Abstract
Developing functional foods through the incorporation of bioactive ingredients presents numerous technological obstacles. Microencapsulation is an effective method for increasing the delivery of bioactive chemicals, including probiotics, minerals, vitamins, etc. Numerous microencapsulation technologies have been developed that maintain probiotic bacteria’s survival during food product processing and storage. Additionally, studies examining the viability and stability of probiotics encapsulated in food matrices are shown and addressed. Although numerous reports have been published on the application of probiotics through its microencapsulation, hardly any exist that have focused on incorporating probiotics with prebiotics and other nutraceuticals. Thus, this analysis not only provides a fundamental understanding of the mechanism of microencapsulation and the properties of functional foods along with its fermented products, but also highlights the microencapsulation technique’s potential as a promising tool for enhancing the viability, growth and activity of probiotics by combining two or more bioactive ingredients to have a synergistic effect. Encapsulation systems, nutraceuticals and probiotics principles and present market condition, microbial microencapsulation and food uses for nutraceuticals and probiotics outside their usage in the industry are discussed. The most recent findings on the nano/microencapsulation of nutraceuticals, prebiotics, and probiotics for use in food items have been summarized.
Keywords
Prebiotics, Prebiotics, Synbiotics, Nutraceuticals, Microencapsulation, Functional food, Fermented food
Introduction
Numerous studies have been advanced to establish a link between gut health and immunological function and to explain how probiotics, prebiotics, nutraceuticals affect the gut microbiota and immune function. A probiotic must possess favourable technical features, be able to live in the upper gastrointestinal system, and operate in the gut environment in order to enhance human health. Probiotic safety is critical, that includes the antibiotic resistance criteria, non-pathogenicity and the strain’s human origin. However, with probiotics for human consumption, its specificity and capacity to remain viable at the target are more essential than the origin. Probiotics have historically been added to fermented dairy products but are also being added to a variety of other foods. There is an increasing need for functional meals that can aid in weight control, sleep management, and even the treatment of chronic conditions such as diabetes, hypertension, and cardiovascular illnesses [1]. There is a growing recognition of the critical nature of immunity in the aftermath of the continuing COVID-19 epidemic. As a result, functional meals that attempt to enhance immunity have gained appeal in recent years. Functional foods, such as probiotics and prebiotics, have been shown to modify the gut microbiota, consequently enhancing the host’s immune system. Prebiotics and probiotics are frequently ingested to help prevent and treat disorders such as obesity, diabetes, and poor digestion, as well as to increase immunity [1].
The most often used probiotic strains recorded are from the genera Bifidobacterium and Lactobacillus. Most commonly found in the human large intestine, Bifidobacteria are grampositive, anaerobic bacteria that thrive at a pH ranging from 4.5 to 8.5. Lactobacilli are a diverse collection of gram-positive, anaerobic and microaerobic bacteria that exhibit a wide range of growth and metabolic properties.
Industrial food production frequently necessitates the inclusion of useful additives. Typically, they are employed to regulate flavour, colour, texture and preservation qualities, but increasingly, they include compounds with possible health advantages. Including bioactive components in functional foods involves a number of issues, most notably the stability of the bioactive compounds throughout processing and storage, as well as the requirement to avoid undesired interactions with the matrix of the carrier food. With respect to this, the microencapsulation of bioactive compounds may aid in resolving some of these issues.
Biological Mechanisms
Gut and gut microbiota
The gastrointestinal tract, also known as the ‘gut,’ is divided into two parts. The upper region contains the jaw, pharynx, oesophagus, liver, and duodenum, while the lower region contains the small and large intestine [1]. The microflora of the gastrointestinal tract is complex, with 100 trillion microorganisms and 150,000 microbial genomes [2]. The individual-specific diversity of microflora is responsible for host metabolism and immune modulation [3]. Genetics, water, medicine, farming, and other factors all have an effect on the gut microbiota [4]. The majority of this microbial community is found in the GI colon and some parts of the upper area. As a result, an organism with a specific metabolic constraint favours specific sections of the GI tract [4]. The majority of the microbial species belonged to one of the four phyla mentioned below. Firmicutes (31.1%), Actinobacteria (25.9%), Proteobacteria (29.5%), and Bacteroidetes (7.1%) are the most common bacteria [5]. Lactic acid bacteria (LAB) and Bifidobacteria have a lot of promise, so they’re being studied a lot. Probiotics are the generic name for them. This microbiota is essential for the body’s metabolism as well as its immune response system. It has been discovered that there is a connection between different diseases in other major body systems and gut microbiota. Certain chronic disorders linked to digestion and immunity have been linked to the composition of microbiota and their end-products [3]. The gut microbiota’s role is to control and synthesise metabolites such as bile acids, short-chain fatty acids (SCFA) and indoleshu [6] These molecules, which are generated or produced by microorganisms, are critical for immune cell communication [7].
Immune system
The immune system can be classified as innate (non-specific) or adaptive (specific) [1]. The immune system is stimulated by altering the expression of a wide number of genes which can also result in the development of cytokines, lipid mediators, and tissue remodelling enzymes, among other things. Immune cells produce energy through a variety of metabolic mechanisms, including the TCA cycle, the pentose phosphate cycle, fatty acid oxidation, amino acid metabolism, and fatty acid synthesis. Immune cells are classified into two types: T-cells and B-cells. Immunoglobin (Ig) is generated by B cells in response to antigenic stimuli such as allergens and pathogens. Ig is further classified into three classes: Ig A, IgG, and IgE. IgA can be found in mucosal secretions, breast milk, and tears, among other places. T cells can be further divided into CD4 or CD8 cells based on their surface expression [8] . CD4+ T cells or helper T cells (Th) support other immune cells in their roles. They contribute to the development of a variety of cytokines, including interleukin-4 (IL-4), interleukin-17 (IL-17), and interferons (IFN-). These helper T cells protect the mucosal barrier while still producing antimicrobial peptides [6].
While the study of microbiota and its composition, as well as its relationship to immunity, is complicated, numerous studies have shown a substantial link between gut health and the onset of other diseases. Given the diet, exercise, and vitamins can change the microflora, functional foods such as prebiotics and probiotics can be used to improve gut health and therefore immunity.[9]
Functional Foods
Functional foods are distinguished from conventional foods by their unique physiological and biochemical benefits. However, since functional food cannot be described in a single way, a wide range of foods may be classified as functional foods. Functional foods are an entirely distinct and distinct group from nutraceuticals, medi-foods, and dietary supplements [10]. Although functional foods can have therapeutic properties, they are not considered as drugs [11]. The aim of functional food is to reduce the risk of illness, rather than to prevent or cure it entirely. Functional foods are everyday foods that are eaten as part of a balanced diet. They are made up of natural or synthetic compounds found in food that would not necessarily have the same benefits. In addition to their nutritional value, functional foods have an uplifting impact. They can improve health or aid in disease prevention. They make legitimate and statistically supported assertions. According to European consensus, a food is considered functional if it has been suitably shown to beneficially affect one or more target functions in the body, in addition to sufficient nutritional effects, in a way that is important to either risk mitigation or an improved stage of health and well-being. A functional food must remain food and show its effects in quantities that are typically eaten in the diet: it is not a pill or capsule, but rather a component of the usual food sequence.” [12]. It should be remembered, however, that this type of diet might not be helpful to the entire population, as each human has specific biochemical requirements.
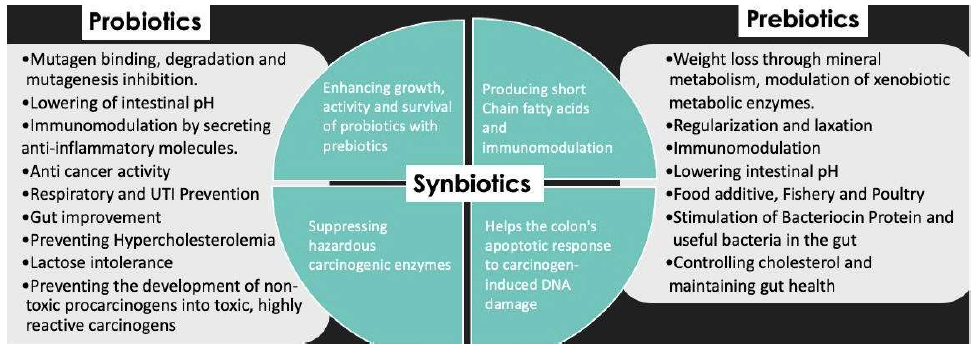
Functional foods are classified according to the active functional compounds they contain, which may include fibres, vitamins, flavonoids, and electrolytes [13]. They may also be classified according to their intended application, which could include boosting immunity, improving sleep and stress control, boosting energy, and increasing stamina. Probiotics and prebiotics are functional foods that are used to help people lose weight, increase their digestion, lower their chance of developing hypertension, and enhance their immunity and resistance to disease. They are made of dairy and non-dairy ingredients. The adage “Let food be thy medicine and medicine be thy food” is often repeated [14]. Historically, many practical foods included a variety of nutrients and phytochemicals to boost immunity, including vitamin C, vitamin D, zinc, iodine, andrographis paniculate, purpura, and astragalus radix. Probiotics and prebiotics, on the other hand, are gaining traction in the consumer market as a result of increased research and their immense potential [14] (Figure 1).
Figure 1. Summary of the uses of Probiotics, Prebiotics and Synbiotics.
Probiotics
Probiotics were originally classified as ‘growth-promoting factors formed by microorganisms.’ However, this concept changed over time. The United Nations World Health Organization (WHO) and Food and Agricultural Organization (FAO) have followed a rigorous definition, which is as follows: “living microorganisms that, when administered in adequate amounts, confer a health benefit on the host.” Historically, probiotics were used to ferment and preserve milk. It was later discovered that it possessed excellent sensory properties [15]. Numerous microorganisms may be categorised as prebiotics but probiotics are mostly included in the genera Lactobacillus and Bifidobacterium. Several strains of yeast, such as Saccharomyces cerevisiae var. boulardii, and bacteria, such as Bacillus pumilus, Bacillus clausii, Bacillus cereus, Bacillus subtilis, and Escherichia coli, have also been considered as possible probiotics [15]. Nonpathogenic yeasts are considered probiotic, and scientific interest in probiotic yeasts has grown in recent years due to their potential for product production in the field of functional foods [16]. Yeast strains such as S. cerevisiae are used to fortify beer, cheese, and wine as probiotics. Baker’s and brewer’s yeasts- Saccharomyces cerevisiae (S. cerevisiae) have been used as a food supplement due to its nutritional advantages such as calcium, peptide, high vitamin B content, and amino acids. V. Zoumpourtikoudi’s [17] study of the relationship between yeast and other probiotic bacteria demonstrated that yeast supplements increase the hydrophobicity of probiotics, decrease the pH by raising the lactic acid content, and thus improve immune response to infections [17]. As awareness regarding the value of immunity has grown, probiotics have become a common addition to the diet. Consumers prefer milk and fermented milk drinks. Additionally, probiotics are prescribed as part of a healthy diet to boost immunity against SARS COVID-19 [18].
Prebiotics
Prebiotics were described in the twentieth century as “a nondigestible food element that benefits the host by selectively stimulating the growth and/or activity of one or a small number of bacteria in the colon, thereby improving host health.” The concept has since been refined, and the International Scientific Association of Probiotics and Prebiotics (ISAPP) now defines prebiotics as “a substrate that is selectively used by a host microorganism conferring a health benefit” [19]. Insulin, galactooligosaccharides (GOS), fructooligosaccharides (FOS), and xylooligosaccharides (XOS) are carbohydrate-based prebiotics, while vitamins, polyphenols, and polyunsaturated fatty acids are non-carbohydrate-based prebiotics [20,21].
The gut microbiota’s primary role is to metabolise branched chain fatty acids (BCFA), short chain fatty acids (SCFA), biogenic amines, branched chain amino acids (BCAAs), bile acids (BAs), vitamins, and xenobiotics, in addition to producing gases [9,12]. The metabolised compounds contribute to barrier function maintenance and immune control [3,9,22]. While the interaction between optimal health and the gut microbiota is critical and complex, many factors may result in regulation of signalling defects associated with disease onset. Thus, prebiotics can play a significant role in preserving the richness of the total microbiota [3]. Beneficial microbes in the intestine degrade and metabolise prebiotics in the diet [2]. These probiotic metabolites (SCFA, BCFA, BA, etc.) are beneficial for primary degraders because they provide a substrate for other bacterial groups that are incapable of metabolising parent compounds such as long chained fatty acids. Thus, it is feasible to modulate individual gut bacteria with the aim of producing specific metabolites or regulating the bacterial population size. This can be extremely useful in terms of preserving and restoring host fitness. Prebiotics are described as fermentable fibres, fatty acids and micronutrients. In vivo experiments indicate that they could be a candidate to confer health benefits on the host [15]. Prior to choosing a prebiotic, a few criteria must be met [1] such as susceptibility to gut fermentation, beneficial to the consumer’s wellness, growthselective stimulation for probiotics, stability inside food matrix during fermentation, preservation, storage and resistance against breakdown in the alimentary canal’s upper portion.
When prebiotics such as maltodextrin, pectin and fructose are added to practical drinks and baby formulas, probiotic viability and development are enhanced. In one sample, fermented milk was supplemented with two probiotic strains of Bifidobacterium lactis Bi-07 and Lactobacillus acidophilus, as well as a prebiotic (isomaltooligosaccharide), and delivered to 100 healthy volunteers at a dosage of 480g/day for two weeks. It was discovered that both participants had a significantly higher faecal population of Lactobacilli and Bifidobacteria than the control sample. Additionally, the population of Enterobacilli decreased significantly. Thus, those formulations have the ability to alter the host’s gut intestinal flora and immune system.
