understanding of how administration affects ISV responses.
For PVNPs and other immune stimulating nanoparticles, the tendency to be ingested by phagocytes makes them more valuable for ISV [7]. Phagocytes such as monocytes, dendritic cells, macrophages, and neutrophils are almost always found in tumors where they have immune suppressive phenotypes. The tendency of phagocytes to ingest nanoparticles focuses the immunostimulatory properties on those cells, where that immune stimulation can change their phenotype from suppressor cells to myeloid effector cells that directly attack the tumor, and antigen presenting cells that ingest tumor antigens, travel to the draining lymph nodes and present antigen to activate antitumor T cell responses [23]. These stimulated tumor antigen-recognizing T cells expand in numbers and circulate to find and attack other tumors, generating systemic antitumor immunity and eventually immune memory.
Induction of Innate Immune Responses by PVNPs
Innate immune cells are activated primarily by exogenous pathogen-associated molecular patterns (PAMPs) or endogenous damage-associated molecular patterns (DAMPS), that stimulate pattern recognition receptors (PRRs) [24,25]. PRRs include membrane, endosomal, cytoplasmic and soluble PRRs that upon interaction with their ligands alter gene expression of the cell [25,26]. One well-studied class of PRRs are the toll-like receptors (TLRs) that primarily recognize PAMPs. TLRs are localized on the cell surface (e.g. TLR1/2/4/5/6) and within endosomes (e.g. TLR3/7/8/9). Binding of their ligands activates signaling pathways that in turn activate transcription factors such as nuclear factor-kappa B (NF-κB) and IFN regulatory factors (IRFs) which stimulate transcription and secretion of inflammatory cytokines, chemokines and type I IFNs [27]. A variety of lab-generated molecules have been developed as TLRs agonists such as polyinosinic: polycytidylic acid (poly (I: C)) for TLR3, imiquimod (R837) and resiquimod (R848) for TLR7/8 and CpG oligodeoxynucleotides (ODNs) for TLR9 [28]. In general, the endocytic TLRs recognize nucleic acids and the surface TLRs recognize proteins or other complex molecules, like lipopolysaccharides.
Many PVNPs are recognized by innate immune cells as nonself, although with varying levels of stimulatory responses. This recognition is perhaps not surprising, since they are viruses, although mechanistically it is minimally studied. Questions such as why some PVNPs are strongly immune stimulatory while other apparently quite similar PVNPs have minimal recognition are not yet well understood. Immune recognition of PVNP depends on various characteristics such as particulate nature, repetitive protein structure, and nucleic acid content [29]. It was shown that PVNP capsids with an organized regular spatial structure are more immunogenic than disassembled capsids and their associated coat proteins (CP) [11,29]. The nanoparticle nature of PVNPs stimulates phagocytosis by various phagocytic cells, including antigen presenting cells (APC) [29,30] but tendency for phagocytic uptake also varies for unclear reasons. A variety of PVNPrelated structures including native virions, VLP, spherical nanoparticle (SNP) and CP of some PVNP (Figure 1A) can serve as adjuvants and induce APC activation and expression of antiviral and proinflammatory cytokines [7,29,31]. PVNPs can activate surface TLRs, while endocytosis of PVNPs containing nucleic acid by APCs contribute to an immune response by stimulating endosomal TLRs (Figure 1B) [7,32,33]. One aspect of note is that unlike most animal viruses, plant viruses are not enveloped, which may facilitate recognition by some PRR receptors, such as TLR4, that generally do not recognize enveloped mammalian viruses.
