Abstract
The standard treatment options for Coronavirus disease 2019 (COVID-19) remain challenging despite community vaccinations and reduced mortality. As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus continues to evolve and new strains emerge, diversity in the use of existing antiviral drugs has become a crucial therapeutic tool in combating the COVID-19 epidemic. Many types of infectious diseases, including DNA and RNA viruses, have traditionally been treated with ivermectin, a broad-spectrum anti-parasitic, and anti-viral drug. In spite of this, the effectiveness of ivermectin as a treatment for SARS-CoV-2 is still controversial, based on currently available data. The aim of this study was to provide comprehensive information on ivermectin, including its safety and efficacy. We hypothesized that ivermectin prophylactic effects may enhance vaccine effectiveness against SARS-CoV-2 infection in this study. Also, the combination of ivermectin with other drugs to reduce its adverse effects could be beneficial and we suggest that it can be evaluated in future studies.
Keywords
COVID-19, SARS-CoV-2, Ivermectin, Vaccine, Prophylactic effects, Variants
Commentary
For the first time, in the winter of 2019, an unprecedented virus was detected in China that spread worldwide in a blink of an eye [1-3]. In February 2020, it was declared that the pathogen of the pandemic, causing severe pneumonia, was a coronavirus named SARS-CoV-2. Moreover, it has been suggested that SARS-CoV-2 infection can lead to progression of multiple diseases such as neurodegenerative diseases, and cancers [4-10]. Studies on SARS-CoV-2 genome have revealed that spike protein, the most important structural protein of the virus, is responsible for the virus entry into host cells via binding to human angiotensin converting enzyme (ACE) 2 receptor [11,12]. Thus, spike protein was considered as a specific target for vaccine design during the pandemic [3,13].
Since the beginning of the outbreak, several noticeable mutations in the spike protein have been detected which alter the virus phenotype, intensify its pathogenicity/infection rate and subsequently, create more aggressive variants. In this line, variants of Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Epsilon (B.1.427 and B.1.429), Kappa (B.1.617.1), Mu (B.1.621, B.1.621.1), Zeta (P.2), Delta (B.1.617.2) and now, Omicron (B.1.1.529) have been emerged, respectively [14]. Unfortunately, studies on the efficacy of vaccines developed against SARS-CoV-2 have indicated that the evolution of these mutated variants, particularly Alpha, Beta, Delta, and Gamma variants, lead to reduction of the effectiveness of the vaccines. In this regard, multiple studies have declared that even the most efficient vaccines such as Moderna, Pfizer, and Novavax (with the efficiency of approximately 95%) can partially lose their effectiveness against variants of Alpha, Beta (ranged from 93.7 to 72%) and delta (88% efficacy for Pfizer) compared to the original/wild virus. Consequently, it is likely that the newly emerged variant of SARS-CoV-2, Omicron, could have even more negative effect on the vaccines’ efficiency [15,16].
In the mid-November 2021, samples collected in Botswana and South Africa were the first ones in which the novel SARS-CoV-2 variant Omicron was detected [17]. As of 26 November 2021, the World Health Organization (WHO) formally announced the SARS-CoV-2 Omicron variant (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage designation B.1.1.529) as a new variant of the outbreak [18].
Since then, a rapid expansion of the novel Omicron variant in different countries has happened in a way that warns us how to face such a new crisis with the COVID-19 outbreak. Omicron appears to be competing with the Delta variant. It has been suggested that this could be attributed to an advantage in increased replication [19].
Ivermectin, as an anti-parasitic drug, has been also proposed as a potential agent against SARS-CoV-2. Interestingly, it has been demonstrated that the multifunctional effects of ivermectin against SARS-CoV-2 are exerted through targeting different mechanisms/pathways (Table 1) [20].
|
Strategy |
Target/Action |
Ivermectin Mechanism/Function |
|---|---|---|
|
Direct effects on SARS-CoV-2 |
SARS-CoV-2 cell entry |
1) The highest binding affinity to the predicted active site of the S glycoprotein and RdRp, NSP14, and TMPRSS2 proteins. 2) Targets spike protein, main protease, replicase, and human TMPRSS2 receptors for executing its antiviral efficiency by disrupting binding.