Synbiotics
Synbiotics, which are described as a mix of a probiotic and a prebiotic, are intended to boost the survival and activity of established probiotics in vivo while also promoting indigenous Bifidobacteria and Lactobacilli. Again, efficacy data in human disease are scarce, while some small trials in irritable bowel syndrome indicate some promise [23-26]. Synbiotics facilitate apoptotic response to carcinogen-induced DNA damage in the colon, enhance colonization, stimulate growth, survival and activity of probiotics in the presence of selective prebiotic substrate. They also increase SCFA production and immunomodulation. The anti proliferative activity of synbiotics initiates downregulation of inducible NO-synthase and cycloxygenase-2 enzymes, involved in colon carcinogenesis. Additionally, they play a role in modifying colonic bacterial ecosystem, leading to an overall improvement in metabolic activity of the colon and cecum.
Nutraceuticals
Stephen DeFelice invented the phrase “nutraceutical” in 1989 by combining the phrases “nutrition” and “pharmaceutical.” Nutraceuticals, he stated, are “foods (or components of food) that provide medicinal or health benefits, such as illness prevention and/or therapy” [27]. They are often available in capsules, tinctures and tablets. Nutraceuticals encompass a diverse range of items, including drinks, dietary supplements, separated nutrients, foods produced with genetic engineering, herbal items, and processed meals. Nutraceuticals are classified into three broad categories: dietary supplements, functional foods along with beverages and nutraceutical components (oils or raw minerals) [27].
However, Nutraceuticals as a concept are not well established due to a lack of a well-defined industry, i.e., if they belong to the food, food supplement, or pharmaceutical sector [28]. Additionally, each country has its own set of laws, which makes it difficult to develop standard rules to assess the safety, mechanism of action, and efficacy of such drugs using proper clinical data. Additionally, nutraceuticals have been defined as food supplements that “provide a concentrated version of a putative bioactive ingredient from a food, presented in a nonfood matrix, and utilised to improve health”. T’elessy [29] also views nutraceuticals to be dietary supplements from a legal standpoint and says they are not disclosed as pharmaceutical products due to the time and expense associated with preclinical (safety) and clinical (efficacy) investigations.
Nonetheless, Santini et al. [30] assert that nutraceuticals vary from food supplements in that they are backed up by clinical data demonstrating their efficacy and safety.
Japan was one of the first countries to regulate food supplements in 1991, using the term Foods for Specified Health Use (FOSHU). This term later evolved into the concept of functional food [30], which refers to food products that contain compounds that support specific body functions in addition to their nutritional properties [31]. According to Diplock et al., functional foods are those that have the potential to benefit organism functions, such as enhancing health and well-being or lowering the chance of acquiring disease. Additionally, functional foods have been characterised as food items that include one or more bioactive compounds that have physiological effects and may potentially improve a consumer’s health. Functional foods vary from fortified foods in that the latter have a higher concentration of certain components, whilst the former are of higher quality than ordinary foods.
Nutraceuticals are gaining traction in Europe and North America, where they are becoming a staple of the consumer diet. The primary cause for this is the rising prevalence of lifestyle-related illnesses, which has increased public awareness of preventative health measures. The functional foods category accounts for the lion’s share of the natural goods industry, followed by functional beverages and food supplements. According to a BBC study [32] , the worldwide nutraceutical industry reached $230.9 billion in 2018 and is expected to expand at a 7.8 percent compound annual growth rate (CAGR) between 2018 and 2023. Currently, the greatest nutraceutical market is focused in Europe; however, the Asian industry is rising at the fastest rate [33].
Numerous modes of action have been ascribed to the various substances included in nutraceutical formulations, including antioxidant, antibacterial, anticancer, antihypertensive, antihypercholesterolemic, anticoagulant, anti-inflammatory, and bone protective properties. Nonetheless, there are several obstacles to their inclusion into food items, including low water solubility, chemical instability, temperature, light, and oxygen sensitivity, as well as issues with GIT absorption. Numerous unfavourable interactions between nutraceuticals and food matrix chemicals are conceivable, which can result in alterations to the sensory qualities and shelf life of food items [34].
Microencapsulation Technology
The fact that the human digestive tract offers such a wide variety of different environmental conditions makes it difficult to design a probiotic delivery system, but also presents a chance to make a system highly tailored to the targeted region. Despite this, the fate of a product’s probiotic bacteria is also dependent on its survival during the encapsulation process. The current devices vary in size from tens of microns to millimetres and their ability to boost probiotic survival varies depending on device size [35]. A dry powder, as a microencapsulated probiotic product, would be preferred for long-term storage, while a wet gel with a lengthy shelf life is the better option for being able to stay stable in food.
Microcapsules are extruded or emulsified in three principal ways: extrusion, emulsion and spray drying (including spouted bed drying). Cross-linking is done via emulsification and extrusion. When it comes to extrusion research, needles and syringes are typically utilised. However, in the spray method category, for example, air-atomizing nozzles [36], spinning-disk atomization [37] and vibrating nozzles [38] have all been used. Industries routinely use multi-nozzle arrays to scale up extrusion operations [39]even while batch processing is involved. Additionally, spray systems lower the size of microcapsules, which are normally a millimetre or two in size, to several hundred microns [40,41]and are useful for laboratory-scale production.
It is recommended to separate dry-powder particles after preparing them since they are easier to handle and store. The material has been sprayed, air dried, frozen, then fluid bed dried to prepare it for injection moulding. The techniques of manufacturing that directly lead to a dried product, like spouted bed drying and spray drying, occur less frequently and result in significantly increased cell damage and death. Probiotic bacteria was encapsulated in microcapsules through intricate coacervation [42]which was then followed by spray drying or spouted bed drying . Unlike spray drying, where the fluid is applied to a bed of drying particles, in spouted bed drying, the fluid is poured over a large fluid bed-type drying drum that suspends and dries the particles [43]. In high acid conditions, spray drying yielded bacteriologically protected microcapsules of B. lactis and L. acidophilus, with a loss of 2 logCFU/mL due to the drying procedure. However, it should be noted that the spouted bed drying method proved unsuccessful at preventing bacteria loss. The increase in acidity was due to the bacteria’s susceptibility to damage from the spouted bed drier, causing it to become more acid sensitive. Since these spray and spouted bed techniques have a cheap cost, they have a widespread use in the pharmaceutical and biotechnology industries [44]. Increasing the storage qualities and decreasing the size of the microcapsule are possible through the drying process [45]. Drying procedures in earlier studies have produced proven issues such as agglomeration and the creation of cracks during air drying [46].
Freeze drying is the process which utilises a vacuum for the sublimation of ice to dry a frozen sample. Due to the availability of cryoprotectants, freeze drying techniques have the added benefit of supporting bacterial growth. However, freeze drying is time-consuming; also, due to the retention of the hydrated structure, it does not reduce particle size as significantly as the various other drying processes. By contrast, fluid bed drying achieves drying by suspending the load in air with extra heat to aid in solvent evaporation [47]. This is an efficient approach since it is fast, commonly utilised in granulation and will break up any grain size.
A separate study was conducted on the effect of drying the fluid bed on various microcapsules and demonstrated that drying the fluid bed does not affect the ability of various microcapsules to protect encapsulated bacteria from low pH [48]. One problem of fluid bed drying is the aerobic environment required for drying, which may be harmful to strict anaerobic organisms. However, this drawback may be circumvented if a device could be built to circulate an atmosphere which does not contain oxygen. Additionally, germs may be harmed by the high temperatures employed in the drying process (80°C and more). While oven drying and vacuum oven drying are simpler processes, they have a number of disadvantages, including the lengthy drying durations and particle clumping. [46]
Encapsulation applied in probiotics and nutraceuticals
In the recent decade, much research has been conducted on the encapsulation of bioactive substances, particularly those with nutraceutical claims. Numerous comprehensive studies have accumulated data on the utilisation of various encapsulation methods and encapsulating materials for a variety of bioactive compounds [32,33,49,50]. Several plant-derived nutraceuticals with established anticancer activity, including resveratrol, quercetin, curcumin, genistein, and epigallocatechin gallate, have been encapsulated in nanoliposomes and biocompatible polymeric nanoparticles [49,51]. Anticancer nutraceuticals loaded into nanoparticles have exceptional outcomes in terms of solubility, absorption, bioavailability, and anticancer activity when compared to nutraceuticals alone. It is worth noting, however, that until recently, only few studies successfully evaluated these nanostructures in real food systems [52]. The series includes several instances of food uses for nanostructured nutraceuticals.
Polyphenols and vitamins that have been encapsulated can be added to a variety of edible goods while preserving their stability. Certain factors, however, must be addressed, including the optimal encapsulation matrix, the optimal core-to-carrier ratio, and the most appropriate operating parameters. For example, folic acid (FA) is a synthetic vitamin that is often used for food fortification; FA may be effectively encapsulated with silica particles and added to commercial citrus juices such as apple or orange juice. FA may benefit from encapsulation during processing and storage, leading in more precise fortification. Additionally, prolonged release following ingestion would aid in vitamin metabolism and assist avoid any harmful effects associated with FA overdosing [53].
The food sector has mostly used lipid transporters, such as nanoliposomes, to encapsulate flavorful, nutritional, and antibacterial chemicals, hence boosting product quality and shelf life. Recent research indicates that tocosomes and nanoliposomes may be used as possible carriers for encapsulating and delivering nutraceuticals in food systems. Several examples include the synergistic delivery of tocopherol and ascorbic acid, or tocopherol and glutathione, to increase the antioxidant activity of foods, and the stability of certain minerals (such as calcium and iron) in milk and other liquids [54]. Additionally, it has been claimed that nanostructured polyunsaturated fatty acids (PUFAs) have been incorporated into bread and milk. The formulations comprising docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) encapsulated in nanoliposomes demonstrated losses of 4.2– 6.5 percent and 5.6–5.9 percent in bread and milk, respectively, while no additional losses occurred during storage [55-57].
At the moment, probiotic cells in their encapsulated form are mostly employed for the marketing of nutraceutical goods. However, the need of developing novel foods that are viable carriers for these bacteria has been widely explored.
Incorporating probiotics into food items presents a number of issues, including ensuring the viability of live cells throughout processing and storage, as well as avoiding undesired interactions with the food matrix. Thus, encapsulation of probiotics attempts to boost their stability and vitality, as well as to offer a regulated release that allows them to attach and colonise the gut efficiently. In this regard, polymers utilised for microencapsulation should be capable of protecting cells during stomach acidity and releasing them during the small intestine’s neutral to alkaline circumstances [58]. To ensure the survivability of probiotics in microcapsules, it is critical to consider specific physical and chemical features, such as coating material concentration, culture type, starting cell count, particle size, and water solubility [59-61].
Although several methodologies and wall materials have been described for successful probiotic encapsulation, maintaining bacterial viability remains a major difficulty, necessitating more study [62].
Prebiotics such as inulin, polydextrose, fructooligosaccharides (FOS), and galactooligosaccharides (GOS) have been characterised as a highly intriguing and promising technique for extending the shelf life of probiotic microbes. Along with boosting probiotic viability during microcapsule manufacture and storage, symbiotics are an approach for food functionalization [63]. In addition to co-encapsulating probiotics and prebiotics, the incorporation of other bioactive compounds, such as omega-3 fatty acids, curcuminoids, antioxidants from fruit juice and green tea extracts, and agroindustrial by-products, can help increase the stability and bioactivity of these microorganisms [64].
Because bacteria are only a micron in size, probiotics encapsulation is only achievable via microencapsulation; nanotechnology is not a viable alternative (particles smaller than 1 m, although microbial cells diameters are generally in the range of around 1–10 m). Additionally, administration channels should be enhanced to ensure optimal protection during storage and transit through the GIT, allowing probiotics to be released into the colon and exercise their healthpromoting effects. Then, a number of critical challenges must be addressed, including the development of novel encapsulating methods to boost the heat resilience of probiotic strains and the enhancement of colloidal distribution systems for probiotics of various sizes. Alginate, starch, chitosan, xanthan gum, k-carrageenan, cellulose acetate phthalate, gelatin, and milk proteins are the most frequently used polymers for probiotic encapsulation [65], whereas the most frequently used techniques are gelling, spray-drying, spraycooling, extrusion, and emulsification (Cor Biopolymer beads made by extrusion in a water-in-oil emulsion are a frequently used encapsulation technique for probiotic bacteria [66]. Due to its simplicity, cheap cost, and formulation conditions that ensure high cell viability, extrusion is the most widely used approach for probiotic microencapsulation [67]. Coacervation, electrospinning/electrospraying, vibrating methods, and impinging aerosol are other potential approaches [63].
Extrusion microencapsulation involves the addition of a hydrocolloid solution containing probiotic microorganisms to a hardening solution containing cations such as CaCl2, therefore producing a three-dimensional network by cross-linking. The emulsification process is defined by the development of a water-in-oil emulsion by dispersing the cell hydrocolloid suspension in an immiscible liquid in which the polymer becomes insolubilized following calcium chloride addition. The first technique offers advantages such as increased cell viability and consistent microcapsule size, but the second method has the disadvantage of being difficult to create evenly shaped microcapsules [68]. Complex coacervation is an intriguing method for encapsulating probiotic bacteria since it does not involve the use of organic solvents or high temperatures. However, this approach may impair the functioning of microencapsulated bacteria, even if it increases their survival rate.
Microencapsulating probiotics with biopolymers is a promising technique since it increases their storage stability and vitality throughout upper GIT transit (Marcial-Coba et al., 2019). However, encapsulating probiotics in simple microgels may not protect them from stomach conditions, as hydrogen ions and digestive enzymes can penetrate into microgelsowing to the high hole size of the biopolymer network. Recent research has demonstrated that microgels containing cellulose nanoparticles (CNCs) can greatly boost the viability of probiotics in simulated GIT settings by plugging holes and preventing stomach acid entrance.