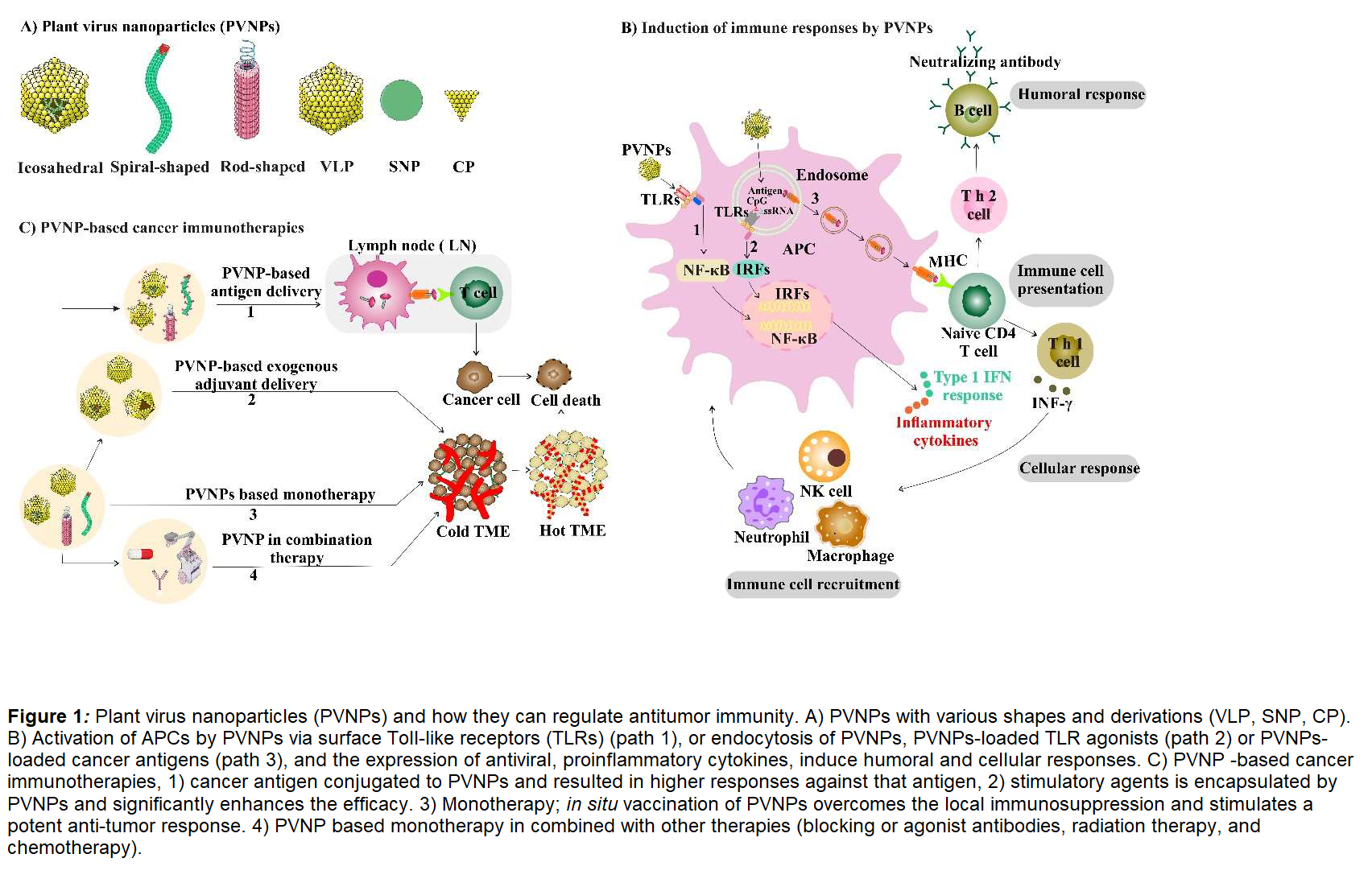
Basic mechanisms of PVNP ISV in treating solid tumors can be summarized by a series of mechanistic steps: 1) the PVNP is delivered directly into the tumor; 2) the PVNP is taken up by various innate immune cells, particularly phagocytes, which become activated; 3) the activated innate cells release cytokines and chemokines that attract greater numbers of activated innate cells to infiltrate the tumor and attack tumor cells 4) T-lymphocytes are presented antigen by the activated APCs in tumor draining lymph nodes, become activated, are attracted to the tumor and attack tumor cells carrying their cognate antigens leading to tumor lysis, 5) activated T-lymphocytes travel systemically and attack metastatic tumors throughout the body [8].
Multifunctional PVNPs for Cancer Immunotherapies
Antitumor immunity efficacy of PVNP is achieved via disruption of the immunosuppressive TME because of immune adjuvant activity with or without cancer antigen delivery [7]. PVNP-based cancer vaccines can be used to induce tumor associated antigen-specific immune responses by targeting tumor cells expressing cancer-driving receptors. Recent studies reported on human epidermal growth factor receptor-2 (HER2)-specific cancer vaccines using different PVNPs such as icosahedral cowpea mosaic virus (CPMV), cowpea chlorotic mottle virus (CCMV), Sesbania mosaic virus (SeMV), Physalis mottle virus (PhMV) and filamentous potato virus X (PVX) [3,34-36]. CPMV has also been used for delivering immunogenic cancer-associated testis antigen NY-ESO-1 [37].
