Abstract
We recently reported that influenza infection is associated with drastic, depot-specific changes in white adipose tissue (WAT), notably the occurrence of thermogenic brown-like adipocytes within the subcutaneous depot, a process referred to as WAT browning. In mammals, induction of the thermogenic circuit increases heat production and consumes energy, consequently improving host’s metabolism. Importantly, we also demonstrated that mouse and human preadipocytes commit to the thermogenic differentiation program upon in vitro influenza virus infection; this signifies that infection-associated WAT browning may partly rely on a direct effect of the virus on fat.
Herein, after a short review of the physiological and cellular mechanisms that have been described to regulate WAT browning, including immune-cell-dependent ones, we will comment on the role that white adipose tissue, which is at the crossroads of metabolism and immunity, may play in influenza pathophysiology.
Keywords
Adipose tissue, Browning, Influenza A virus
Adipose Tissue: A Highly Dynamic and Flexible Organ
Mammals have two main types of adipose tissue (commonly defined as “fat”): the white adipose tissue (WAT), which represents the main energy reservoir of the body and provides lipids as substrates for other tissues, and the brown adipose tissue (BAT), which uses lipids for heat production (a process called “thermogenesis”). WAT and BAT are mainly composed of white and brown adipocytes, respectively. Whereas white adipocytes contain few mitochondria and a single large lipid droplet (unilocular), brown adipocytes contain many mitochondria and multiple small lipid droplets (multilocular). More recently, another type of adipocytes has been uncovered: the beige/brite (“BRown-in-whITE”) adipocytes, which locate in WAT and share some morphological and functional features with brown adipocytes. Indeed, alike brown adipocytes, beige/ brite cells contain many mitochondria that express high levels of the thermogenic uncoupling protein 1 (UCP1) in their inner membrane [1-4] (Figure 1a).
(b) Inter-organ regulation of white adipose tissue browning: The central nervous system (CNS), sympathetic nervous system (SNS), skeletal muscles, adipose tissues, liver and thyroid regulate white fat browning (detailed in the text). Additionally, upon cold exposure cardiac tissues secrete natriuretic peptides, and gut microbiota composition is altered. Adapted from [58].
FGF21: Fibroblast Growth Factor-21; Metrnl: Meteorin-like; NE: Norepinephrine; T4: Thyroxine (tetra-iodothyronine); T3: tri-iodothyronine.
UCP1 was first observed in BAT mitochondria [5], and has a key role in mediating thermogenesis since it dissociates mitochondrial respiration (oxidative phosphorylation) from ATP production [6].
The adipose tissue, mostly the WAT, possesses the remarkable capacity to quickly adapt its size and function in response to a variety of internal and external cues, including nutritional status, ageing, and infection [7,8]. WAT’s tremendous plasticity is notably exemplified by the large increase in the number of thermogenic beige/ brite adipocytes (a process called “WAT browning” or “WAT beiging”) upon conditions such as e.g. chronic cold exposure, regular physical exercise, intermittent fasting or caloric restriction [3,9-11]. In addition, numerous “browning inducers” have now been described (certain food components or drugs), and have been expertly listed in several reviews [12,13]. It should be noted that the subcutaneous fat depots are more likely to undergo browning than visceral fat because subcutaneous adipocytes are predominantly smaller and have a greater potential to differentiate [14].