Finally, certain in vivo studies confirm that encapsulation can significantly increase the viability and effects of probiotics. When a commercial powdered probiotic cocktail including Lactobacillus fermentum, Lactobacillus acidophilus, and Bacillus spp. was evaluated in duck feed [69]. In comparison to powdered probiotic, formulations containing 0.2 percent and 0.4 percent encapsulated probiotic performed better on relative carcass, villus height, and villus weight. Gunzburg et al. [70] demonstrated that cellulose sulphate encapsulation enhanced probiotic strain distribution to the digestive system. Five probiotic bacteria (Lactobacillus and Bifodobacterium species) and one yeast (Saccharomyces boulardii) were encapsulated in cellulose sulphate microspheres and were found to survive for at least 4 hours in low pH without significant loss of viability when compared to their nonencapsulated counterparts. Bioluminescent bacteria were used to demonstrate effective intestine transport and release of encapsulated cells in mice. The cecum of mice receiving encapsulated bacteria had much more bacteria than those getting non-encapsulated bacteria, and this difference was considerably more pronounced in the large intestine. Similar changes in the quantity of live bacteria were also seen 24 hours after dosing in faecal pellets. Given that a mouse’s gastrointestinal transit time is approximately 4–6 hours, the results indicate that encapsulated bacteria were protected during passage through the stomach and that they were also released and colonised in the intestine, as evidenced by the continued presence of marked bacteria at a constant level in the faeces 24 hours later [70].
To encapsulate probiotic Bacillus coagulans, a layerby- layer method was adopted, alternating layers of the cationic polysaccharide chitosan and the anionic polymer alginate via electrostatic interactions. The effect of coating on probiotic survival and delivery was examined in vivo by oral gavage administration of an equivalent amount of free and encapsulated bioluminescent bacteria. One hour after treatment, the encapsulated probiotic generated a bioluminescence signal that was almost sixfold that of the free bacteria in the GIT [71]. In vivo, the chitosan-alginate coated B. coagulans outperformed free B. coagulans in terms of survivability against acid and bile damage, adherence to intestinal surfaces at short time periods, and direct growth on intestinal surfaces. In another work, chitosan-coated alginate beads encapsulating L. plantarum MTCC2621 were produced and assessed for their ability to ameliorate alcohol-induced liver damage (ALD) in rats. When bacterial cells were subjected to gastrointestinal tract conditions, viability was preserved when the strain was encapsulated, but free probiotics had a substantial fall in viable counts. After continuous alcohol treatment, the animals developed endotoxemia; however, supplementation with encapsulated probiotics dramatically decreased endotoxemia, serum transaminases, NF-κB, and cytokines, accompanied by restoration of normal intestinal and liver histology. Thus, the encapsulated probiotic was more effective in restoring liver and intestine histology, decreasing endotoxemia, and decreasing inflammation in the ALD rat model.
Delivery Systems of Microencapsulation
Prebiotics with probiotics
Proven by change in bacterial metabolism and an increase in the relative numbers of Lactobacillus and Bifidobacterium, an/a increased/higher synthesis of short-chain fatty acids such as butyrate and propionate was seen [72].
A short-chain fatty acid also has antibacterial activity and supports colonic homeostasis in a number of ways, primarily via its influence on the integrity of the colonic epithelium along with the metabolic, immunological activities of the short-chain fatty acids [42-45]. Some of the positive benefits of prebiotics may be mediated via interactions with the epithelium or immune system. These interactions may have effects on bile acid metabolism [75] and neuroendocrine responses. In addition, data has also demonstrated influences that extend outside the gastrointestinal tract, all the way to the central nervous system [76]. Over time, more precise probiotic compounds will be developed, making it easier to specifically affect certain biological functions.
Inulin and oligosaccharides: Bifidobacteria and lactobacilli ferment inulin and fructo-oligosaccharides, which results in their stimulating action. Bifidobacteria only partially digest fructo-oligosaccharides and inulin, and the importance of enzyme concentration in this process varies by species. For instance, the enzyme from B. adolescentis G1 has a substrate selectivity for fructooligomers that is unique from inulin. Additionally, B. longum and B. animalis were capable of hydrolyzing a broad range of oligosaccharides, including FOS with a DP of 2–4 and inulin with a DP>8. The biggest number of viable bifidobacteria (3.59–2.25 billion CFU/g) was found in the product, which included B. animalis and FOS and was largest when the corn starch was high in amylose. For 21 days, a live bacterium was detected in yoghurt with FOS. Bifidobacterium is less tolerant to milk preservation at low temperatures than Lactobacillus acidophilus. Bifidobacteria are currently acknowledged to be subspecies of animal bifidobacteria. Bifidobacterium animalis ssp. lactis Bb12 is the predominant strain discovered in dairy products in Europe. Bifidobacterium lactis contains the required enzyme, b-glucosidase which is employed to utilise oligosaccharides and cleave related b-(2,6) linked fructans in both MRS and fermented milk, thereby increasing the Bifidobacterium’s growth and metabolic activity.
The digestive enzyme activity and gut microbe ecology in rats were examined by Suh-Ching et al. [77] using a symbiotic. SD rats, were organised into three groups, according to the dosage of prebiotics and probiotics in the feed: control, low and high dose. After 8 weeks it was investigated that high and low dose groups significantly increased the counts of Lactobacillus and Bifidobacterium while lowering counts of Coliform organisms. Lactase activity only increased in the lowdose group, attritubed to the lower count of Lactobacillus. The increase in sucrose, lipase, isomaltase and lactase was particularly pronounced in the high dose group. Probiotics consumed in low and high doses changed the ecology of the digestive tract by increasing the probiotic population.
The antidiarrheal properties of synbiotic plum juice containing prebiotic inulin were also investigated to determine the effect of prebiotic meals comprising of oligosaccharides on the formation and activity of probiotic bacteria [78]. Plum juice contains Lactobacillus kefiranofaciens, Saccharomyces boluradii and Candida kefir probiotic strains. Synbiotic plum juice was evaluated for its antibacterial effectiveness against diarrhea-causing organisms such as Escherichia coli, Staphylococcus aureus, Salmonella paratyphi A, Vibrio cholerae and Shigella dysenteriae. The compound found in synbiotic plum juice was analysed using gas chromatography(GS)- mass spectrometry(MS). The research established that oligofructose-enriched inulin is the most appropriate prebiotic in spray drying microencapsulation of Bifidobacterium BB-12, with high potential of being a beneficial ingredient in dairy meals [79].
Currently, a human clinical investigation is taking place to see if probiotic Bifidobacterium lactis Bb12 and Lactobacillus rhamnosus GG supplements together with prebiotic oligofructose-enriched inulin would impact colon cancer risk biomarkers [80-83]. It has been claimed that this synbiotic combination affects the balance of gut flora, as demonstrated by an increase in the numbers of lactobacilli and bifidobacteria along with a decrease in the numbers of coliforms. It remains to be seen whether or not this approach will lower cancer rates [83].
In animal study, it is postulated that synbiotics have a protective effect on colorectal cancer. There is only one human study to date showing that a synbiotic prevents colon cancer. A synbiotic mixture combining oligofructose-enriched inulin, B. lactis (BB12) and L. rhamnosus GG (LGG) was shown to reduce the risk of colon cancer in a 12-week randomised, double-blinded, placebo-controlled human research [84].
Bifidobacteria and lactobacilli were both raised and Clostridium perfringens was lowered when synbiotic intervention took place. Additionally, this intervention greatly reduced the number of new cells in the colon and increased the capacity of faecal water to induce cell death in colonic cells, while simultaneously increasing the integrity of the epithelial barrier in polypectomized patients. Supplementation with synbiotic ingredients not only increased interferon production but also inhibited interleukin-2 (IL-2) secretion by peripheral blood mononuclear cells(PBMC) [84].
The evidence till date suggests that synbiotics are much more effective at preventing and treating colorectal cancer than prebiotics or probiotics alone. Combining the strains of Lactobacillus and Bifidobacteria with oligosaccharides such as galacto-oligosaccharides(GOS), fructo-oligosaccharides (FOS) and inulin increased its efficacy compared to administering the probiotics in isolation [85].
Freeze-dried fruit and vegetable extracts administered to mice reduces the number of intestinal adenomas, a tumourinducing condition, showing that eating less and changing diet could also alter the intestinal microbiota and lower the risk of cancer [86]. Similar beneficial changes in bacterial metabolic activity and cecal physiology, as well as decreased tumour risk and the likelihood of azoxymethane-induced preneoplastic colonic lesions, were found in the mouse model when B. longum (a probiotic) and a derivate of inulin prebiotic (“Raftiline HP”) were co-administered [87].
The viability of probiotics remained higher in the control batch than in any of the yoghurts with additional prebiotics after storage. Inulin, Hi-maize and FOS (fructooligosaccharides) were found to be beneficial in preserving the viability of probiotic organisms in fresh yoghurt during storage but were found to be detrimental in freeze-dried yoghurt. Increased viability in fresh yoghurt may be a result of prebiotics supplying more solids, which protect cells from damage. The oligosaccharides utilised in this investigation are hydrocolloids that have been shown to protect probiotic bacteria. Although FOS was found to be the most efficient prebiotic in preserving the viability of probiotic organisms in yoghurt, the addition of FOS lowered the survivability of probiotics when the yoghurt was freeze-dried.
It has been demonstrated that the inclusion of oligosaccharides such as -glucan and other plant extracts enhances probiotic distribution [88-93]. Although oligosaccharides are not normally degraded by enzymes found in gastrointestinal fluids, they can be utilised by certain bacteria, particularly Lactobacillus species in the colon. Recently, it was demonstrated that microencapsulating Lactobacillus fermentum with oligosaccharides protects probiotics from low temperatures [90]. Additionally, it has been observed that incorporation of sea buckthorn (having non-digestible oligosaccharides) extract into microgels protected L. casei during heat treatment and GIT passage, [94] which was attributed to its antioxidative activity and effects on the size and structure of the microgels [92].
Resistant starch (RS): While studying the encapsulation of bifidobacteria in stirred yoghurt, a granular texture was noted with particles ranging from 22 to 50 millimetres. However, the probiotic bacteria could not be retained in the microencapsulation due to the sensory issues, which lead to poor customer approval [95].
Prebiotics such as resistant starch or cryoprotectants like glycerol may be used to encapsulate probiotic cells, thereby enhancing their viability [96,97]. It was reported that this technique increases probiotic viability within the product but not in simulated GI environments.
A combination of Bacillus lactis and resistant starch (RS) improved the tumour’s sensitivity to a genotoxic carcinogen (AARGC) and colonic fermentative processes in rats [98,99]. B. lactis has the capability to exert its pro-apoptotic activity when used as a metabolic substrate for RS. To assess the effectiveness of treatment in patients, a research group utilised a rat model of colorectal cancer (AOM-induced rat colon cancer) [100]. When used individually and in combination, B. lactis and RS were effective against AOMinduced rat colon cancer. RS is proven to have an impact on fermentation [101], while AOM-induced colorectal adenomas and carcinogenesis are inhibited by synbiotic combinations containing fructans, B. lactis (Bb12) and L. rhamnosus GG. This resulted in the production of SCFAs, a decrease in proliferation and an increase in expression of Pi-type, cyclooxygenase-2 enzymes implicated in the pathogenesis of collateral cancer and inducible NO-synthase. [102] Researchers theorised that synbiotic dietary supplements that feature oligofructoseenriched inulin, Lactobacillus rhamnosus and Lactobacillus lactis have antitumorigenic properties because they regulated immune responses, affecting Peyer’s patches (PP) and peripheral blood mononuclear cells (PBMC) [102].
There was a larger number of live bacteria present in microcapsules with the addition of Hi-Maize starch (a kind of Resistance Starch) [103]. Probiotic viability and effectiveness are both improved by increasing the alginate content and capsule size [104]. According to Chen et al. [105] this solution of 2% sodium alginate and 1% gellan gum offers the best protection for Bifidobacterium bifidum when heat-treated. In addition, peptides were found to have a prebiotic effect. A new study discovered that extruding nonfat milk increases/ boosts the number of viable cells.
Fructo-oligosaccharides: Colorectal cancer has received more attention from scientists since the research suggested that prebiotics and probiotics are critical for treating this disease. Probiotic supplementation significantly reduced cytotoxicity compared to control, as well as to prebiotic supplemented groups. In the case of Peyer’s patches (PP), NK cell-like cytotoxicity was reduced when synbiotic supplementation was used [106]. Synbiotics and prebiotics administered to the groups also enhanced IL-10 production, whereas the opposite was observed in Peyer’s patches (PP) groups, with interferon production decreasing. This synbiotic treatment strengthened the immune system, as demonstrated by higher immunoglobulin levels in the Peyer’s patches (PP) and was correlated with reduced incidence of colon cancer in rats treated with carcinogens [106]. The study also mentioned in vitro and in vivo models that included Fructooligosaccharides in conjunction with a Bifidobacterium strain and lactitol [107].
Live/living cells from the abrasive conditions are associated with a low pH in the stomach. Geneflora™ was developed in the United States of America by the BioPlus Corporation. It is a synbiotic probiotic composed of lactobacillus sporogenes encapsulated in a fructo-oligosaccharide prebiotic which sparked interest in the use of prebiotics and fibre as probiotic cell protectors in the last several years.
Fructooligosaccharides and isomaltooligosaccharides, as well as peptide and growth stimulant sodium alginate were employed as coating materials to encapsulate four probiotics (Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium longum, and Bifidobacterium bifidum). To investigate the survival of probiotics in simulated gastric fluid, various proportions of the prebiotic, peptide sodium alginate were adjusted using response surface methodology (RSM). This was followed by sequential quadratic programming (SQP) to optimise the model it was determined that samples comprising 3% fructooligosaccharides, 1% sodium alginate and 1% peptide, produced the best results. It was also discovered that prebiotics within the walls of probiotic microcapsules enhanced the protection of the active organisms [108].
The effect of isomalto-oligosaccharides on foods such as honey, soy, sauce and miso was examined. Bacteroides fragilis and Bifidobacteria in miso, soy, sauce, sake, and honey were able to utilise isomalto-oligosaccharides, unlike E. coli. After two weeks of daily administration of 13.5 grammes of isomalto-oligosaccharides to healthy adults, the abundance of bifidobacteria increased [109].
Though treated with simulated stomach juice and bile salt, the probiotic counts were maintained between 106 and 107 colony-forming units (CFU)/gram for microcapsules that had been stored for 1 month. The probiotics that were encapsulated in prebiotic microcapsules in milk persisted for two weeks when the milk was stored in the refrigerator. Similar outcomes were discovered in fermented frozen dairy desserts owing to the probiotics microencapsulated in alginate microcapsules in yoghurt [96,110]. Thus it was concluded that probiotics in food products can be safeguarded by microencapsulation with calcium alginate [111]. Prebiotics also increased the protection of the microorganisms included in the capsules of probiotic alginate.