While these PVNPs vary in ability to serve as immune adjuvants, they differ in other properties, including ability to be manipulated to carry other molecules. PVNPs can be modified to improve antitumor efficacy via encapsulation of a soluble adjuvant so that it has nanoparticle properties, which include preferential ingestion by phagocytes. For example, loading CCMV with CpG oligonucleotides promotes activation of tumor associated macrophages (TAMs) ex vivo and in vivo [38]. While in-situ vaccination using PVNPs produces significant treatment efficacy as cancer therapy, effects of systemic administration are weak because they are sequestered away from the tumor by the mononuclear phagocyte system [13-15].
One outcome of repeated delivery of foreign proteins like PVNPs is generation of antibodies against the proteins. This will “neutralize” oncolytic viruses since their impact depends on productive infection of the mammalian cells. However, since PVNPs do not infect mammalian cells, they are not “neutralized” by anti-PVNP antibodies. Interestingly, the presence of anti-CPMV antibodies not only did not inhibit the efficacy of CPMV-based ISV, but rather improved the antitumor efficacy [14]. While not extensively studied, this makes sense since antibody coating (opsonization) of a nanoparticle is expected to increase both ingestion by phagocytes as well as stimulation and activation of the ingesting phagocytes.
CPMV based monotherapy
CPMV has the been the most well-studied PVNP and is a more potent immune stimulatory reagent than most PVNPs, for reasons that are not fully understood [21]. CPMV is an icosahedral single-stranded RNA (ssRNA) virus that is rapidly ingested by phagocytes in vitro and in vivo [40]. CPMV is recognized by MyD88-dependent toll-like receptors (TLRs). The assembled capsid is recognized by TLR2 and TLR4 and the encapsidated ssRNA is recognized by TLR7 which uniquely induces secretion of type I interferons (IFNs), and contributes to CPMV’s local tumor efficacy [31]. CPMV used for ISV upregulates immunostimulatory cytokines including IL-1β, IL-12, interferon (IFN)-γ, chemokine ligand 3, macrophage inflammatory protein-2, and granulocytemacrophage colony-stimulating factor as well as suppressing IL-10 and transforming growth factor β [11,30,41]. These changes in intratumoral cytokines are generated by the changed phenotype of intratumoral myeloid cells and also mediate further activation, repolarization and recruitment of macrophages, DCs and neutrophils with an effector antitumor phenotype [41]. CPMV-ISV treatment significantly improves effector and memory CD4+ and CD8+ T cell responses and promotes systemic tumor-specific cytotoxic CD8+ T cell activity [41]. CPMV-based ISV treatment efficacy has been shown in mouse models of ovarian, breast, colon cancer, glioma, and melanoma [11,30,42-45] as well as in companion dogs with spontaneous tumors of multiple types [46]. CPMV, as a prophylactic and therapeutic immunotherapy can be used to target S100A9, a calcium-binding protein and suppressor of the tumor microenvironment, preventing manifestation of lung metastasis [47].
In vitro stimulation of CD14+ human monocytes with CPMV resulted in the induction of HLA-DR, CD86, PD-L1, IL-15R, CXCL10, MIP-1a and MIP-1b. CPMV also caused activation of dendritic cells and monocyte-derived macrophages. These findings demonstrated that CPMV activates human monocytes via Syk signaling, endosomal acidification, and recognition by Toll-like Receptor (TLR) 7/8. These findings support the potential for CPMV ISV to be an effective immunebased approach in humans [48].
Empty CPMV (eCPMV) based monotherapy
eCPMV is an RNA-free VLP that induces an antitumor response that requires Th1-associated cytokines IL-12 and INF-γ, adaptive immunity, and neutrophils for full effect [12]. eCPMV treatment was superior in direct comparison to high dose LPS, poly(I:C), and STING agonist [11]. Thus, the immunostimulatory effect does not require on TLR stimulation by RNA and eCPMV is recognized by MyD88-dependent TLR2 and TLR4 but not TLR7 [31]. However, recent studies clearly show that RNAcontaining CPMV is more effective in treating local tumors since it does stimulate TLR7 and generate type I IFN [15]. The value of TLR7 and associated type I IFN in generating systemic antitumor immunity is not yet demonstrated.