|
|
Importin α (IMPα) |
interact with IMPα preventing interaction with IMP β1, and blocks transporting the viral proteins in to the host cell nuclear and subsequently, decreases the virus replication. |
|
|
Role as an ionophore |
Two ivermectin molecules, reacting with each other in a “head-tail” mode, can create a complex suitable to be considered as an ionophore and neutralize the virus at an early stage of the infection before its adhesion to the host cells and enters it. |
|
|
viral replication |
Anti-viral, inhibition of replication and assembly |
Interacts with RNA-dependent RNA polymerase (RdRp), NSP14, N phosphoprotein, and M protein. |
|
posttranslational processing of the virus polyproteins |
1) inhibits 3 chymotrypsin-like proteases 2) binds to both proteins, Mpro, and to a lesser extent to PLpro of SARS-CoV-2 |
|
|
Host inflammation related factors |
Levels of interferon (IFN) |
Upregulates several IFN-related genes, such as IFIT1, IFIT2, IF144, ISG20, IRF9, and OASL |
|
Toll- like receptors (TLRs) |
Inhibits toll-like receptor 4 (TLR4) signaling and lipopolysaccharide (LPS)-induced production of inflammatory cytokines via NF-kappa B pathway blockage |
|
|
JAK-STAT pathway |
Blocks the PD-L1 receptors presenting on the endothelial cells by STAT-3 inhibition |
|
|
Interleukin-6 (IL-6) |
Suppresses IL-6 and TNF-α production |
|
|
CD147 on the RBCs |
Disables S protein of the SARS-CoV-2 binds to CD147 |
Ivermectin's efficacy will be determined by more clinical trials since there is insufficient human clinical evidence. While some clinical outcomes from numerous randomized and observational controlled trials of ivermectin have consistently shown significant improvement against COVID-19 (Table 1). In 18 randomized controlled trials of ivermectin in COVID-19 patients, it has been demonstrated that this drug can play a significant role in reductions of time to viral clearance, time to recovery, and mortality. Additionally, numerous controlled prophylaxis trials have found that regular use of ivermectin significantly reduces the risk of contracting COVID-19 [21]. Additionally, Behera et al. have found that two doses (300 μg/kg at 72 hours intervals) of ivermectin chemoprophylaxis significantly decrease the risk of contracting COVID-19 by 83% when administered to healthcare workers for one month [22].
In Omicron, there are a number of mutations. Compared to the Delta variant, its spike protein alone contains at least 32 mutations. Aside from these newly discovered mutations, the Omicron variant requires NSP12 to reproduce the virus and NSP14 in order to function as a methyltransferase and exoribonuclease. Consequently, they can play a role in viral RNA replication. As a result, it is believed that the Omicron variant is at least three times more infectious than the original SARS?CoV?2 and potentially more infectious in comparison with the delta variant [23,24]. On the one hand, ivermectin has a high affinity for binding to the predicted active site of NSP14 and RNA-dependent RNA polymerase (RdRp) [20] which are significantly necessary for replication of the SARS-CoV-2. On the other hand, we suggest that ivermectin potentially can bind to the Spike protein of Omicron, as seen in previous variants. To assessing these potencies of ivermectin, we simulate ivermectin potential for binding to the active site of NSP14, RdRp, TMPRSS2, Delta, and Omicron variant spike protein by using molegro virtual docker to, which we found that not only ivermectin can have a high affinity for binding to these proteins but also it can bind to the S protein of Omicron with a higher affinity in comparison with Delta S protein (Figure 1).