Prebiotic fibres: The ability of various prebiotic fibres to protect the stability and viability of Lactobacillus rhamnosus probiotic strains was crucial in the setting of probiotic encapsulation. It was described how this type of protection is maintained throughout the freeze-drying, storage, formulation processes for apple juice and chocolate-coated breakfast cereals [112]. According to studies conducted, fibres are classified as prebiotics because they contain an indigestible component that aids the survivability of probiotics and acts as an optimal carrier for probiotics in the route of administration [113].
Additionally, psyllium and fenugreek have been demonstrated to be excellent prebiotics, enhancing the viability and growth of probiotics in the gastrointestinal tract [114]. During freeze drying, polydextrose and wheat dextrin-based prebiotics have demonstrated effectiveness as preservatives for Lactobacillus rhamnosus[112].
Raftilose: Numerous studies have been conducted on the use of raftilose, a prebiotic, to co-encapsulate two probiotic strains (Bifidobacterium infantis CSCC 1912 and Lactobacillus acidophilus CSCC 2409) with the goal of enhancing bacterial growth and protecting cells from dangerous environmental conditions. Due to the synergy between the prebiotic and probiotic components, this concept of co-encapsulation results in an increase in the efficiency of functional foods. Utilization of prebiotic compounds also contributes to the viability of probiotic cells. It was demonstrated that coating the capsule with chitosan protects probiotic cells better than alginate does in yoghurt and in simulated GI transit conditions [115].
Probiotic bacteria such as Bifidobacterium spp., Lactobacillus casei, Lactobacillus acidophilus and Lactobacillus rhamnosus were examined following processing and storage of yoghurt and freeze-dried yoghurt. Additionally, the effectiveness of microencapsulating probiotic organisms, as well as the addition of prebiotics and cryoprotectants was explored in order to increase their viability. The viability of L. rhamnosus GG and Bifidobacterium infantis 17930 was reduced at a lesser extent than L. casei 1520 and Bifidobacterium longum 1941. L casei 1520’s viability increased by 7% when 2.5 percent (w/v) cryoprotectant UnipectineTM RS 150-was added to it. When added to yoghurt at a concentration of 1.5 percent w/v, the prebiotic RaftiloseP95 increased the viability of the combined probiotic organisms after four weeks of storage at 4°C. Alginate microencapsulation increased the viability of a mixture of selected probiotic organisms in freeze-dried yoghurt stored at 21°C [97]. Due to the availability of prebiotics, the presence of these oligosaccharides led to reduction in the rate of cell death of the saccharolytic bacteria during storage. A marginal increase in the viability of probiotic organisms was also discovered in yoghurt containing FOS after four weeks of refrigerated storage [116].
Although when prebiotics were added to freeze dried yoghurt, the viability of the probiotic organisms was significantly lower than the control. According to the research, lactic acid bacteria are subjected to environmental challenges [117]. The oligosaccharides may have been unable to shield the cells from damage, resulting in a decrease in probiotic viability.
Galacto-oligosaccharide: A recent study examined the effect of incorporated Bifidobacterium breve into a multiparticulate made up of poly D,L-lactic-co-glycolic acid microcapsules containing prebiotic galactooligosaccharides embedded in an matrix consisting of a combination of alginate and chitosan [88]. After one hour of exposure to stomach conditions at pH 2, the viable cell count of the multiparticulate system coated with chitosan decreased significantly. This method results in a low viability of B. breve in a gastrointestinal environment when compared to the fluid-bed drying techniques [48]. However, the chitosan-coated multiparticulate system significantly increased cell viability in the intestinal portion, possibly due to the release of prebiotics (galactooligosaccharides) that stimulated cell development.
Encapsulating prebiotics within the core of microgels has recently been proven to increase their viability without impairing their release [90]. Prebiotics are typically indigestible or only partially digestible substances that promote probiotic growth un the human gut [101].
| Lactobacillus, Bifidobacterium | Inulin, Fructo-oligosaccharides. | increased probiotic growth and metabolic activity | [118] |
| Lactobacillus kefiranofaciens, Candida kefir, Saccharomyces boluradii | Inulin | effective against diarrhea-causing organisms | [78] |
| Encapsulated bifidobacteria | Resistant Starch or cryoprotectants like glycerol | increases probiotic viability within the product | [96,97] |
| Lactobacillus acidophilus CSCC 2409 Bifidobacterium infantiis CSCC 1912 | Raftilose | viability of probiotic cells and efficiency of functional foods | [115] |
| Lactobacillus rhamnosus | Prebiotic fibres | optimal carrier for probiotics may be contingent on the final route of administration | [119] |
| Lactobacillus sporogenes | Fructo-oligosaccharide | development of Geneflora | [109] |
| Lactobacillus acidophilus,Lactobacillus casei, Bifidobacterium bifidum, Bifidobacterium longum | Fructooligosaccharides, isomalto-oligosaccharides, peptide | enhanced the protection of the active organisms. | [108] |
| Lactobacillus acidophilus,Lactobacillus casei, Lactobacillus rhamnosus,Bifidobacterium spp | RaftiloseP95, oligosaccharides | increased the viability of the probiotic after four weeks of storage,reduced the rate of cell death of the saccharolytic bacteria during storage | [97] |
| L. acidophilus 33200, L. casei 279, B. longum 536, L. rhamnosus GG |
Hi-maize, FOS and inulin | improved viability of probiotics in freeze-dried yoghurt with FOS being the most effective in yoghurt | [114] |
| Bifidobacterium BB-12 | Oligofructose-enriched inulin | beneficial ingredient in dairy meals | [79] |
| Bifidobacterium breve B-3 | Galactooligosaccharides | increased cell viability in the intestine | [88,120] |
| Lactobacillus fermentum | Oligosaccharides such as glucan, plant extracts | enhanced probiotic distribution | [90] |
| Lactobacillus species | Psyllium, Fenugreek | enhancing the viability, growth of probiotics in the gastrointestinal tract | [114] |
| Lactobacilluscasei | Sea buckthorn extract | attributed to probiotic's antioxidative activity | [94] |
| Lactobacillus rhamnosus GG , Bifidobacterium lactis Bb12 | Oligofructose-enriched inulin | affects the balance of gut flora, increases probitotic strains, decreases coliforms | [121] |
| Bifidobacterium Longum | Raftiline HP(derivative of inulin) |
Nutraceuticals with probiotics
To have a favourable impact, it is considered that an active probiotic meal should contain around 105 CFU/g [59]. According to Yao et al. [62], a research on the GIT survivability of probiotics revealed a decrease of 106 CFU in all commercial products evaluated after 5 minutes incubation in stomach secretions. Probiotic-containing nutraceuticals are derived from biomass produced in large-scale industrial fermentation and then microencapsulated and dried. Typically, these products are marketed in the form of powder, capsules, or chewable tablets. Encapsulation can be accomplished by spray coating the probiotic microbes with fat-based polymers, therefore improving their bioavailability in the stomach environment. STARTM and ProbiocapTM technology are two examples available on the market [68]. Fatty acids (Omega-3, Omega-6): Health benefits of omega-3 and omega-6 fatty acids have been widely documented, however, major obstacles to these oils’ flavour and odour, as well as their tendency to oxidise rapidly, have emerged [124.] It was proven that eating foods enriched with spray-dried microencapsulated fish oil was equivalently effective to consuming fish oil gelatine capsules on a regular basis owing to the equal amounts of omega-3 long-chain fatty acid in both. Fourteen species of live probiotic bacteria were employed, which include, Acetobacter, Lactobacillus and Lactococcus, Propionibacterium and Bifidobacterium. Animal groups received combinations comprising of Symbiter-Smectite and Symbiter-Omega complicated mixes that included, in addition to probiotics, smectite gel. Another group of rats also got flax oil and wheat germ oil, along with an omega-3 unsaturated fatty acid. These oils include alpha-linoleic acid, which acts as a substrate for eicosapentaenoic and docosahexaenoic acids [125]. Combining nutraceuticals and probiotics yields an overall enhanced cytoprotection of the mucosa and a simultaneous improvement in the relationship between the intestinal bacteria and the physiological microflora of the gut. The micro-capsulation technique combining probiotics with nutraceuticals such as omega-3 or smectite is more effective than simply using probiotics alone in a rat model of obesity developed from the ingestion of monosodium glutamate (MSG). To conclude, it preserves the bioactive and non-stable food ingredients and helps to prevent their fast degradation before the products are delivered to specific locations [126- 128]. Encapsulating probiotic bacteria in an oil emulsion containing omega-3 fatty acids was in order to help with probiotic bacteria adhesion to the intestinal epithelium, as well as for the benefits of its synergy [129]. It has been difficult to combine pharmacological agents and nutraceuticals in the same bioactive preparation, until now, since the hydrophobic nature of omega-3 fatty acids and the hydrophilic nature of probiotic mass do not mix well. Vitamins: Most generally used methods for encapsulating water-soluble vitamins are fluidized-bed coating and spraychilling, whereas spray-drying emulsions are the more commonly recommended for lipid-soluble vitamins (e.g. b-carotene, vitamins A, D and E). Ensuring appropriate calcium-protein interactions was made achievable by encapsulating calcium lactate in lecithin liposomes and fortifying soymilk with quantities of calcium comparable to those seen in cow’s milk. As with probiotics, vitamin and mineral co-encapsulation may be helpful [130]. Co-encapsulation of minerals and vitamins may be more effective. Since calcium in a hydrophilic phase is coupled with vitamins A or D in a hydrophobic phase, it may help the bodyabsorb calcium. Synergistic antioxidant activity has been seen in the situation when vitamin E is co-encapsulated within the liposome wall, whereas ascorbic acid is located within the aqueous space [131]
Whey protein, protein isolate and casein hydrolysate :
An encapsulation method used to keep live probiotic cells maintained in fermented dairy products in the gastrointestinal system was reported to be capable of transporting Bifidobacteria cells as well [132]. Since using the spray drying method necessitates high temperatures, heat resistance must be examined before the process is initiated. No change in yoghurt appearance, colour, flavour, taste, or acidity was noted when probiotic capsules were added. In contrast, including probiotic organisms changed the textural characteristics such as smoothness and also resulted in customers noting a gritty texture in yoghurts. However, it was discovered that after the acidification step, the post-acidification process was comparatively slow. While it is difficult to preserve probiotics in acidic fluids, whey protein matrices encapsulated with bacteria survived for several weeks in acid environments. Probiotics were shielded from the low pH environment due to whey protein’s ability to create a colloidal matrix. In addition to that, Reid et al. [96] proved that microencapsulation in a milk matrix also increased L. rhamnosus survival time in vegetable juice by two weeks as compared to free cells stored in a whey protein matrix. In cranberry juice (pH 2.3), which has a low viable count, a milk matrix offered the maximum survival rate. Bioactive peptides are peptides produced from various protein sources, for example, milk or soy. Exemplying this, milk peptides are well-known for their anti-inflammatory and blood pressure-lowering effects as well as for helping to increase mineral absorption [133]. Ensuring the effectiveness of peptides in food can often need concealing their bitter taste and also handling their hygroscopic character through encapsulation techniques. The soybean protein isolate and pectin complex coacervation resulted in a casein hydrolysate that was less bitter than the hydrolysate that was not encapsulated. Besides, the encapsulated casein was less hygroscopic than the free hydrolysate. Similar improvements were obtained when a casein hydrolysate was produced by spray drying gelatin and soy protein carriers
Alginic acid:
A blend of 1,4-linked D-mannuronic and L-guluronic acids is known as alginic acid. Interfacial polymerization also uses cationic monomers, such as calcium, which combines with the water-soluble polymer, alginate, to form a calcium alginate hydrogel. The hydrogel then slowly dries and polymerizes, forming an impermeable barrier. On a laboratory or industrial scale, alginates are commonly used in microencapsulation. This is a common property of powdery matrix capsules, however, the capsules are exceedingly porous, allowing water to flow in and out of the matrix. Alginate encapsulation enhanced survival by one log as compared to free cell counts when bacteria were maintained in skim milk for 24 hours. To ensure their survivability during storage, Lactobacillus bulgaricus can be encapsulated with alginate and a chitosan coating. Kebary et al. [134] demonstrated that Bifidobacteria encased in alginate showed higher survival after being frozen. The alginate beads were prepared by adding glycerol and mannitol, while glucose had no affect.
Glucan:
Also, in apple juice, fresh L. rhamnosus grown on an oat flour with 20% -glucan medium fared better than freeze-dried cells. When L. rhamnosus with fibres as carriers was stored for three months, there was no change in the pH of juice [135]. Bifidobacteria encapsulated in potato starch, which are difficult to cultivate in fermented oat drinks, show no increase in cultivability when stored in nondistilled or distilled beverages. However, when encapsulated in a lipid matrix (cocoa butter) and polydextrose, the bifidobacteria do benefit from improved storage stability.