WAT browning is an adaptive response to certain environmental stimuli, including cold exposure, exercise, or restriction in nutrient supply, as well as various peptides and hormones [3,9-15]. In response to such cues, thermogenic beige/brite adipocytes will emerge in WAT either from de novo differentiation of newly recruited precursor cells [16] and/or from transdifferentiation of white adipocytes towards beige/brite adipocytes [17]. WAT browning is a reversible phenomenon: it ceases upon termination of the external stimulus. In the context of prolonged cold-exposure, it has been demonstrated that cold-induced beige/brite adipocytes are gradually replaced by UCP1-negative adipocytes with unilocular lipid droplets (i.e. white adipocytes) when cold-exposed mice are returned to room temperature or thermoneutrality [18]. The operating mechanisms of WAT browning are diverse and may depend on the nature of the stimuli. However, it is acknowledged that WAT browning is mostly a sympathetic event: sustained adrenergic stimulation is central to inducing the occurrence of thermogenic beige/ brite adipocytes in WAT; lipolysis being a major regulator of catabolic activity [19]. Upon prolonged cold exposure or exercise, norepinephrine (NE, the main neurotransmitter of the sympathetic nervous system) is released from the sympathetic nerves that innervate the WAT. Then, NE acts on β-adrenoreceptors (predominantly subtype β3) on the surface of adipocytes, thereby promoting mitochondrial biogenesis and peroxisome proliferatoractivated receptor activation [20]. In addition, chronic cold exposure or exercise increase the secretion of irisin (a myokine/adipokine/neurokine) and fibroblast growth factor-21 (a hepatokine/myokine/adipokine), which both promote the differentiation of white adipocyte precursors into mature beige/brite adipocytes [21,22]. More recently, meteorin-like was identified as a circulating hormone that is induced in skeletal muscles after exercise and in adipose tissue upon cold exposure [23]. Besides, other factors have been proposed to contribute to WAT browning, such as thyroid hormones [24], insulin and leptin [25], viperin (an interferon-inducible protein) [26], and erythropoietin [27]. All these secreted factors contribute to WAT browning; illustrating the complex inter-organ connections that occur in response to certain cues to dissipate heat and thus, improve energy metabolism (Figure 1b).
It is worth mentioning that recent findings indicate that brown and beige adipocytes can also carry out thermogenesis through UCP1-independent mechanisms that are still incompletely unraveled [28,29].
Immune Cells control White Adipose Tissue Browning
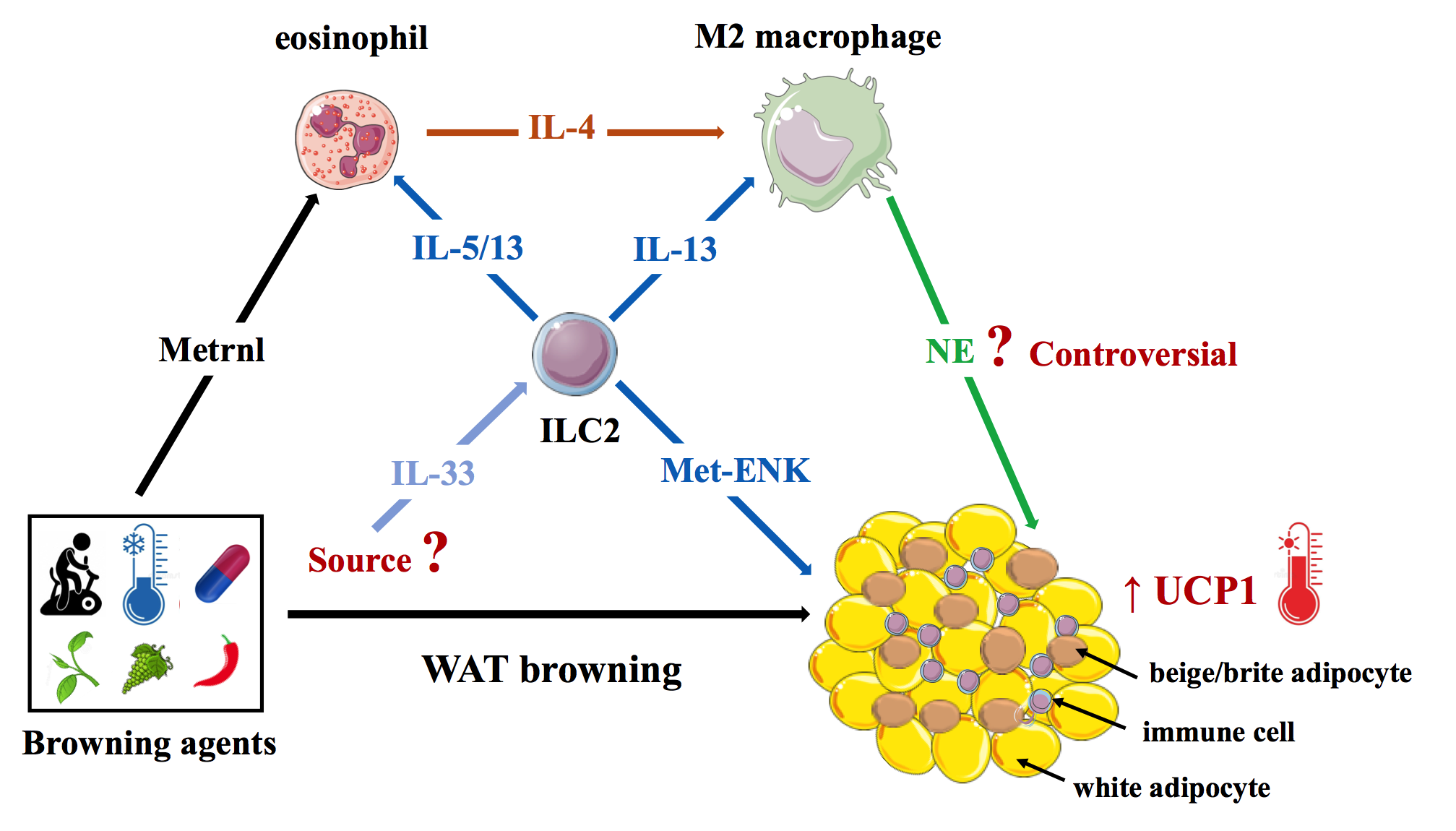