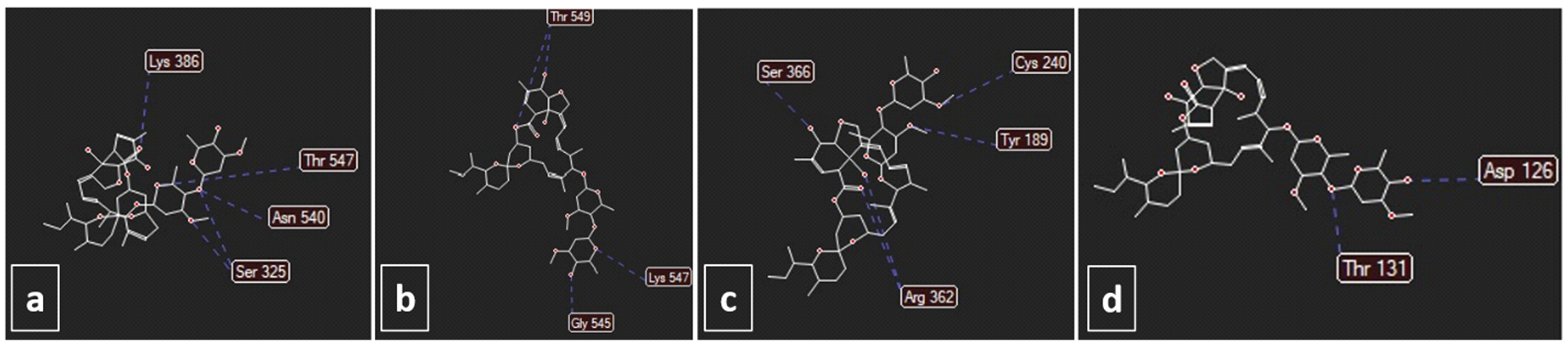
Figure 1. Docking of a) spike delta (score: 120), b) spike omicron (score: 124), c) RdRp (score: 137), and d) NSP-14 (score: 147) with ivermectin. The unit of scoring function is moldockscore. The energies were obtained using molegro virtual docker. The figure was used from Nabi-Afjadi et al. [25].
Accordingly, we hypothesize that ivermectin potentially can hinder the Omicron variant replication in host cells via targeting NSP14 and RdRp. Additionally, ivermectin can hinder both virus entry to host cells and stimulation of intracellular signaling pathways via attachment of Omicron Spike protein to its receptors (e.g., ACE2, CD147, Neuropilin-1, etc.), thereby reducing the severity of disease and risk of emergence of new super-variants.
The safety of ivermectin in the prevention and treatment of COVID-19 has been demonstrated by increasing evidences [21]. According to a study by Okumu? et al. [26], ivermectin administration to patients without MDR-1/ABCB1 and/or CYP3A4 gene mutations (genes that affect the metabolism of ivermectin and result in toxic effects in patients) is considered to be safe and without serious adverse effects. They also have suggested that by providing appropriate treatment, unwanted effects can be alleviated.
Recent studies have shown the Omicron spike is associated with significant evasion of vaccine-induced neutralizing antibodies, which is more pronounced for ChAdOx-1 than for BNT162b2 vaccine. There is a support for these data from vaccine effectiveness measurements in the UK (UKHSA report Dec 2021) [19].
It seems that there is a high risk of Omicron variant affliction even with a first and second dose of vaccination [19]. Unfortunately, ChAdOx-1 is more commonly used in low-income countries where third doses of mRNA-containing vaccines are not commonly available, thereby it may accelerate the wide expansion of Omicron and increase hospitalization and severe disease in these countries.
Based on these, we propose that ivermectin administration potentially can be beneficial for these populations:
- People under 10 years old with no vaccination.
- Old people.
- People with an autoimmune disease or who experienced an autoimmune response and adverse effects resulting from vaccination.
- People with a high risk of COVID-19 infection, primary diseases and any kinds of immune system deficiencies.
- People with high-risk jobs such as healthcare workers.
- People who were vaccinated with ChAdOx-1, without a third dose of mRNA-containing vaccines.