Combination of various nutraceuticals:
: Eratte et al. [136] was the first to use microcapsules created by the coacervation of whey protein isolate (WPI) and gum Arabic to encapsulate probiotic bacteria (GA) and omega-3 fatty acids. The omega-3 fatty acid tuna oil (O) and the probiotic bacterium Lactobacillus casei 431 (P) were utilised as models, respectively. Through freeze and spray drying, the WPI-P-O-GA Comicrocapsules and L. casei microcapsules were converted into powder. The L. casei bacterium was more vigorous in co-microcapsules of WPI-P-O-GA than in co-microcapsules of WPI-P-GA(without omega-3-fatty acids). For this work, concentrated probiotic bacteria biomass was employed, enhanced with sterile smectite gel and omega-3 polyunsaturated fatty acids derived from flax seeds and wheat embryos. Eicosapentaenoic and docosahexaenoic acids can be formed from alpha-linoleic acid by using it as a substrate. These pharmacological forms were found to be more efficacious than microcapsules. Due to the bacterium’s biologically active state being present in preparations of varying quantities, these bacteria were ready to operate immediately upon oral administration of the preparation. On the other side, the inclusion of smectite gel was designed to enhance probiotic efficacy, as the gel in its active state acts as an enterosorbent. The limitation of this model was that it caused an early-onset obesity due to MSG-induced lesions in newborn animals’ arcuate nuclei. This model was defined by an elevated fat-tobody weight ratio as a result of VAT buildup, an abnormally high HOMA index, severe hyperinsulinemia and hyperleptinemia, all of which indicated the development of diabetes mellitus [137].In the rat MSG obesity model, probiotics and nutraceuticals resulted in a substantial decrease in obesity prevalence, total VAT weight and insulin resistance. The study established that supplementing probiotics with omega-3 fatty acids may be the most helpful anti-obesity strategy. Probiotic bacteria are commonly stabilised by embedding them in food biopolymeric gel matrices (e.g., alginate, milk protein gels, carrageenan and gellan gum). This process entails extruding a hydrocolloid-probiotic cell combination into a solution which hardens the capsules through a nozzle and maintains a protective environment for the desired microorganisms until they reach the desired location in the intestinal system. Synbiotic formulations including protein-carbohydrate-oil matrices and prebiotics preserve probiotic bacteria during nonrefrigerated storage when they are exposed to low pH, while also enabling their release in simulated intestinal fluids [138]. Resveratrol and curcumin are insoluble in water and oils, and so they are easily destroyed. Due to this, the challenges that prevent these chemicals from entering water-based meals and transportation across the gastrointestinal system are stabilisation and increasing their solubility in aquatic conditions. A wide range of delivery vehicles is conceivable for polyphenols, including, but not limited to, protein, liposomal preparations, cyclodextrin, and polysaccharide (pectinate, chitosan). They are efficient transporters of polyphenols due to their protein-based capability to transport polyphenols. A different option is to use partially-separated buttermilk, which is the byproduct of creating butter. During the separation process, certain components of the butter stay in the buttermilk’s oil phase.The application of gum acacia soluble fibre prior to spray drying results in an increase in the growth of Lactobacillus Paracasei, while a modified waxy maize starch coating does not [139].
Most water-soluble polyphenols (such as tea catechins) can be absorbed directly into the body but they must be protected from oxidation and maintained at a high pH. Epigallocatechin gallate (EGCG) is bound to natural food-derived components, such as apple pomace, to be used as a carrier in functional food formulations. Studies conducted in vitro have proven that chitosan-tripolyphosphate nanoparticles that are coated with catechin and EGCG remain stable in the acidic digestive system environment. In the small intestine, anthocyanins have also been proven to be protected from degradation by being encapsulated in polysaccharide (pectin amide) or whey proteins.
Fermented Foods
Probiotics in food not only have additional health benefits but also improve its taste. Their high stability during manufacturing and storage for the duration of the food’s shelf life, on the other hand, is critical for maintaining the food’s probiotic impact [115]. The majority of probiotic-containing foods on the market are enhanced with probiotic microbes in their natural state, with just a small percentage using their microencapsulated form [61]. Additionally, probiotics can be incorporated into edible polymeric matrices to create (bio)active food packaging, acting as a natural alternative to antibiotics in terms of preventing pathogenic/spoilage microorganisms and enhancing food safety, in addition to their role in promoting consumer health (Espitia et al., 2016). The most common food products functionalized with probiotic microcapsules are dairy (yoghurt and cheese), bakery (bread, cakes, and cookies), meat (fermented sausages), juices (fruits and vegetables), and others (ice cream, mayonnaise, fermented drinks).
Lactose-based
Probiotic milk products, fresh and ripened cheese, have all been around for a long time. Prebiotic lactulose increases probiotic count (especially Bifidobacterium lactis), lactic acid concentration and acidification rate in skimmed milk while also shortening fermentation time [146]. The use of prebiotic milk whey culture can help with the treatment of inflammatory bowel disease [141]. Probiotic starter cultures, fermented milk products and yoghurt powder are used to make these probiotic ice creams since the twentieth century. Lactobacillus casei and 2.5 percent inulin were added to this ice cream, which had favourable nutritional and sensory properties [147]. Other probiotic-rich fermented milk products, such as sour milk, sour whey, sour cream, buttermilk, or kefir, are less common. Compared to yoghurt, probiotic-enriched unfermented milks (bifidus milk, sweet acidophilus milk) are far less popular.
Baked products
Bakery goods, which include breads, cakes, and pastries, are critical to the European diet, so they allow for functionality in any form to be provided to the customer in an acceptable meal. Concentrated forms of bioactive substances such as curcumin or omega-3 fatty acids are frequently encapsulated in breads [148]. The use of probiotic microorganisms in this kind of food remains unexplored. The only major hurdle to probiotic survival is the heat involved in cooking food and how long some probiotic-containing foods have to be stored afterward (e.g. biscuits). Ensuring that probiotic cultures remain viable until product consumption is vital for the successful production of bakery products. Probiotic resilience and stability are improved through microencapsulation.Concurrently, the applicability of probiotics in breads remains unexplored [149]. Altamirano-Fortoul et al. [150] used Lactobacillus acidophilus probiotic microcapsules was to bind to the surface of partially baked bread. Immediately after baking, the final product had a survival rate of 63% with a 10% drop over time. Although Lactobacillus rhamnosus GG cells are used to produce edible films which are applied to cooked bread, they do microencapsulate probiotic cells. Instead, they were given via edible films composed of a mixture of two biopolymers, whey proteins and sodium alginate, often employed as microencapsulating agents [151]. Probiotic viability was increased after using an edible film that contained alginate and whey proteins during the drying and storage of bread, as opposed to an alginate-only film that caused higher in vitro digestion survival results. Lactobacillus rhamnosus R011 was encapsulated in whey protein for inclusion in baked goods [143]. The viability of microencapsulated cells decreased by two log cycles during the baking process but a total of five log cycles were lost by the end of storage. Malmo et al. [152] implanted Lactobacillus reuteri DSM 17938 into chitosan-alginate microcapsules within a chocolate soufflé prior to baking at 180°C for 10 minutes (the core of the product at 80°C). Following chitosan-alginate microencapsulation treatment, the scientists obtained a 10% survival rate for the probiotic population, compared to the 1% survival rate of the unencapsulated cells. Moreover, the study found that encapsulated Lactobacillus reuteri DSM 17938 is more heat resistant in actual food than in vitro. Using the hypothesis that food matrices protect probiotic cells, cakes were stuffed with Lactobacillus acidophilus ATCC 39392 microencapsulated in a cream. They had a reduced viability of about 3 log cycles, indicating that microencapsulation has a significant protective impact on probiotic bacteria in bakery goods [153].
Cereal-based
Cereals, particularly barley and oat, are essential in the manufacturing of functional foods. Cereals’ numerous health benefits can be leveraged in a variety of ways, resulting in the development of novel cereal foods and cereal ingredients aimed at specific demographics. To make an easy-to-use carrier for dried probiotics, cereals (“flakes”) were combined with sugar and lyophilized probiotic cultures [154]. Breads with dietary fibres have better sensory perception, more resistant and less digestible starch [155]. Damen et al. [156] investigated the synthesis of prebiotic AXOS in-situ during the bread-making process. Also, the in vivo prebiotic potential of barley -glucan was found to have a strong bifidogenic effect [157].
Meat-based
Human health and wellness has likewise influenced interest in functional meat products because of various studies regarding the fat and salt content. Probiotic microorganisms may have additional benefits in functional meat production. Probiotic use has been studied in many different types of fermented sausage, pork where novel probiotic strains are isolated. The lactobacilli are the main microorganisms used in the fermented meat products as starter cultures; it has also been shown that certain probiotic or potentially probiotic lactobacillus strains retain viability and fermentative activity in sausages, resulting in a final product. Probiotic bacteria is used in meat products to create an antibacterial impact on spoilage and pathogenic populations. In a meat-fermented environment, however, it was discovered that Lactobacillus reuteri ATCC 55730 is particularly susceptible to severe conditions. Low pH and competing organisms have all been identified as intrinsic factors that can lead to the failure of probiotic cells. Microencapsulation process still requires improvements on a commercial scale, representing a challenge to be overcome in order to obtain immobilized products that are economically viable. Probiotic sausages were created by encapsulating live Lactobacillus reuteri ATCC 55730 and Bifidobacterium longum, which remained viable during the manufacturing, fermentation and drying processes. Microencapsulation had no effect on the antimicrobial activity of the sausage against E. coli O157:H. Lactobacillus casei ATCC 393 in wheat grains was used to functionalize fermented sausage. Their goals were to preserve the probiotic strain’s survival at levels high enough to impart health benefits, improve the qualitative aspects of the new goods and extend their shelf life. Additionally, L. casei was found in sausages in the free and immobilised state, both of which had levels high enough to be responsible for imparting a probiotic effect during ripening. Besides demonstrating a protective effect on cell viability, L. casei cells with chemical preservatives have a greater effect in retarding sausage deterioration than free L. casei cells. The effect of L. casei and its inoculum concentration was also studied on the sausage’s aroma. Despite being underutilised, microencapsulation technologies may be a possible way to use probiotic microorganisms in meat products.
From fruits and vegetables
Fermented vegetable products (for example, “sauerkraut,” “kimchi,” or “lacto-pickles”) contain probiotic lactobacilli [158]. The probiotic impact of fermented cashew and apple juice is assessed by using Lactobacillus johnsonii B-2178 [159]. By combining probiotic bacteria, Lactobacillusrhamnosus GG with the prebiotics oligofructose and inulin, potentially synbiotic fresh-cut apple wedges have also been created [160].
From roots and tubercule
Sinki and Sunki is a fermented radish product and themost common probiotic bacteria identified are Lactobacillus fermentum, Lactobacillus brevis and Lactobacillus plantarum [161]. The Japanese product “sunki” is made by fermenting otaki-turnip without the addition of salt. Lactobacillus delbrueckii, Lactobacillus plantarum and Lactobacillus fermentum dominated the bacterial community when it was profiled using PCR-denaturing gradient gel electrophoresis throughout fermentation [132]. Kanji is a product made from fermented purple carrots. This carrot kanji yielded one genotype of Lb. pentosus and two genotypes of Lb. paraplantarum after addition of water and being brine-salted for 7-10 days [133]. Spray-dried probiotic bacteria can be added to the production of sweets, confectionery pastries [164]. Milk powder based products skimmed milk, yoghurt family, buttermilk, or whey on the other hand, can be used as a delivery mechanism. Little attention has been paid to the long-term viability of probiotic microorganisms in powdered milk products [165].
Beverages
Non-alcoholic beverage is a significant functional foood fortified with vitamins A, C, and E or other functional additives. There are numerous cholesterol-lowering beverages (including a mix of omega-3 and soy), beverages for eye health (including lutein) and bone health (containing calcium and inulin) available as functional foods [130,136]. Fortified juices with inulin, vitamins, L-carnitine, magnesium and calcium are also marketed.
Challenges while Incorporating Nutraceuticals in Food and Its Solutions
While delivering an effective dose of nutrients is important, it is necessary to consider its impact on the completed product’s flavour and fragrance. Ensuring that the food is free of contamination is also a challenge, especially as food can sometimes harbour contaminants. Additionally, there are several concerns: a) avoiding undesirable interactions between the nutraceutical, the environment and the food matrix components; b) limiting nutraceutical degradation during processing of food; c) stabilising the nutraceutical during shelf life; and d) preventing contamination of the food containing the nutraceutical. As many bioactives are found only in their natural food source, they need to be encapsulated before being used in the preparation of designed functional food. In order to encapsulate an unstable component, the encapsulation system should take into account the component’s compatibility for the final food application and the substance’s anticipated health impact. Establishing the need for microencapsulation before manufacturing microencapsulated nutraceuticals that give advantages over direct usage and incorporating a nutraceutical into a food product is of paramount importance. When administering nutraceuticals in their natural state (i.e., unencapsulated), special consideration needs to be given to the difficulties associated with effectively distributing and incorporating them into food products. Overcoming the restrictions in the application of nonencapsulated nutraceuticals in functional foods is a must in order to find creative solutions. At certain instances, while using natural carriers like food components, it may be possible to encapsulate micronutraceuticals for a specific application.
High levels of omega-3 fatty acids found in certain oils
This ingredient has gained a lot of attention because of its multiple health benefits. As far as health benefits go, theseindividuals are also more protected from the wide range of inflammatory conditions [186]. Some types of polyunsaturated fatty acids, particularly the long-chain polyunsaturated fatty acids (LCPUFAs) that contain the omega-3 fatty acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are subject to oxidation. Inadequate protection of omega-3 fatty acids from oxygen results in the production of smells and tastes that are objectionable. To protect the oil from oxidation, antioxidants may be added to large-volume oils to help slow down the oxidation process. For this purpose, antioxidants have been used to help keep high-quality fish oils in good condition, where they are added to some shortlife products to provide lower amounts of omega-3 LCPUFAs. To protect them from oxidation, microencapsulation uses two mechanisms: First, it prevents oxidation in the omega-3 fatty acid component; second, it keeps oxidation out of the finished food product. This allows omega-3 LCPUFAs to be utilised in a wider range of dishes [187]. Omega-3 fatty acid formulations that use several patented ways to encapsulate them include spray drying emulsions, fluidized bed coating, extrusion, coacervation, and biopolymer gelation [188]. Stabilised oils-in-water emulsions commonly use proteins or combinations of proteins and carbohydrates that have been spray dried into powders. To obtain Maillard reaction products prior to emulsification, protein-sugar mixtures are heated until the formation of Maillard reaction products occurs. This enables oils stabilised by proteins and carbohydrates to be more resistant to oxidation [189]. Omega-3-rich milk powders or powdered drinks can be made by combining powdered omega-3 oils with other powdered ingredients. It is also possible to include oils into aqueous-based food items like juices because of the availability of omega-3 fatty acids in both powder and emulsion forms. Some investigations have found omega-3 fish oils provided as microencapsulated complex coacervates to be bioequivalent to soft gel capsules [127]. Two experiments designed to examine human ileostomy capabilities showed that both microencapsulated fish oils and fish oil gelatin capsules, whose ingredients included Maillard reaction products, proved equally effective when blended with a variety of meals, including orange juice, yoghurt, and cereal bars. The kinetics of the fish oil microencapsulation in the gastrointestinal tract was modified too [190].