Besides adipocytes, WAT also harbors innate and adaptive immune cells that are crucial for tissue maintenance and homeostasis [30-32]. Recently, immune cells were also found to have an unexpected involvement in WAT browning. In 2011, Nguyen and colleagues indeed reported that short-term (6-hour) cold-exposure rapidly promote eosinophil-derived interleukin-4 (IL-4)-dependent alternative activation of WAT macrophages (M2 typemacrophages), which then recruit and activate beige/brite adipocytes in the subcutaneous adipose tissue (SCAT) [33]. In addition, eosinophils have been reported to be the target of meteorin-like, which is induced in skeletal muscle and adipose tissue in response to exercise or cold exposure [23]. Accordingly, genetic loss of eosinophils or IL4/13 signaling blunted cold-induced WAT browning through lack of alternative activation of macrophages [34]. However, whether M2 macrophages do synthesize NE is still a matter of debate [35].
Type 2 innate lymphoid cells (ILC2s) are critical regulators of type 2 immune cells: M2 macrophages, eosinophils, mast cells, T regulatory cells (Treg), and T-helper type 2 cells (Th2) [36]. It has been reported that, upon IL- 33 elicitation, ILC2s can induce beige/brite adipocyte activation in the SCAT [37]. Independent of the immune system, IL-33-activated ILC2s can also drive the browning process by producing methionine-enkephalin that directly acts on adipocytes to upregulate UCP1 expression [38].
Overall, the current consensus is that type 2 immunity (anti-inflammatory cytokines) promote WAT beiging (at least in rodents) through the complex interplay between eosinophils, M2 macrophages, and ILC2s (Figure 2). Conversely, recent reports suggest that pro-inflammatory factors, such as Tumor necrosis factor-alpha (TNF-α) and endotoxins, may negatively affect WAT browning [39,40]. This may partly explain why WAT browning is impaired in obesity, which is associated with a chronic low-grade inflammation of adipose tissues characterized by elevated local and systemic levels of TNF-α and LPS [41].
Influenza A Virus: A Recently Described White Adipose Tissue Browning Inducer
As stated above, WAT contains a wide variety of innate and adaptive immune cells with unique properties and functions [30-32]. Beside contributing to homeostatic tissue function, WAT immune cells also provide defense against infection [42,43]. Importantly, even though innate immune sensors (i.e. pattern recognition receptors (PRRs), which sense invading microbes) are mainly expressed by innate immune cells, several PRRs (such as Toll-like receptor 2 (TLR2) and TLR4) are also broadly expressed by adipocytes and their progenitors (preadipocytes) [44], not to mention that these cells can secrete a large range of pro-inflammatory (e.g. IL-1, IL-6, TNFα, leptin) and antiinflammatory (e.g. IL-10, adiponectin) factors, as well as antimicrobial peptides (e.g. cathelicidins) [45,46].