Despite of these, some desirable effects have been reported for ivermectin indicating that the drug usage is dependent on several conditions/factors such as geographical location, stage of the diseases, patient’s conditions, sex and etc. For instance, apoptosis of mitochondria and S phase arrest are caused by ivermectin in colorectal cancer cells [27]. As a result of mitochondrial dysfunction, ivermectin increases mitochondrial superoxide levels in glioblastoma cells and brain endothelial cells. The presence of antioxidants reversed the inhibition of ivermectin in glioblastoma and endothelial cells, showing that antioxidants are required for this to occur [27,28]. Moreover, placentation may also be affected by ivermectin during early pregnancy due to constant exposure and accumulated levels. A multitude of adverse effects were found in pTr and pLE cells caused by ivermectin, including cell cycle arrest, apoptosis, mitochondrial permeabilization, calcium accumulation, and endothelial dysfunction [29]. It has been shown that ivermectin stimulates the P2X7 receptor, resulting in inflammatory and pathological effects in SARS-CoV-2 infection [30]. So, until data from randomized trials of sufficient size, dose, duration, and patients’ conditions are available, healthcare providers should be prohibited from prescribing ivermectin to treat or prevent COVID-19 [31,32].
Generally, some clinical trials on the effects of ivermectin on the SARS-COVE-2 infected patients with different results have been reported that are listed in Table 2.
|
|
Number of patients |
Country of study |
Ivermectin dose |
Description/Outcome |
Ref |
|---|---|---|---|---|---|
|
Positive responded studies |
280 |
United States |
200 mg/kg/day |
|
[33] |
|
69 |
Iran |
0.2 mg/kg/day |
Significantly reduced mean dyspnea, persistent cough, hospitalization, and incidence of lymphopenia. |
[34] |
|
|
72 |
Bangladesh |
12 mg |
The safety and effectiveness of Ivermectin were confirmed as well as the effect of Ivermectin on the virus clearance rate and improvement of clinical symptoms. |
[35] |
|
|
66 |
Turkey |
200 µg/kg/day |
Improved clinical recovery, improve prognostic test indicators, and reduce mortality. |
[26] |
|
|
Negative responded studies |
125 |
India |
12 and 24 mg/day |
Increased negative RT-PCR tests or decreased viral loads in patients receiving Covid19, but no statistically significant difference compared with the placebo group. |
[36] |
|
490 |
Malaysia
|
0.4 mg/kg/day
|
No significant difference compared to the control, so treatment with Ivermectin in the early stages of Covid19 could not prevent the disease from progressing to more serious stages. |
[37] |
|
|
501 |
Argentina
|
12 and 18 mg/day |
No significant difference compared to the control. |
[38] |
|
|
476 |
Colombia |
300g/kg/day
|
No significant improved symptom remission of COVID19. |
[39]
|
|
|
100 |
Lebanon |
9 mg, 12 mg, and 150 µg/kg
|
|
[40] |
Also, in simultaneous use of ivermectin, some drugs have been proposed to reduce its side effects. For instance, COVID-19 viral loads were reduced by concomitant administration of ivermectin, nitazoxanide, ribavirin and zinc [41]. It has been found that azithromycin inhibits the production of cytokines, attenuates inflammation, and increases immunoglobulin release in clinical studies [42]. Generally, the proof likewise recommends that early utilization of ivermectin may decrease grimness and mortality from Coronavirus. This depends on (1) decreases in Coronavirus contaminations when ivermectin was utilized as prophylaxis, (2) the more positive impact gauges for gentle to direct illness contrasted and serious sickness for death because of any reason, and (3) on the proof exhibiting decreases in decay [43].
In conclusion, we postulate the prophylactic effects of ivermectin may boost the effectiveness of vaccination against newly emerged variants and future variants. Furthermore, it might have a possible role by reducing the probability of a novel super variant emergence from probable co-infection of Omicron and the Delta variants. Based on currently available data, different clinical trial studies have shown positive and negative therapeutic responses to ivermectin for COVID-19 (Table 1). Hence, as long as more reliable data emerge from randomized trials with sufficient size, dose, and duration, healthcare providers should refrain from prescribing ivermectin to treat or prevent COVID-19. Also, combined treatment of ivermectin with other drugs may minimize adverse effects and demonstrate its therapeutic efficacy in the treatment of COVID-19, as we suggested in our previous study [25]. Thus, we hope that this hypothesis will encourage further investigations and consideration of the possible prophylactic roles of ivermectin against Omicron and future variants of SARS-CoV-2 to save the lives of many people and prevent hospitalization and morbidity.