Fat-soluble bioactives
Additionally, phytosterol, carotenoids, phytostanol and fatsoluble vitamins such as vitamins A, D, E, and K can be added to functional foods. The major problems with included dietary fats in the diet are their sensitivity to oxidation and low water solubility. They are insoluble in water, which means that they are unable to be absorbed into the human body. Because encapsulation technologies (emulsions, microemulsions, nanoemulsions, solid lipid nanoparticles, and liposomes) have successfully incorporated these lipids and fat-soluble bioactives into functional meals, these may be more bioavailable than previously thought [191,192].
Emerging Trends and Future Perspectives
The technically and economically viable method of microencapsulating probiotic bacteria and omega-3 fatty acids is suitable for incorporation into foods. Food and beverage items must be rigorously tested for their effects on shelf life and sensory qualities before they can be released to the market. While omega-3 fatty acids are typically found in functional foods, probiotic bacteria are rarely included in the formulas. Because of the significant savings in money and increased product quality, it is essential to co-encapsulate numerous substances into a single microcapsule. Unstable substances, including numerous reagents, have been shown to benefit from the use of coacervation, which utilises a complex coacervation. Finally, given the global demand for prebiotics, probiotics and nutraceuticals, there has been a concerted effort to meet that demand over the last decade. Developed nations should work together to develop a common standard for probiotic, prebiotic, and nutraceutical manufacturing practises. Because many prebiotic sources are geographically limited, the quality manufacturing guideline may help to reduce trade barriers between countries and continents for prebiotics, probiotics and nutraceuticals. Given that it is widely accepted that a person’s nutritional and medical requirements alter as they age, the functional food and nutraceutical sectors may offer products targeted at specific age groups. (for example, omega-3 fatty acids), immunological health (for example, probiotics), digestive health (for example, probiotics, fibre), skeletal and bone health (e.g.vitamin D3, calcium), the ageing process (for example, resveratrol, antioxidants) and eye health (for example, zeaxanthin, lutein). For babies and very young children, products that stimulate immune health (e.g., probiotics) and brain development (e.g., omega-3 fatty acids). Infant formula is the fastest growing category of functional foods on the planet, followed by energy drinks and pre- and probiotic yoghurts. Due to the increased prevalence of dietary intolerances and health concerns connected with soy and dairy, nondairy milk substitutes such as rice, hemp nut and oat milks are predicted to be the fastest-growing functional beverage category. Functional foods and beverages that promote general well-being perform best, followed by those that promote digestive health, weight management, endurance and energy boost. Among these categories, the fastest growing occupation is Energy-boosting foods [193]. Due to the potential for synergistic health benefits, foods containing a combination of nutraceutical ingredients are fast gaining appeal in the market [194]. This hampers the evolution of encapsulation technologies and their placement in functional meals in the future. Advances in nutrigenomics, combined with a greater understanding of nutrient-geneinteractions along with the impact of diet and lifestyle on health and well-being, will spur the industry to formulate more personalised products for certain demographic segments and people.
Conclusion
By incorporating nutraceuticals into meals, customers gain access to a broader variety of healthier food options that can assist them in meeting some of their health and wellness goals. Customer trust in functional food items containing nutraceuticals will rise as a result of evidence-based research, as will the regulations governing functional foods and nutraceuticals. These regulations vary by country [195] and must be taken into account when developing functional foods for international markets. Encapsulating a nutraceutical component before adding it to a food product is a common practise in the food industry. It is employed to overcome challenges associated with the addition of nutraceutical components at an acceptable dosage without impairing the sensory features or storage stability of the meal. There are numerous encapsulation methods available, and the method chosen is dictated by the nutraceutical component’s properties and intended usage. When food is used as a vehicle for delivery, novel formulation processes and food shapes have been investigated. Whichever methodology is used, it is vital to understand the interaction between the nutraceutical component and the food matrix during processing. These interactions may have an effect on the extra nutraceutical component’s bioavailability and bioefficacy. Additional research is critical for understanding the interactions of nutraceuticals with complex food matrices and to emphasise both the health advantages of nutraceutical products in food and the challenges associated with their transit from the intestines to the body.
Abbreviations
LAB: Lactic Acid Bacteria; Ig: Immunoglobin; Th: T cells; IL: Interleukin; IFN: Interferon; GOS: Galactooligosaccharides; FOS: Fructooligosaccharides; XOS: Xylooligosaccharides; BCFA: Branched Chain Fatty Acids; SCFA: Short Chain Fatty Acids; BCAA: Branched Chain Amino Acids; BA: Bile Acids; GS: Gas Chromatography; MS: Mass Spectrometry; LGG: L. rhamnosus GG; PBMC: Peripheral Blood Mononuclear Cells; RS: Resistant Starch; PP: Peyer’s Patches; MSG: Monosodium Glutamate; LCPUFA: Long-Chain Polyunsaturated Fatty Acids.
Competing Interest
The author has no financial interests or conflict of interests.
Authors’ Contribution
The first author is Ekta Jagtiani and second author is Sachin Adsare. They have written the entire review article.
References
2. Jones M, Defever E, Ayland Letsinger J, Weaver RG, Hunt E, et al. The Catalogue of Journal of Sport and Health Science 2020. The Catalogue of Journal of Sport and Health Science. 2020;2095:2546.
3. Ballan R, Battistini C, Xavier-Santos D, Saad SM. Interactions of probiotics and prebiotics with the gut microbiota. Progress in Molecular Biology and Translational Science. 2020 Jan 1;171:265-300.
4. Spencer SP, Fragiadakis GK, Sonnenburg JL. Pursuing humanrelevant gut microbiota-immune interactions. Immunity. 2019 Aug 20;51(2):225-39.
5. Butorac K, Novak J, Bellich B, Terán LC, Banić M, Leboš Pavunc A, et al. Lyophilized alginate-based microspheres containing Lactobacillus fermentum D12, an exopolysaccharides producer, contribute to the strain’s functionality in vitro. Microbial Cell Factories. 2021 Dec;20(1):1-7.
6. Lu D, Huang Y, Kong Y, Tao T, Zhu X. Gut microecology: Why our microbes could be key to our health. Biomedicine & Pharmacotherapy. 2020 Nov 1;131:110784.
7. Michaudel C, Sokol H. The gut microbiota at the service of immunometabolism. Cell Metabolism. 2020 Oct 6;32(4):514-23.
8. Atarashi K, Umesaki Y, Honda K. Microbiotal influence on T cell subset development. Seminars in Immunology. 2011 Apr 1;23(2):146-153.
9. Peredo-Lovillo A, Romero-Luna HE, Jiménez-Fernández M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Research International. 2020 Oct 1;136:109473.
10. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. British Journal of Nutrition. 2010 Aug;104(S2):S1-63.
11. Ashaolu TJ. A review on selection of fermentative microorganisms for functional foods and beverages: the production and future perspectives. International Journal of Food Science & Technology. 2019 Aug;54(8):2511-9.
12. Grumezescu AM, Holban AM. Nutrients in Beverages. Academic Press. 2019.
13. da Silva JM, Barão CE, Esmerino EA, Cruz AG, Pimentel TC. Prebiotic frozen dessert processed with water‐soluble extract of rice byproduct: Vegan and nonvegan consumers perception using preferred attribute elicitation methodology and acceptance. Journal of Food Science. 2021 Feb;86(2):523-30.
14. Han B, Hoang BX. Opinions on the current pandemic of COVID-19: Use functional food to boost our immune functions. Journal of Infection and Public Health. 2020 Dec 1;13(12):1811-7.
15. Illanes A, Guerrero C, Vera C, Wilson L, Conejeros R, Scott F. Lactose-derived prebiotics: a process perspective. Academic Press; 2016 Jul 6.
16. Foligné B, Dewulf J, Vandekerckove P, Pignède G, Pot B. Probiotic yeasts: anti-inflammatory potential of various nonpathogenic strains in experimental colitis in mice. World Journal of Gastroenterology. 2010 May 7;16(17):2134.
17. Zoumpourtikoudi V, Pyrgelis N, Chatzigrigoriou M, Tasakis RN, Touraki M. Interactions among yeast and probiotic bacteria enhance probiotic properties and metabolism offering augmented protection to Artemia franciscana against Vibrio anguillarum. Microbial Pathogenesis. 2018 Dec 1;125:497-506.
18. Hu J, Zhang L, Lin W, Tang W, Chan FK, Ng SC. Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends in Food Science & Technology. 2021 Feb 1;108:187-96.
19. Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Archives of Disease in Childhood. 2006 Oct 1;91(10):814-9.
20. Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Humanmilk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. The Journal of Nutrition. 2005 May 1;135(5):1304-7.
21. Shah BR, Li B, Al Sabbah H, Xu W, Mráz J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends in Food SCience & Technology. 2020 Aug 1;102:178-92.
22. Michaudel C, Sokol H. The gut microbiota at the service of immunometabolism. Cell metabolism. 2020 Oct 6;32(4):514-23.
23. Andriulli A, Neri M, Loguercio C, Terreni N, Merla A, Cardarella MP, et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. Journal of Clinical Gastroenterology. 2008 Sep 1;42:S218-23.
24. Colecchia A, Vestito A, La Rocca A, Pasqui F, Nikiforaki A, Festi D. Effect of a symbiotic preparation on the clinical manifestations of irritable bowel syndrome, constipation-variant. Results of an open, uncontrolled multicenter study. Minerva Gastroenterologica e Dietologica. 2006 Dec 1;52(4):349-58.
25. Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, Marotta F. Single‐blind follow‐up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chinese Journal of Digestive Diseases. 2004 Oct;5(4):169-74.
26. ughera L, Elia C, Navino M, Cisarò F. Effects of symbiotic preparations on constipated irritable bowel syndrome symptoms. Acta Bio-Medica: Atenei Parmensis. 2007 Aug 1;78(2):111-6
27. El Sohaimy SA. Functional foods and nutraceuticals-modern approach to food science. World Applied Sciences Journal.2012;20(5):691-708.
28. Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, Pani L, et al. Nutraceuticals: Opening the debate for a regulatory framework. British Journal of Clinical Pharmacology. 2018 Apr;84(4):659-72.
29. Télessy IG. Nutraceuticals. In: The role of functional food security in global health. Academic Press. 2019; pp. 409-421.
30. Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, Pani L, et al. Nutraceuticals: Opening the debate for a regulatory framework. British Journal of Clinical Pharmacology. 2018 Apr;84(4):659-72.
31. Sarao LK, Arora M. Probiotics, prebiotics, and microencapsulation: A review. Critical Reviews in Food Science and Nutrition. 2017 Jan 22;57(2):344-371.
32. Shishir MR, Xie L, Sun C, Zheng X, Chen W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology. 2018 Aug 1;78:34-60.
33. acobsen C, García-Moreno PJ, Mendes AC, Mateiu RV, Chronakis IS. Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annual Review of Food Science and Technology. 2018 Mar 25;9:525-49.
34. Chen J, Hu L. Nanoscale delivery system for nutraceuticals: Preparation, application, characterization, safety, and future trends. Food Engineering Reviews. 2020 Mar;12(1):14-31.
35. Sun W, Griffiths MW. Survival of bifidobacteria in yogurt and simulated gastric juice following immobilization in gellan–xanthan beads. International Journal of Food Microbiology. 2000 Oct 1;61(1):17-25.
36. Cui JH, Goh JS, Kim PH, Choi SH, Lee BJ. Survival and stability of bifidobacteria loaded in alginate poly-l-lysine microparticles. International Journal of Pharmaceutics. 2000 Dec 4;210(1-2):51-9.
37. Senuma Y, Lowe C, Zweifel Y, Hilborn JG, Marison I. Alginate hydrogel microspheres and microcapsules prepared by spinning disk atomization. Biotechnology and Bioengineering. 2000 Mar 5;67(5):616-22.
38. Chandramouli V, Kailasapathy K, Peiris P, Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. Journal of Microbiological Methods. 2004 Jan 1;56(1):27-35.
39. Ryu JS, Lee YJ, Choi SI, Lee JW, Heo TR. Optimization of screw pumping system (SPS) for mass production of entrapped bifidus. Food Science and Biotechnology. 2005;14(5):566-71.
40. Mokarram RR, Mortazavi SA, Najafi MH, Shahidi F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Research International. 2009 Oct 1;42(8):1040-5.
41. Heidebach T, Först P, Kulozik U. Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocolloids. 2009 Oct 1;23(7):1670-7.
42. Oliveira RP, Florence AC, Silva RC, Perego P, Converti A, Gioielli LA, Oliveira MN. Effect of different prebiotics on the fermentation kinetics, probiotic survival and fatty acids profiles in nonfat symbiotic fermented milk. International Journal of Food Microbiology. 2009 Jan 15;128(3):467-72.
43. Jono K, Ichikawa H, Miyamoto M, Fukumori Y. A review of particulate design for pharmaceutical powders and their production by spouted bed coating. Powder Technology. 2000 Dec 6;113(3):269-77.
44. Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Research International. 2007 Nov 1;40(9):1107-21.
45. Ding WK, Shah NP. An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. Journal of Food Science. 2009 Mar;74(2):M53-61.
46. Graff S, Hussain S, Chaumeil JC, Charrueau C. Increased intestinal delivery of viable Saccharomyces boulardii by encapsulation in microspheres. Pharmaceutical Research. 2008 Jun;25(6):1290-6.
47. Faure A, York P, Rowe RC. Process control and scale-up of pharmaceutical wet granulation processes: a review. European Journal of Pharmaceutics and Biopharmaceutics. 2001 Nov 1;52(3):269-77.
48. Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules. 2011 Jul 11;12(7):2834-40.
49. Illahi AF, Muhammad F, Akhtar B. Nanoformulations of nutraceuticals for cancer treatment. Critical Reviews™ in Eukaryotic Gene Expression. 2019;29(5).
50. Speranza B, Petruzzi L, Bevilacqua A, Gallo M, Campaniello D, Sinigaglia M, et al. Encapsulation of active compounds in fruit and vegetable juice processing: current state and perspectives. Journal of food science. 2017 Jun;82(6):1291-301.