Thus, WAT has the potential to fight against infection through mounting a local immune response and, most importantly, through contributing to the systemic immune response. Recently, the WAT’s contribution to long-term protective defense against infection was very elegantly demonstrated, and constituted a major scientific breakthrough. Indeed, in models of acute mucosal parasite (Toxoplasma gondii) and bacterial (Yersinia pseudotuberculosis) infection, the authors reported that WAT is a site for the accumulation of pathogen-specific memory T cells (including resident ones) that can protect against subsequent challenge infections [47].
At the same time, WAT can also be a target for several pathogens; parasites (Plasmodium spp., Toxoplasma spp., and Trypanosoma spp.), bacteria (Mycobacterium tuberculosis and Staphylococcus aureus), and viruses (human and simian immunodeficiency virus, lymphocytic choriomeningitis virus, adenovirus and vaccinia virus) were reported to target murine and human WAT directly, wherein they induce metabolic and immune perturbations [48-50].
We lately reported on the (short- and long-term) metabolic consequences of influenza A virus (IAV) infection in mice, and on the impact of infection on the two main fat depots: the subcutaneous (i.e. inguinal) adipose tissue (SCAT), and the visceral (i.e. epididymal) adipose tissue (EWAT) [51]. Surprisingly, the viral genome was detected in both SCAT and EWAT (albeit at lower levels than in lungs), thus prompted a search for infected cells in fat tissues. Viral-antigen-harboring immune cells (characterized as hemagglutinin- and CD45-coexpressing cells) were detected, predominantly in the SCAT. The presence of virus-infected immune cells was concomitant with the occurrence of (thermogenic UCP1-expressing) beige/ brite adipocytes in the SCAT (Figure 3). To the best of our knowledge, this is the first demonstration of a selective WAT beiging triggered by an acute viral infection.
We are currently investigating the mechanism(s) behind influenza-associated WAT browning. As mentioned before, WAT browning occurs in response to e.g. coldexposure or β3- selective adrenergic agonists [3,9-11]. Interestingly, a sympathetic-nervous-system control of anti-influenza immune responses has been described [52], and stimulation of α2-adrenergic receptor (α2-AR) impairs influenza virus infection in vitro [53]. Thus, it remains to be determined whether influenza infection stimulates β3- AR in fat cells, with the consequent enhanced lipolysis, which is followed by a greater capacity for lipid oxidation and thermogenesis in mitochondria, ultimately leading to WAT browning. Infection may also have favored the accumulation and activation of eosinophils, M2 macrophages and/or ILC2 in the SCAT; cell-types known to support WAT browning [33,34,37]. Moreover, the long-term maintenance of low blood glucose levels postinfluenza infection may help promoting type 2 immune cells since these cells predominantly depend on fatty acid rather than glucose metabolism for their energy supply [54]. The phenotyping of WAT’s (innate and adaptive) immune cells is presently under investigation, as well as the determination of their contribution to influenza pathophysiology (through adoptive cell-transfer and graft experiments). Apart from these indirect mechanisms which could explain SCAT browning associated with influenza infection, we showed that the virus on its own can directly promote beige adipogenesis in preadipocytes. Identification of virus-derived factors involved in this process may lead to the identification of novel browning inducer agents targeting key transcription factors in the browning process.
Conclusions and Perspectives
It is commonly acknowledged that induction of WAT browning confers metabolic benefits since it decreases adiposity and increases energy expenditure. Hence, the phenomenon of WAT browning has received much attention for its potential to raise body energy expenditure and, subsequently, reverse/treat obesity and its associated co-morbidities [55]. However, it has to be mentioned that detrimental effects of WAT browning have also been reported, including e.g. heart failure, edema, bone loss, cachexia or cancer, as recently reviewed by Tamucci and colleagues [15]. Thus, there is a need to further investigate factors and mechanisms that regulate WAT browning activation and deactivation.
Considering influenza infection, we showed that influenzainduced SCAT browning is transient since IAV-infected mice were not protected from diet-induced-obesityassociated body weight gain and glucose intolerance. In fact, we propose that influenza-induced SCAT browning may participate to the initial steps of immune defense against the infection. SCAT browning leads to increased local temperature, and a growing body of evidence suggests that immune cells are highly sensitive to thermal stress [56,57]. Thus, influenza-induced SCAT thermogenesis might help to controlling antiviral immune defenses, locally. From the virus side, the temporal weakness of the host (increased thermogenesis reduces energy savings) may help ensuring its propagation in infected cells.