Funding
Not applicable
Declarations
Conflict of interest
All other authors declare no conflict of interest.
Consent for publication
All other authors declare no conflict of interest.
References
2. Samidoust P, Delshad ME, Talemi RN, Mojtahedi K, Samidoust A, Jahangiri S, et al. Incidence, characteristics, and outcome of COVID-19 in patients on liver transplant program: a retrospective study in the north of Iran. New Microbes and New Infections. 2021 Nov 1;44:100935.
3. Sedighimehr N, Fathi J, Hadi N, Rezaeian ZS. Rehabilitation, a necessity in hospitalized and discharged people infected with COVID-19: a narrative review. Physical Therapy Reviews. 2021 May 4;26(3):202-10.
4. Zalpoor H, Akbari A, Samei A, Forghaniesfidvajani R, Kamali M, Afzalnia A, et al. The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies. Cellular & Molecular Biology Letters. 2022 Dec;27(1):1-21.
5. Zalpoor H, Bakhtiyari M, Liaghat M, Nabi?Afjadi M, Ganjalikhani?Hakemi M. Quercetin potential effects against SARS?CoV?2 infection and COVID?19?associated cancer progression by inhibiting mTOR and hypoxia?inducible factor?1? (HIF?1?). Phytotherapy Research. 2022 Mar 20.
6. Zalpoor H, Bakhtiyari M, Shapourian H, Rostampour P, Tavakol C, Nabi-Afjadi M. Hesperetin as an anti-SARS-CoV-2 agent can inhibit COVID-19-associated cancer progression by suppressing intracellular signaling pathways. Inflammopharmacology. 2022 Oct;30(5):1533-9.
7. Zalpoor H, Akbari A, Nabi-Afjadi M, Forghaniesfidvajani R, Tavakol C, Barzegar Z, et al. Hypoxia?inducible factor 1 alpha (HIF?1?) stimulated and P2X7 receptor activated by COVID-19, as a potential therapeutic target and risk factor for epilepsy. Human Cell. 2022 Sep;35(5):1338-45.
8. Zalpoor H, Shapourian H, Akbari A, Shahveh S, Haghshenas L. Increased neuropilin-1 expression by COVID-19: a possible cause of long-term neurological complications and progression of primary brain tumors. Human Cell. 2022 Jul;35(4):1301-3.
9. Zalpoor H, Rezaei M, Yahyazadeh S, Ganjalikhani-Hakemi M. Flt3-ITD mutated acute myeloid leukemia patients and COVID-19: potential roles of autophagy and HIF-1? in leukemia progression and mortality. Human Cell. 2022 Jul;35(4):1304-5.
10. Zalpoor H, Akbari A, Nayerain Jazi N, Liaghat M, Bakhtiyari M. Possible role of autophagy induced by COVID-19 in cancer progression, chemo-resistance, and tumor recurrence. Infectious Agents and Cancer. 2022 Jul 18;17(1):38.
11. Nabi-Afjadi M, Heydari M, Zalpoor H, Arman I, Sadoughi A, Sahami P, et al. Lectins and lectibodies: potential promising antiviral agents. Cellular & Molecular Biology Letters. 2022 Dec;27(1):37.
12. Zalpoor H, Akbari A, Nabi-Afjadi M. Ephrin (Eph) receptor and downstream signaling pathways: a promising potential targeted therapy for COVID?19 and associated cancers and diseases. Human Cell. 2022;35(3):952-4.
13. Yano M, Matsuda F, Sakuntabhai A, Hirota S. Socio-Life Science and the COVID-19 Outbreak: Public Health and Public Policy. Springer Nature; 2022.
14. Castro GM, Sicilia P, Bolzon ML, López L, Barbás MG, Pisano MB, et al. Strategy for a rapid screening and surveillance of SARS-CoV-2 variants by real time RT-PCR: a key tool that allowed control and delay in Delta spread in Cordoba, Argentina. medRxiv. 2021 Nov 20:2021-11.