51. Dutta S, Moses JA, Anandharamakrishnan C. Encapsulation of nutraceutical ingredients in liposomes and their potential for cancer treatment. Nutrition and Cancer. 2018 Nov 17;70(8):1184-98.
52. Lopes NA, Brandelli A. Nanostructures for delivery of natural antimicrobials in food. Critical Reviews in Food Science and Nutrition. 2018 Sep 2;58(13):2202-12.
53. Ruiz-Rico M, Pérez-Esteve É, Lerma-García MJ, Marcos MD, Martínez-Máñez R, Barat JM. Protection of folic acid through encapsulation in mesoporous silica particles included in fruit juices. Food Chemistry. 2017 Mar 1;218:471-8.
54. Zarrabi A, Alipoor Amro Abadi M, Khorasani S, Mohammadabadi MR, Jamshidi A, et al. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and dietary molecules. Molecules. 2020 Feb 1;25(3):638.
55. Rasti B, Jinap S, Mozafari MR, Yazid AM. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chemistry. 2012 Dec 15;135(4):2761-70.
56. Sarao LK, Arora M. Probiotics, prebiotics, and microencapsulation: A review. Critical reviews in food science and nutrition. 2017 Jan 22;57(2):344-71.
57. Corona‐Hernandez RI, Álvarez‐Parrilla E, Lizardi‐Mendoza J, Islas‐Rubio AR, de la Rosa LA, Wall‐Medrano A. Structural stability and viability of microencapsulated probiotic bacteria: a review. Comprehensive Reviews in Food Science and Food Safety. 2013 Nov;12(6):614-28.
58. Coghetto CC, Brinques GB, Ayub MA. Probiotics production and alternative encapsulation methodologies to improve their viabilities under adverse environmental conditions. International Journal of Food Sciences and Nutrition. 2016 Nov 16;67(8):929-43.
59. Sarao LK, Arora M. Probiotics, prebiotics, and microencapsulation: A review. Critical Reviews in Food Science and Nutrition. 2017 Jan 22;57(2):344-71.
60. Lopes NA, Brandelli A. Nanostructures for delivery of natural antimicrobials in food. Critical Reviews in Food Science and Nutrition. 2018 Sep 2;58(13):2202-12.
61. De Prisco A, Mauriello G. Probiotication of foods: A focus on microencapsulation tool. Trends in Food Science & Technology. 2016 Feb 1;48:27-39.
62. Yao M, Xie J, Du H, McClements DJ, Xiao H, Li L. Progress in microencapsulation of probiotics: A review. Comprehensive Reviews in Food Science and Food Safety. 2020 Mar;19(2):857-74.
63. De Prisco A, Mauriello G. Probiotication of foods: A focus on microencapsulation tool. Trends in Food Science & Technology. 2016 Feb 1;48:27-39.
64. Misra S, Pandey P, Mishra HN. Novel approaches for coencapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends in Food Science & Technology. 2021 Mar 1;109:340- 51.
65. Mahmoud M, Abdallah NA, El-Shafei K, Tawfik NF, El-Sayed HS. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon. 2020 Mar 1;6(3):e03541.
66. Zhang Y, Lin J, Zhong Q. The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Research International. 2015 May 1;71:9-15.
67. Martín MJ, Lara-Villoslada F, Ruiz MA, Morales ME. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innovative Food Science & Emerging Technologies. 2015 Feb 1;27:15-25.
68. Kwak HS, editor. Nano-and microencapsulation for foods. John Wiley & Sons; 2014 Apr 2.
69. Ardiansah I, Sholiha K, Sjofjan O. Dietary supplementation of powdered and encapsulated probiotic: In vivo study on relative carcass, giblet weight and intestinal morphometry of local duck. Acta Scientiarum. Animal Sciences. 2020 Feb 3;42.
70. Gunzburg WH, Aung MM, Toa P, Ng S, Read E, Tan WJ, et al. Efficient protection of microorganisms for delivery to the intestinal tract by cellulose sulphate encapsulation. Microbial Cell Factories. 2020 Dec;19(1):1-4.
71. Anselmo AC, McHugh KJ, Webster J, Langer R, Jaklenec A. Layer‐ by‐layer encapsulation of probiotics for delivery to the microbiome. Advanced Materials. 2016 Nov;28(43):9486-90.
72. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017 Mar 4;8(2):172-84.
73. CODEX GUIDELINES ON NUTRITION LABELLING [Internet]. [cited 2021 Jul 12]. Available from: http://www.fao.org/3/y2770e/ y2770e06.htm
74. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nature reviews Gastroenterology & hepatology. 2015 May;12(5):303-10.
75. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nature Reviews Gastroenterology & Hepatology. 2020 Nov;17(11):687-701.
76. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiotagut- brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological Psychiatry. 2017 Oct 1;82(7):472-87.
77. Yang SC, Chen JY, Shang HF, Cheng TY, Tsou SC, Chen JR. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World Journal of Gastroenterology. 2005 Dec 21;11(47):7413.
78. Sheela T, Suganya RS. Studies on anti-diarrhoeal activity of synbiotic plums juice. International Journal of Scientific and Research Publications. 2012 Feb;2(2):1-5.
79. Fritzen-Freire CB, Prudêncio ES, Amboni RD, Pinto SS, Negrão- Murakami AN, Murakami FS. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Research International. 2012 Jan 1;45(1):306-12.
80. Malkov SV, Markelov VV, Polozov GY, Barabanschikov BI, Kozhevnikov AY, Trushin MV. Significant delay of lethal outcome in cancer patients due to peroral administration of Bacillus oligonitrophilus KU-1. The Scientific World Journal. 2006 Jul 8;6:2177- 87.
81. Rafter J. Probiotics and colon cancer. Best Practice & Research Clinical Gastroenterology. 2003 Oct 1;17(5):849-59.
82. Rafter J. Lactic acid bacteria and cancer: mechanistic perspective. British Journal of Nutrition. 2002 Sep;88(S1):S89-94.
83. Van Loo J, Clune Y, Bennett M, Collins JK. The SYNCAN project: goals, set-up, first results and settings of the human intervention study. British Journal of Nutrition. 2005 Apr;93(S1):S91-8.
84. Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. The American journal of clinical nutrition. 2007 Feb 1;85(2):488-96.
85. Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017 Sep;9(9):1021.
86. Mai V, Colbert LH, Perkins SN, Schatzkin A, Hursting SD. Intestinal microbiota: a potential diet‐responsive prevention target in ApcMinmice. Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center. 2007 Jan;46(1):42-8.
87. Rowland IR, Rumney CJ, Coutts JT, Lievense LC. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis. 1998 Feb 1;19(2):281-5.
88. Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. International Journal of Pharmaceutics. 2014 May 15;466(1-2):400-8.
89. Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules. 2011 Jul 11;12(7):2834-40.
90. Liao N, Luo B, Gao J, Li X, Zhao Z, Zhang Y, Ni Y, Tian F. Oligosaccharides as co-encapsulating agents: effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract. Biotechnology Letters. 2019 Feb;41(2):263-72.
91. D’Orazio G, Di Gennaro P, Boccarusso M, Presti I, Bizzaro G, Giardina S, et al. Microencapsulation of new probiotic formulations for gastrointestinal delivery: in vitro study to assess viability and biological properties. Applied Microbiology and Biotechnology. 2015 Nov;99(22):9779-89.
92. Pop OL, Dulf FV, Cuibus L, Castro-Giráldez M, Fito PJ, Vodnar DC, Coman C, Socaciu C, Suharoschi R. Characterization of a sea buckthorn extract and its effect on free and encapsulated Lactobacillus casei. International Journal of Molecular Sciences. 2017 Dec;18(12):2513.
93. Yeung TW, Üçok EF, Tiani KA, McClements DJ, Sela DA. Microencapsulation in alginate and chitosan microgels to enhance viability of Bifidobacterium longum for oral delivery. Frontiers in Microbiology. 2016 Apr 19;7:494.
94. Haghshenas B, Abdullah N, Nami Y, Radiah D, Rosli R, Yari Khosroushahi A. Microencapsulation of probiotic bacteria Lactobacillus plantarum 15 HN using alginate‐psyllium‐fenugreek polymeric blends. Journal of Applied Microbiology. 2015Apr;118(4):1048-57.
95. Adhikari K, Mustapha A, Grün IU. Survival and metabolic activity of microencapsulated Bifidobacterium longum in stirred yogurt. Journal of Food Science. 2003 Jan;68(1):275-80.
96. Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate– starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. International Journal of Food Microbiology. 2000 Dec 5;62(1-2):47-55.
97. Capela P, Hay TK, Shah NP. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Research International. 2006 Mar 1;39(2):203-11.
98. Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010 Feb 1;31(2):246-51.
99. Le Leu RK, Brown IL, Hu Y, Bird AR, Jackson M, Esterman A, et al. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. The Journal of Nutrition. 2005 May 1;135(5):996-1001.
100. Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010 Feb 1;31(2):246-51.
101. Kolida S, Gibson GR. Synbiotics in health and disease. Annual Review of Food Science and Technology. 2011 Apr 10;2:373-93.
102. Roller M, Femia AP, Caderni G, Rechkemmer G, Watzl B. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. British Journal of Nutrition. 2004 Dec;92(6):931-8.
103. Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate– starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. International Journal of Food Microbiology. 2000 Dec 5;62(1-2):47-55.
104. Mandal S, Puniya AK, Singh K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. International Dairy Journal. 2006 Oct 1;16(10):1190-5.
105. Chen MJ, Chen KN, Kuo YT. Optimal thermotolerance of Bifidobacterium bifidum in gellan–alginate microparticles. Biotechnology and bioengineering. 2007 Oct 1;98(2):411-9.
106. Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. The Journal of Nutrition. 2004 Jan 1;134(1):153-6.
107. Iannitti T, Palmieri B. Therapeutical use of probiotic formulations in clinical practice. Clinical Nutrition. 2010 Dec 1;29(6):701-25.
108. Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Veterinary immunology and immunopathology. 2016 Apr 1;172:72-84.
109. Sarao LK, Arora M. Probiotics, prebiotics, and microencapsulation: A review. Critical Reviews in Food Science and Nutrition. 2017 Jan 22;57(2):344-71.
110. Shah NP, Ravula RR. Microencapsulation of probiotic bacteria and their survival in frozen fermented dairy desserts. Australian Journal of Dairy Technology. 2000 Oct 1;55(3):139.
111. Hansen LT, Allan-Wojtas PM, Jin YL, Paulson AT. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiology. 2002 Feb 1;19(1):35-45.
112. Saarela M, Virkajärvi I, Nohynek L, Vaari A, Mättö J. Fibres as carriers for Lactobacillus rhamnosus during freeze-drying and storage in apple juice and chocolate-coated breakfast cereals. International journal of Food Microbiology. 2006 Nov 1;112(2):171- 8.
113. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. The Journal of Nutrition. 1995 Jun 1;125(6):1401-12.
114. Yao M, Xie J, Du H, McClements DJ, Xiao H, Li L. Progress in microencapsulation of probiotics: A review. Comprehensive Reviews in Food Science and Food Safety. 2020 Mar;19(2):857-74.
115. Anjani K, Iyer C, Kailasapathy K. Survival of co-encapsulated complementary probiotics and prebiotics in yoghurt. Milchwissenschaft: Milk Science International. 2004.
116. Bruno FA, Lankaputhra WE, Shah NP. Growth, viability and activity of Bifidobacterium spp. in skim milk containing prebiotics. Journal of Food Science. 2002 Sep;67(7):2740-4.
117. Bâati L, Fabre-Gea C, Auriol D, Blanc PJ. Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. International Journal of Food Microbiology. 2000 Sep 10;59(3):241- 7.
118. Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Applied and Environmental Microbiology. 2005 Oct;71(10):6150-8.
119. Saarela MH, Alakomi HL, Puhakka A, Mättö J. Effect of the fermentation pH on the storage stability of Lactobacillus rhamnosus preparations and suitability of in vitro analyses of cell physiological functions to predict it. Journal of Applied Microbiology. 2009 Apr;106(4):1204-12.
120. Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules. 2011 Jul 11;12(7):2834-40.
121. Femia AP, Luceri C, Dolara P, Giannini A, Biggeri A, Salvadori M, Clune Y, Collins KJ, Paglierani M, Caderni G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002 Nov 1;23(11):1953-60.
122. Roller M, Femia AP, Caderni G, Rechkemmer G, Watzl B. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. British Journal of Nutrition. 2004 Dec;92(6):931-8.
123. Malkov SV, Markelov VV, Polozov GY, Sobchuk LI, Zakharova NG, Barabanschikov BI, et al. Antitumor features of Bacillus oligonitrophilus KU-1 strain. Journal of Microbiology, Immunology and Infection. 2005 Apr 1;38(2):96-104.
124. Augustin MA, Sanguansri L. Polyunsaturated fatty acids: delivery, innovation and incorporation into foods. Food Australia. 2003;55(7):294-296.
125. Kobyliak N, Falalyeyeva T, Boyko N, Tsyryuk O, Beregova T, Ostapchenko L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. diabetes research and clinical practice. 2018 Jul 1;141:190-9.
126. de Lara Pedroso D, Thomazini M, Heinemann RJ, Favaro- Trindade CS. Protection of Bifidobacterium lactis and Lactobacillus acidophilus by microencapsulation using spray-chilling. International Dairy Journal. 2012 Oct 1;26(2):127-32.
127. Barrow CJ, Nolan C, Holub BJ. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. Journal of Functional Foods. 2009 Jan 1;1(1):38-43.
128. Chen Q, Zhong F, Wen J, McGillivray D, Quek SY. Properties and stability of spray-dried and freeze-dried microcapsules coencapsulated with fish oil, phytosterol esters, and limonene. Drying Technology. 2013 Apr 26;31(6):707-16.
129. Das UN. Essential fatty acids as possible enhancers of the beneficial actions of probiotics. Nutrition. 2002 Sep 1;18(9):786-9.
130. Favaro-Trindade CS, Grosso CR. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. Journal of Microencapsulation. 2002 Jan 1;19(4):485-94.