Papaya mosaic virus (PapMV) based monotherapy
Rod-shaped PapMV contains single stranded RNA (ssRNA) that is primarily responsible for inducing human peripheral blood mononuclear cells (PBMC) to secrete type I interferon alpha (IFNα), IL-6 and other pro-inflammatory cytokines and chemokines. Internalization and disassembly of PapMV nanoparticles into the endosome leads to the release of ssRNA that can activate TLR7 and/or 8 and induce a strong immune response [33]. Intra-tumoral administration of PapMV significantly prolonged survival and correlated with enhanced chemokine and proinflammatory cytokine production in the tumor and increased immune cell infiltration [10].
PVX based monotherapy
Flexible rod-shaped PVX has been studied as an immunotherapeutic for ISV monotherapy. In the context of B16F10 melanoma, PVX -based ISV can delay tumor progression, and with chemotherapies can be amplified [23].
Tobacco mosaic virus (TMV) based monotherapy
TMV and TMV-short can elicit potent antitumor immunity after intratumoral treatment of dermal melanoma via strong pro-inflammatory cytokines, primarily IL-6, and the recruitment of innate immune cells and T cells. The treatment slowed tumor growth and increased survival time. [30].
Alfalfa mosaic virus (AMV) based monotherapy
AMV is mixture of two bacilliform and spherical phenotypes, which encapsulate the virus genome. We investigated AMV as ISV in 4T1 mouse breast cancer, which is a very difficult mouse cancer model to treat with immunotherapy because of its recruitment of exceptional numbers of suppressive myeloid cells. AMV induced a potent immune response, which significantly delayed growth of this very challenging model. Response was characterized by IFN-γ, INF-α, IL-6, and IL-12 cytokines, and infiltration of CD4+ and CD8+ T cells at the treated site [49].
PVNP-based Cancer Immunotherapies Combined with Other Cancer Therapies
Single reagent immunotherapy (monotherapy) is prevalent using checkpoint blocking antibodies against PD-1 or PD-L1, however most patients do not respond to single-approach immunotherapy. Currently, even responding patients generally eventually develop resistance, disease recurrence, and many patients have the treatment limited by toxicity due to autoimmunity caused by systemic checkpoint blockade administration [50,51]. It is increasingly recognized that combining immune therapies is the next stage of cancer immune therapy, just as combining chemotherapies dominates cancer chemotherapy [51]. The multiple advantages of ISV noted above make this a prime candidate for combination with checkpoint blockade and other developing systemic immunotherapies for solid tumors.
PVNP ISV contributes to combinatorial immune therapy by expanding the pool of antitumor effector T cells. This expanded T cell pool could be combined with immune checkpoint therapy, radiation therapy and chemotherapy to reduce tumor burden, prolong survival, and support expanded tumor-specific immune memory (Figure 1C). For example, intratumoral injected CPMV in combination with selected T cell focused antibodies; PD-1 blocking antibodies, or agonistic OX40-specific antibodies, and myeloid cell-focused CD47- blocking antibodies activates and recruits innate immune cells, thereby reprogramming the immunosuppressive tumor microenvironment toward an immune-activated state [52-54]. Utilizing combination radiation therapy (RT) with immunostimulatory CPMV suggests that CPMV in combination with RT can turn an immunologically “cold” tumor (with low number of tumor infiltrating lymphocytes) into an immunologically “hot” tumor [50]. Combination of CPMV and RT was tested and had efficacy in companion dogs with melanoma [55]. PVX immunotherapy and doxorubicin chemotherapy are best when co-administered separately into the tumor, allowing each drug to act on their own, leading to potent antitumor effects [56]. CPMV based ISV combined with cyclophosphamide reduced breast cancer tumor burden and inhibits lung metastasis [57].