15. Mahase E. Covid-19: Where are we on vaccines and variants?.
16. Mahase E. Covid-19: How many variants are there, and what do we know about them?. Bmj. 2021 Aug 19;374.
17. Threat Assessment Brief: Implications of the further emergence and spread of the SARS CoV 2 B.1.1.529 variant of concern (Omicron) for the EU/EEA first update Stockholm: European Centre for Disease Prevention and Control (ECDC); 2021 [Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-threat-assessment-spread-omicron-first-update.
18. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern Geneva: World Health Organization (WHO); 2021 [Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variantof-concern.
19. Meng B, Kemp SA, Papa G, Datir R, Ferreira IA, Marelli S, et al. SARS-CoV-2 Omicron Spike Mediated Immune Escape, Infectivity and Cell-Cell Fusion. 2021. https://www. biorxiv. org/content/10.1101/2021.12.;17:v2.
20. Zaidi AK, Dehgani-Mobaraki P. The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review. The Journal of antibiotics. 2022 Feb;75(2):60-71.
21. Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. American journal of therapeutics. 2021 May;28(3):e299.
22. Behera P, Patro BK, Padhy BM, Mohapatra PR, Bal SK, Chandanshive PD, et al. Prophylactic role of ivermectin in SARS-CoV-2 infection among healthcare workers.
23. Raj R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochemistry and biophysics reports. 2021 Mar 1;25:100847.
24. Gao SJ, Guo H, Luo G. Omicron variant (B. 1.1. 529) of SARS?CoV?2, a global urgent public health alert!. Journal of medical virology. 2022 Apr;94(4):1255.
25. Nabi-Afjadi M, Mohebi F, Zalpoor H, Aziziyan F, Akbari A, Moradi-Sardareh H, et al. A cellular and molecular biology-based update for ivermectin against COVID-19: is it effective or non-effective?. Inflammopharmacology. 2023 Jan 7:1-5.
26. Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC infectious diseases. 2021 Dec;21(1):1-1
27. Zhou S, Wu H, Ning W, Wu X, Xu X, Ma Y, et al. Ivermectin has new application in inhibiting colorectal cancer cell growth. Frontiers in Pharmacology. 2021:2145.
28. Liu Y, Fang S, Sun Q, Liu B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochemical and biophysical research communications. 2016 Nov 18;480(3):415-21.
29. Lee JY, Lim W, Ham J, Kim J, You S, Song G. Ivermectin induces apoptosis of porcine trophectoderm and uterine luminal epithelial cells through loss of mitochondrial membrane potential, mitochondrial calcium ion overload, and reactive oxygen species generation. Pesticide biochemistry and physiology. 2019 Sep 1;159:144-53.
30. Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. American journal of cancer research. 2018;8(2):317.
31. Molnar A, Lau S, Berges M, Masa RB, Solano JJ, Alter SM, et al. Ivermectin in COVID-19: the case for a moratorium on prescriptions. Therapeutic Innovation & Regulatory Science. 2022 May;56(3):382-5.
32. Furlan L, Caramelli B. The regrettable story of the “Covid Kit” and the “Early Treatment of Covid-19” in Brazil. The Lancet Regional Health–Americas. 2021 Dec 1;4.
33. Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021 Jan 1;159(1):85-92.
34. Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clinical therapeutics. 2021 Jun 1;43(6):1007-19.
35. Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. International Journal of Infectious Diseases. 2021 Feb 1;103:214-6.
36. Mohan A, Tiwari P, Suri T, Mittal S, Patel A, Jain A, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial.
37. Lim SC, Hor CP, Tay KH, Jelani AM, Tan WH, Ker HB, et al. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA internal medicine. 2022 Apr 1;182(4):426-35.
38. Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC infectious diseases. 2021 Dec;21(1):1-1.
39. López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. Jama. 2021 Apr 13;325(14):1426-35.
40. Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021 May 26;13(6):989.
41. Elalfy H, Besheer T, El?Mesery A, El?Gilany AH, Soliman MA, Alhawarey A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS. NRIZ study) on the clearance of mild COVID?19. Journal of medical virology. 2021 May;93(5):3176-83.
42. Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microbial pathogenesis. 2020 Aug 1;145:104228.
43. Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. American journal of therapeutics. 2021 Jul;28(4):e434.