More largely, we believe that the context of this study is important regarding the repeatedly reported associations between viral infections (including influenza) and obesity, a state of excessive (dysfunctional) fat mass and impaired WAT browning.
Acknowledgements
The authors thank Dr. Corinne Grangette for careful editing of the manuscript. JB was supported by Lille University and the Institut Pasteur de Lille (France). IW was supported by the Centre National de la Recherche Scientifique (CNRS).
Author Contributions Statement
JB and IW contributed to writing and editing the manuscript. Both authors approved the final version.
Sources of Funding
We acknowledge support from our funding agencies Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS), the University of Lille (Lille Univ), and the Pasteur Institute of Lille. J.B. received salary support from Lille Univ and Pasteur Institute of Lille, and I.W received salary support from CNRS.
Competing Interests
The authors have no competing interests to declare.
References
2. Rogers PM, Mashtalir N, Rathod MA, Dubuisson O, Wang Z, Dasuri K, et al. Metabolically Favorable Remodeling of Human Adipose Tissue by Human Adenovirus Type 36. Diabetes. 2008 Sep 1;57(9):2321-31.
3. Nedergaard J, Cannon B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metabolism. 2014 Sep 2;20(3):396-407.
4. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: Physiological roles beyond heat-generation. Cell Metabolism. 2015 Oct 6;22(4):546-59.
5. Ricquier D, Kader JC. Mitochondrial protein alteration in active brown fat: a sodium dodecyl sulfatepolyacrylamide gel electrophoretic study. Biochemical and Biophysical Research Communications. 1976 Dec 6;73(3):577-83.
6. Rousset S, Alves-Guerra M-C, Mozo J, Miroux B, Cassard-Doulcier A-M, Bouillaud F, et al. The Biology of Mitochondrial Uncoupling Proteins. Diabetes. 2004 Feb 1;53(Suppl 1):S130-5.
7. Lee Y-H, Mottillo EP, Granneman JG. Adipose tissue plasticity from WAT to BAT and in between. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014 Mar 1;1842(3):358-69.
8. Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016 April 4;59:1075-88.
9. Bargut TCL, Souza-Mello V, Aguila MB, Mandarimde- Lacerda CA. Browning of white adipose tissue: lessons from experimental models. Hormone Molecular Biology and Clinical Investigation. 2017 Jan 18;31(1):1-23.
10. Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat- Durebex C, Stevanovic Dokic A, Colin DJ, et al. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metabolism. 2016 Sept 13;24(3):434-46.
11. Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metabolism. 2017 Oct 3;26(4):672-85.e4.
12. Montanari T, Pošcic N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obesity Reviews. 2017 Feb 10;18(5):495-513.
13. Kaisanlahti A, Glumoff T. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. Journal of Physiology and Biochemistry. 2019 Feb 15;75(1):1-10.
14. Gustafson B, Smith U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis. 2015 Jul 1;241(1):27-35.
15. Tamucci KA, Namwanje M, Fan L, Qiang L. The dark side of browning. Protein Cell. 2018 Feb ;9(2):152-63.
16. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine. 2013 Oct 1;19(10):1338-44.
17. Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown-adipocytes in adult mice. FASEB Journal. 2015 Jan;29(1):286-99.
18. Loncar D. Convertible adipose tissue in mice. Cell Tissue Research. 1991 Oct 1;266(1):149-61.
19. Ramseyer VD, Granneman JG. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte. 2016 Feb 18;5(2):119-29.
20. Wang W, Seale P. Control of brown and beige fat development. Nature Reviews Molecular Cell Biology. 2016 Nov ;17(11):691-702.
21. Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metabolism. 2014 Feb 4;19(2):302-9.
22. Pyrzak B, Demkow U, Kucharska AM. Brown Adipose Tissue and Browning Agents: Irisin and FGF21 in the Development of Obesity in Children and Adolescents. Advances in Experimental Medicine and Biology. 2015 Jan 1;866:25-34.
23. Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014 June 5;157(6):1279-91.