Conclusions
Studies show that PVNPs with their inherent immunostimulatory nature are valuable reagents for in situ vaccination and can be utilized as nanocarriers of tumor antigens to generate strong and sustained anti-tumor immune response without need for additional adjuvants. With nanoengineering, future designs could fuse the tumor antigens into CP of PVNPs, thus providing a means of costeffective manufacture. PVNPs can induce antitumor responses in tumor models when administrated into a TME as an in situ vaccine, which alters the tumor microenvironment to an antitumor state, generating large numbers of tumor-specific effector T cells that supports systemic antitumor immunity and immune memory. Furthermore, significant therapeutic efficacy with prolonged survival can potentially be achieved when PVNPs-ISV combine with chemotherapy, radiotherapy, other option immunotherapies. While the wild type viruses are the current focus of research, PVNP engineering strategies may improve functionality and associated efficacy. Preclinical capabilities of PVNPs indicate that some PVNPs are more suitable for tumor immunotherapies than others and understanding the mechanisms of immune activation and relevant complex differences between PVNPs will set the stage for successful clinical development of PVNPs as a platform for cancer immunotherapy.
Acknowledgements
Support from NIH grants from the National Cancer Institute of the US National Institutes of Health, grant numbers U01CA218292, R01CA224605, 1R01CA253615 to SF and support from Dartmouth Norris Cotton Cancer Center 5P30CA023108-41.
Conflict of Interest
Dr. Fiering is cofounder and has a financial interest in Mosaic Immunoengineering Inc.
References
2. Shahgolzari M, Pazhouhandeh M, Milani M, Yari Khosroushahi A, Fiering S. Plant viral nanoparticles for packaging and in vivo delivery of bioactive cargos. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2020 Sep; 12(5):e1629.
3. Santoni M, Zampieri R, Avesani L. Plant virus nanoparticles for vaccine applications. Current Protein and Peptide Science. 2020 Mar 1; 21(4):344-56.
4. Zampieri R, Brozzetti A, Pericolini E, Bartoloni E, Gabrielli E, Roselletti E, et al. Prevention and treatment of autoimmune diseases with plant virus nanoparticles. Science Advances. 2020 May 6; 6(19):eaaz0295.
5. Zeltins A, West J, Zabel F, El Turabi A, Balke I, Haas S, et al. Incorporation of tetanus-epitope into virus-like particles achieves vaccine responses even in older recipients in models of psoriasis, Alzheimer’s and cat allergy. npj Vaccines. 2017 Oct 23; 2(1):1-3.
6. Ortega-Rivera OA, Shin MD, Chen A, Beiss V, Moreno-Gonzalez MA, Lopez-Ramirez MA, et al. Trivalent subunit vaccine candidates for COVID-19 and their delivery devices. Journal of the American Chemical Society. 2021 Sep 7; 143(36):14748-65.
7. Shahgolzari M, Dianat-Moghadam H, Fiering S. Multifunctional plant virus nanoparticles in the next generation of cancer immunotherapies. InSeminars in Cancer Biology 2021 Aug 8; S1044- 579X (21)00212-1.
8. Chung YH, Cai H, Steinmetz NF. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Advanced Drug Delivery Reviews. 2020 Jan 1; 156:214-35.
9. Nkanga CI, Steinmetz NF. The pharmacology of plant virus nanoparticles. Virology. 2021 Apr 1; 556:39-61.
10. Lebel MÈ, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A. Potentiating cancer immunotherapy using papaya mosaic virusderived nanoparticles. Nano Letters. 2016 Mar 9; 16(3):1826-32.
11. Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature Nanotechnology. 2016 Mar;11(3):295-303.
12. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery. 2021 Feb; 20(2):101-24.
13. Le DH, Méndez-López E, Wang C, Commandeur U, Aranda MA, Steinmetz NF. Biodistribution of filamentous plant virus nanoparticles: pepino mosaic virus versus potato virus X. Biomacromolecules. 2018 Dec 5; 20(1):469-77.
14. Lico C, Giardullo P, Mancuso M, Benvenuto E, Santi L, Baschieri S. A biodistribution study of two differently shaped plant virus nanoparticles reveals new peculiar traits. Colloids and Surfaces B: Biointerfaces. 2016 Dec 1;148:431-9.
15. Nikitin NA, Zenin VA, Trifonova EA, Ryabchevskaya EM, Kondakova OA, Fedorov AN, et al. Assessment of structurally modified plant virus as a novel adjuvant in toxicity studies. Regulatory Toxicology and Pharmacology. 2018 Aug 1; 97:127-33.
16. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016 Apr 26; 1(5):1-2.
17. Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. Journal of Clinical Oncology. 2015 Sep 1;33(25):2780-8.