131. Reineccius GA. Controlled release techniques in the food industry.
132. Picot A, Lacroix C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. International Dairy Journal. 2004 Jun 1;14(6):505-15.
133. Wada Y, Lönnerdal B. Bioactive peptides derived from human milk proteins—mechanisms of action. The Journal of Nutritional Biochemistry. 2014 May 1;25(5):503-14.
134. Kebary KM, Hussein SA, Badawi RM. Improving viability of bifidobacterium and their effect on frozen ice milk. Egyptian Journal of Dairy Science. 1998;26(2):319-337.
135. Matejčeková Z, Liptáková D, Valík Ľ. Functional probiotic products based on fermented buckwheat with Lactobacillus rhamnosus. LWT-Food Science and Technology. 2017 Aug 1;81:35- 41.
136. Eratte D, Dowling K, Barrow CJ, Adhikari BP. In-vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chemistry. 2017 Jul 15;227:129-36.
137. Matyšková R, Maletínská L, Maixnerová J, Pirník Z, Kiss A, Železná B. Comparison of the obesity phenotypes related to monosodium glutamate effect on arcuate nucleus and/or the high fat diet feeding in C57BL/6 and NMRI mice. Physiological research. 2008 Jan 1;57(5):727.
138. Crittenden R, Weerakkody R, Sanguansri L, Augustin M. Synbiotic microcapsules that enhance microbial viability during nonrefrigerated storage and gastrointestinal transit. Applied and Environmental Microbiology. 2006 Mar;72(3):2280-2.
139. Desmond C, Ross RP, O’callaghan E, Fitzgerald G, Stanton C. Improved survival of Lactobacillus paracasei NFBC 338 in spray‐dried powders containing gum acacia. Journal of Applied Microbiology. 2002 Dec;93(6):1003-11.
140. Favaro-Trindade CS, Grosso CR. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. Journal of Microencapsulation. 2002 Jan 1;19(4):485-94.
141. Uchida M, Mogami O, Matsueda K. Characteristic of milk whey culture with Propionibacterium freudenreichii ET-3 and its application to the inflammatory bowel disease therapy. Inflammopharmacology. 2007 Jun;15(3):105-8.
142. Huq T, Khan A, Khan RA, Riedl B, Lacroix M. Encapsulation of probiotic bacteria in biopolymeric system. Critical Reviews in Food Science and Nutrition. 2013 Jan 1;53(9):909-16.
143. Reid AA, Champagne CP, Gardner N, Fustier P, Vuillemard JC. Survival in food systems of Lactobacillus rhamnosus R011 microentrapped in whey protein gel particles. Journal of Food Science. 2007 Jan;72(1):M031-7.
144. Augustin MA, Sanguansri L, Lockett T. Nano‐and micro‐ encapsulated systems for enhancing the delivery of resveratrol. Annals of the New York Academy of Sciences. 2013 Jul;1290(1):107- 12.
145. Zhao W, Liu Y, Latta M, Ma W, Wu Z, Chen P. Probiotics database: A potential source of fermented foods. International Journal of Food Properties. 2019 Jan 1;22(1):198-217.
146. Oliveira RP, Florence AC, Perego P, De Oliveira MN, Converti A. Use of lactulose as prebiotic and its influence on the growth, acidification profile and viable counts of different probiotics in fermented skim milk. International Journal of Food Microbiology. 2011 Jan 31;145(1):22-7.
147. Di Criscio T, Fratianni A, Mignogna R, Cinquanta L, Coppola R, Sorrentino E, et al. Production of functional probiotic, prebiotic, and synbiotic ice creams. Journal of Dairy Science. 2010 Oct 1;93(10):4555-64.
148. Vitaglione P, Barone Lumaga R, Ferracane R, Radetsky I, Mennella I, Schettino R, Koder S, Shimoni E, Fogliano V. Curcumin bioavailability from enriched bread: the effect of microencapsulated ingredients. Journal of Agricultural and Food Chemistry. 2012 Apr 4;60(13):3357-66.
149. Jao CL, Huang SL, Wu SC, Kuo-Chiang H. The study on SFLAB GanedenBC30 viability on baking products during storage. Procedia Food Science. 2011 Jan 1;1:1601-9.
150. Altamirano-Fortoul R, Moreno-Terrazas R, Quezada-Gallo A, Rosell CM. Viability of some probiotic coatings in bread and its effect on the crust mechanical properties. Food Hydrocolloids. 2012 Oct 1;29(1):166-74.
151. Soukoulis C, Singh P, Macnaughtan W, Parmenter C, Fisk ID. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocolloids. 2016 Jan 1;52:876-87.
152. Malmo C, La Storia A, Mauriello G. Microencapsulation of Lactobacillus reuteri DSM 17938 cells coated in alginate beads with chitosan by spray drying to use as a probiotic cell in a chocolate soufflé. Food and Bioprocess Technology. 2013 Mar;6(3):795-805.
153. Zanjani MA, Tarzi BG, Sharifan A, Mohammadi N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iranian Journal of Pharmaceutical Research: IJPR. 2014;13(3):843.
154. Sanders ME. Probiotics: considerations for human health. Nutrition reviews. 2003 Mar 1;61(3):91-9.
155. Angioloni A, Collar C. Physicochemical and nutritional properties of reduced-caloric density high-fibre breads. LWT-Food Science and Technology. 2011 Apr 1;44(3):747-58.
156. Damen B, Pollet A, Dornez E, Broekaert WF, Van Haesendonck I, Trogh I, et al. Xylanase-mediated in situ production of arabinoxylan oligosaccharides with prebiotic potential in whole meal breads and breads enriched with arabinoxylan rich materials. Food Chemistry. 2012 Mar 1;131(1):111-8.
157. Mitsou EK, Panopoulou N, Turunen K, Spiliotis V, Kyriacou A. Prebiotic potential of barley derived β-glucan at low intake levels: A randomised, double-blinded, placebo-controlled clinical study. Food Research International. 2010 May 1;43(4):1086-92.
158. Paramithiotis S. Lactic acid fermentation of fruits and vegetables. CRC press; 2017 Feb 3.
159. Vergara CM, Honorato TL, Maia GA, Rodrigues S. Prebiotic effect of fermented cashew apple (Anacardium occidentale L) juice. LWT-Food Science and Technology. 2010 Jan 1;43(1):141-5.
160. Rößle C, Brunton N, Gormley RT, Ross PR, Butler F. Development of potentially synbiotic fresh-cut apple slices. Journal of Functional Foods. 2010 Oct 1;2(4):245-54.
161. Swain MR, Anandharaj M, Ray RC, Rani RP. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnology research international. 2014;2014.
162. Endo A, Mizuno H, Okada S. Monitoring the bacterial community during fermentation of sunki, an unsalted, fermented vegetable traditional to the Kiso area of Japan. Letters in Applied microbiology. 2008 Sep;47(3):221-6.
163. Kingston JJ, Radhika M, Roshini PT, Raksha MA, Murali HS, Batra HV. Molecular characterization of lactic acid bacteria recovered from natural fermentation of beet root and carrot Kanji. Indian Journal of Microbiology. 2010 Sep;50(3):292-8.
164. Corcoran BM, Ross RP, Fitzgerald GF, Stanton C. Comparative survival of probiotic lactobacilli spray‐dried in the presence of prebiotic substances. Journal of Applied Microbiology. 2004 May;96(5):1024-39.
165. Gardiner GE, O’sullivan E, Kelly J, Auty MA, Fitzgerald GF, Collins JK, et al. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Applied and Environmental Microbiology. 2000 Jun 1;66(6):2605-12.
166. Singh RP, Singh A. Functional Foods for Health Status and Social Well Being. Food & Nutrition Journal. Gavin Publishers; 2017;5.
167. Zhao S, Han J, Bie X, Lu Z, Zhang C, Lv F. Purification and characterization of plantaricin JLA-9: a novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage. Journal of Agricultural and Food Chemistry. 2016 Apr 6;64(13):2754-64.
168. Woraprayote W, Pumpuang L, Tosukhowong A, Roytrakul S, Perez RH, Zendo T, et al. Two putatively novel bacteriocins active against Gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control. 2015 Sep 1;55:176-84.
169. Gao Y, Li D, Liu S, Zhang L. Garviecin LG34, a novel bacteriocin produced by Lactococcus garvieae isolated from traditional Chinese fermented cucumber. Food Control. 2015 Apr 1;50:896-900.
170. Xin LŘ, Yi L, Dang J, Dang Y, Liu B. Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibioticresistant microorganisms. Food Control. 2014 Dec 1;46:264-71.
171. Sawa N, Koga S, Okamura K, Ishibashi N, Zendo T, Sonomoto K. Identification and characterization of novel multiple bacteriocins produced by L actobacillus sakei D98. Journal of applied microbiology. 2013 Jul;115(1):61-9.
172. Han EJ, Lee NK, Choi SY, Paik HD. Bacteriocin KC24 produced by Lactococcus lactis KC24 from kimchi and its antilisterial effect in UHT milk. Journal of Dairy Science. 2013 Jan 1;96(1):101-4.
173. Vieira AD, Biscola V, de Albuquerque MA, Bedani R, Saad SM. Impact of Acerola (Malpighia emarginata DC) Byproduct and Probiotic Strains on Technological and Sensory Features of Fermented Soy Beverages. Journal of Food Science. 2019 Dec;84(12):3726-34.
174. Maldonado-Barragán A, Cárdenas N, Martínez B, Ruiz-Barba JL, Fernández-Garayzábal JF, Rodríguez JM, et al. Garvicin A, a novel class IId bacteriocin from Lactococcus garvieae that inhibits septum formation in L. garvieae strains. Applied and Environmental Microbiology. 2013 Jul 15;79(14):4336-46.
175. Şahingil D, İşleroğlu H, Yildirim Z, Akcelik M, Yildirim M. Characterization of lactococcin BZ produced by Lactococcus lactis subsp. lactis BZ isolated from boza. Turkish Journal of Biology. 2011 Feb 4;35(1):21-33.
176. Akkoc N, Ghamat A, Akcelik M. Optimisation of bacteriocin production of Lactococcus lactis subsp. lactis MA23, a strain isolated from Boza. International Journal of Dairy Technology. 2011 Aug;64(3):425-32.
177. Todorov SD, Wachsman M, Tomé E, Dousset X, Destro MT, Dicks LM, et al. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiology. 2010 Oct 1;27(7):869-79.
178. Gong HS, Meng XC, Wang H. Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1. 0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control. 2010 Jan 1;21(1):89-96.
179. Alegría Á, Delgado S, Roces C, López B, Mayo B. Bacteriocins produced by wild Lactococcus lactis strains isolated from traditional, starter-free cheeses made of raw milk. International Journal of Food Microbiology. 2010 Sep 30;143(1-2):61-6.
180. Stoyanovski S, Chipeva V, Dimov SG, Danova S, Dimitrova I, Yocheva L, et al. Characterization of lactic acid bacteria from dry sausages. Biotechnology & Biotechnological Equipment. 2009 Jan 1;23(sup1):870-3.
181. Powell JE, Witthuhn RC, Todorov SD, Dicks LM. Characterization of bacteriocin ST8KF produced by a kefir isolate Lactobacillus plantarum ST8KF. International Dairy Journal. 2007 Mar 1;17(3):190-8.
182. Albano H, Todorov SD, van Reenen CA, Hogg T, Dicks LMT, Teixeira P. Characterization of two bacteriocins produced by Pediococcus acidilactici isolated from “Alheira”, a fermented sausage traditionally produced in Portugal. International Journal of Food Microbiology. 2007;116(1):239-247.
183. Audisio MC, Terzolo HR, Apella MC. Bacteriocin from honeybee beebread Enterococcus avium, active against Listeria monocytogenes. Applied and Environmental Microbiology. 2005 Jun;71(6):3373-5.
184. Todorov SD, Dicks LM. Partial characterization of bacteriocins produced by four lactic acid bacteria isolated from regional South African barley beer. Annals of Microbiology. 2004 Jan 1;54(4):403-13.
185. Ullah N, Wang X, Wu J, Guo Y, Ge H, Li T, Khan S, Li Z, Feng X. Purification and primary characterization of a novel bacteriocin, LiN333, from Lactobacillus casei, an isolate from a Chinese fermented food. LWT. 2017 Oct 1;84:867-75.
186. Ruxton C, Reed S, Simpson M, Millington K. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. Journal of Human Nutrition and Dietetics: the Official Journal of the British Dietetic Association. 2007 Jun 1;20(3):275-85.
187. Taneja A, Singh H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annual Review of Food Science and Technology. 2012 Apr 10;3:105-23.
188. Drusch S, Mannino S. Patent-based review on industrial approaches for the microencapsulation of oils rich in polyunsaturated fatty acids. Trends in Food Science & Technology. 2009 Jul 1;20(6-7):237-44.
189. Augustin MA, Sanguansri L, Bode O. Maillard reaction products as encapsulants for fish oil powders. Journal of Food Science. 2006 Mar;71(2):E25-32.
190. Sanguansri L, Shen Z, Weerakkody R, Barnes M, Lockett T, Augustin MA. Omega-3 fatty acids in ileal effluent after consuming different foods containing microencapsulated fish oil powder–an ileostomy study. Food & Function. 2013;4(1):74-82.
191. Chen B, McClements DJ, Decker EA. Design of foods with bioactive lipids for improved health. Annual Review of Food Science and Technology. 2013 Feb 28;4:35-56.
192. Yao M, Xiao H, McClements DJ. Delivery of lipophilic bioactives: assembly, disassembly, and reassembly of lipid nanoparticles. Annual Review of Food Science and Technology. 2014 Feb 28;5:53-81.
193. Datagraphic: Health and Wellness Performance Overview 2013 | Market Research Blog [Internet]. [cited 2021 Jul 12]. Available from: https://blog.euromonitor.com/datagraphic-health-andwellness- performance-overview-2013
194. Shahidi F. Nutraceuticals, functional foods and dietary supplements in health and disease. Journal of Food and Drug Analysis. 2012 Apr 1;20(1):226-30.
195. Brown PN, Chan M. An overview of functional food regulation in North America, European Union, Japan and Australia. Functional Food Product Development. 2010 Jun 11:257-92.