18. Sheen MR, Fiering S. In situ vaccination: Harvesting low hanging fruit on the cancer immunotherapy tree. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2019 Jan;11(1):e1524.
19. Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nature Reviews Cancer. 2004 Oct;4(10):806-13.
20. Goins B, Phillips WT, Bao A. Strategies for improving the intratumoral distribution of liposomal drugs in cancer therapy. Expert Opinion on Drug Delivery. 2016 Jun 2;13(6):873-89.
21. Boone CE, Wang C, Lopez-Ramirez MA, Beiss V, Shukla S, Chariou PL, et al. Active microneedle administration of plant virus nanoparticles for cancer in situ vaccination improves immunotherapeutic efficacy. ACS Applied Nano Materials. 2020 Aug 7;3(8):8037-51.
22. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nature Reviews Drug Discovery. 2019 Mar;18(3):175-96.
23. Gorbet MJ, Singh A, Mao C, Fiering S, Ranjan A. Using nanoparticles for in situ vaccination against cancer: mechanisms and immunotherapy benefits. International Journal of Hyperthermia. 2020 Dec 15;37(3):18-33.
24. Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, et al. The function and clinical application of extracellular vesicles in innate immune regulation. Cellular & Molecular Immunology. 2020 Apr;17(4):323-34.
25. Hu HG, Li YM. Emerging adjuvants for cancer immunotherapy. Frontiers in Chemistry. 2020 Jul 30;8:601.
26. Liwinski T, Zheng D, Elinav E. The microbiome and cytosolic innate immune receptors. Immunological Reviews. 2020 Sep;297(1):207-24.
27. Medzhitov R, Janeway Jr C. The Toll receptor family and microbial recognition. Trends in Microbiology. 2000 Oct 1;8(10):452-6.
28. Xu Y, Ma S, Si X, Zhao J, Yu H, Ma L, et al. Polyethyleneimine‐CpG Nanocomplex as an In Situ Vaccine for Boosting Anticancer Immunity in Melanoma. Macromolecular Bioscience. 2021 Feb;21(2):2000207.
29. Evtushenko EA, Ryabchevskaya EM, Nikitin NA, Atabekov JG, Karpova OV. Plant virus particles with various shapes as potential adjuvants. Scientific Reports. 2020 Jun 25;10(1):1-0.
30. Murray AA, Wang C, Fiering S, Steinmetz NF. In situ vaccination with cowpea vs tobacco mosaic virus against melanoma. Molecular Pharmaceutics. 2018 May 25;15(9):3700-16.
31. Mao C, Beiss V, Fields J, Steinmetz NF, Fiering S. Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors. Biomaterials. 2021 Aug 1;275:120914.
32. Albakri MM, Veliz FA, Fiering SN, Steinmetz NF, Sieg SF. Endosomal toll‐like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus. Immunology. 2020 Feb;159(2):183-92.
33. Carignan D, Herblot S, Laliberté-Gagné MÈ, Bolduc M, Duval M, Savard P, et al. Activation of innate immunity in primary human cells using a plant virus derived nanoparticle TLR7/8 agonist. Nanomedicine: Nanotechnology, Biology and Medicine. 2018 Oct 1;14(7):2317-27.
34. Hu H, Steinmetz NF. Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine. Cancers. 2021 Jan;13(12):2909.
35. Shukla S, Jandzinski M, Wang C, Gong X, Bonk KW, Keri RA, et al. A viral nanoparticle cancer vaccine delays tumor progression and prolongs survival in a HER2+ tumor mouse model. Advanced Therapeutics. 2019 Apr;2(4):1800139.
36. Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, et al. Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials. 2017 Mar 1;121:15- 27.
37. Patel BK, Wang C, Lorens B, Levine AD, Steinmetz NF, Shukla S. Cowpea Mosaic Virus (CPMV)-Based Cancer Testis Antigen NY-ESO-1 Vaccine Elicits an Antigen-Specific Cytotoxic T Cell Response. ACS Applied Bio Materials. 2020 Jun 25;3(7):4179-87.
38. Cai H, Shukla S, Steinmetz NF. The antitumor efficacy of CpG oligonucleotides is improved by encapsulation in plant virus‐like particles. Advanced Functional Materials. 2020 Apr;30(15):1908743.
39. Czapar AE, Tiu BD, Veliz FA, Pokorski JK, Steinmetz NF. Slowrelease formulation of cowpea mosaic virus for in situ vaccine delivery to treat ovarian cancer. Advanced Science. 2018 May;5(5):1700991.
40. Gonzalez MJ, Plummer EM, Rae CS, Manchester M. Interaction of Cowpea mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivo. PloS One. 2009 Nov 23;4(11):e7981.
41. Wang C, Fiering SN, Steinmetz NF. Cowpea mosaic virus promotes anti‐tumor activity and immune memory in a mouse ovarian tumor model. Advanced Therapeutics. 2019 May;2(5):1900003.
42. Stump CT, Ho G, Mao C, Veliz FA, Beiss V, Fields J, et al. Remission- Stage Ovarian Cancer Cell Vaccine with Cowpea Mosaic Virus Adjuvant Prevents Tumor Growth. Cancers. 2021 Jan;13(4):627.
43. Shukla S, Wang C, Beiss V, Steinmetz NF. Antibody response against cowpea mosaic viral nanoparticles improves in situ vaccine efficacy in ovarian cancer. ACS Nano. 2020 Mar 5;14(3):2994-3003.
44. Cai H, Shukla S, Wang C, Masarapu H, Steinmetz NF. Heterologous prime-boost enhances the antitumor immune response elicited by plant-virus-based cancer vaccine. Journal of the American Chemical Society. 2019 Apr 16;141(16):6509-18.
45. Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PL, Sloan AE, et al. Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers. 2019 Apr;11(4):515.
46. Mao C, Gorbet MJ, Singh A, Ranjan A, Fiering S. In situ vaccination with nanoparticles for cancer immunotherapy: understanding the immunology. International Journal of Hyperthermia. 2020 Dec 15;37(3):4-17.
47. Chung YH, Park J, Cai H, Steinmetz NF. S100A9‐Targeted Cowpea Mosaic Virus as a Prophylactic and Therapeutic Immunotherapy against Metastatic Breast Cancer and Melanoma. Advanced Science. 2021 Nov;8(21):2101796.
48. Albakri MM. Modulation of Monocyte/Macrophage Activation and Maturation by Plant Virus Nanoparticles and Free Fatty Acids: Implications for Tumor Immunotherapy: Case Western Reserve University; 2022.
49. Shahgolzari M, Pazhouhandeh M, Milani M, Fiering S, Khosroushahi AY. Alfalfa mosaic virus nanoparticles-based in situ vaccination induces antitumor immune responses in breast cancer model. Nanomedicine. 2020 Nov;16(2):97-107.
50. Patel R, Czapar AE, Fiering S, Oleinick NL, Steinmetz NF. Radiation therapy combined with cowpea mosaic virus nanoparticle in situ vaccination initiates immune-mediated tumor regression. ACS Omega. 2018 Apr 2;3(4):3702-7.
51. Boone CE, Wang L, Gautam A, Newton IG, Steinmetz NF. Combining nanomedicine and immune checkpoint therapy for cancer immunotherapy. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2022 Jan; 14(1):e1739.
52. Wang C, Steinmetz NF. A combination of cowpea mosaic virus and immune checkpoint therapy synergistically improves therapeutic efficacy in three tumor models. Advanced Functional Materials. 2020 Jul;30(27):2002299.
53. Wang C, Steinmetz NF. CD47 blockade and cowpea mosaic virus nanoparticle in situ vaccination triggers phagocytosis and tumor killing. Advanced Healthcare Materials. 2019 Apr;8(8):1801288.
54. Gautam A, Beiss V, Wang C, Wang L, Steinmetz NF. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. International Journal of Molecular Sciences. 2021 Jan; 22(18):9733.
55. Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, et al. Treatment of canine oral melanoma with nanotechnologybased immunotherapy and radiation. Molecular pharmaceutics.2018 Apr 3;15(9):3717-22.
56. Lee KL, Murray AA, Le DH, Sheen MR, Shukla S, Commandeur U, et al. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Letters. 2017 Jul 12;17(7):4019-28.
57. Cai H, Wang C, Shukla S, Steinmetz NF. Cowpea mosaic virus immunotherapy combined with cyclophosphamide reduces breast cancer tumor burden and inhibits lung metastasis. Advanced Science. 2019 Aug;6(16):1802281.