24. Guilherme A, Yenilmez B, Bedard AH, Henriques F, Liu D, Lee A, et al. Control of Adipocyte Thermogenesis and Lipogenesis through ß3-Adrenergic and Thyroid Hormone Signal Integration. Cell Reports. 2020 May 5;31(5):107598-611.
25. Dodd G, Descherf S, Loh K, Simonds SE, Wiede F, Balland E, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015 Jan 15;160(1-2):88-104.
26. Eom J, Kim JJ, Yoon SG, Jeong H, Son S, Lee JB, et al. Intrinsic expression of viperin regulates thermogenesis in adipose tissues. Proceedings of the National Academy of Sciences of the United States of America. 2019 Aug 27;116(35):17419-28.
27. Dutescu RM, Li QX, Crowston J, Masters CL, Baird PN, Culvenor JG. Amyloid precursor protein processing and retinal pathology in mouse models of Alzheimer’s disease. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2009 Sep 1;247(9):1213-21.
28. Roesler A, Kazak L. UCP1-independent thermogenesis. Biochemical Journal. 2020 Feb 14;477(3):709-25.
29. Chang S-H, Song N-J, Choi JH, Yun UJ, Park KW. Mechanisms underlying UCP1 dependent and independent adipocyte thermogenesis. Obesity Reviews. 2019 Feb;20(2):241-51.
30. LaMarche NM, Kohlgruber AC, Brenner MB. Innate T Cells Govern Adipose Tissue Biology. The Journal of Immunology. 2018 Oct 1;201(7):1827-34.
31. DiSpirito JR, Mathis D. Immunological contributions to adipose tissue homeostasis. Seminars in immunology. 2015 Sep 1;27(5):315-21.
32. Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring). 2015 Mar;23(3):512-8.
33. Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011 Dec 1;480(7375):104-8.
34. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014 June 5;157(6):1292-308.
35. Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature medicine. 2017 Apr 17;23(5):623-30.
36. von Moltke J, Locksley RM. I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Current Opinion in Immunology. 2014 Dec;31:58-65.
37. Lee M-W, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated Type 2 Innate Lymphoid Cells regulate Beige Fat Biogenesis. Cell. 2015 Jan 15;160(1-2):74-87.
38. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015 Mar 12;519(7542):242-6.
39. García M del C, Pazos P, Lima L, Diéguez C. Regulation of Energy Expenditure and Brown/Beige Thermogenic Activity by Interleukins: New Roles for Old Actors. International Journal of Molecular Sciences. 2018 Aug 29;19(9):2569-607.
40. Valladares A, Roncero C, Benito M, Porras A. TNFalpha inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Letters. 2001 Mar 1;493(1): 6-11.
41. Cui XB, Chen SY. White adipose tissue browning and obesity. Journal of Biomedical Research. 2017 Jan;31(1):1- 2.
42. Hegde V, Dhurandhar NV. Microbes and obesity— interrelationship between infection, adipose tissue and the immune system. Clinical Microbiology and Infection. 2013 Apr 1;19(4):314-20.
43. Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, et al. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Frontiers in Microbiology. 2019 Dec 17;10(4):2837-62.
44. Jin C, Flavell RA. Innate sensors of pathogen and stress: Linking inflammation to obesity. Journal of Allergy and Clinical Immunology. 2013 Aug ;132(2):287-94.
45. Carrillo JLM, Campo JOMD, Coronado OG, Gutiérrez PTV, Cordero JFC, Juárez JV. Adipose Tissue and Inflammation. Adipose Tissue. 2018 Feb 22;DOI:10.5772/ interchopen.74227.
46. Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. Journal of Clinical Investigation. 2019 Dec 2;129(12):5327-42.
47. Han S-J, Zaretsky AG, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, et al. The white adipose tissue is a reservoir for memory T cells that promotes protective memory responses to infection. Immunity. 2017 Dec 19;47(6):1154-68.e6.
48. Damouche A, Lazure T, Avettand-Fènoël V, Huot N, Dejucq-Rainsford N, Satie A-P, et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathogens. 2015 Sept 24;11(9):e1005153.
49. Beigier-Bompadre M, Montagna GN, Kühl AA, Lozza L, Iii JW, Kupz A, et al. Mycobacterium tuberculosis infection modulates adipose tissue biology. PLoS Pathogens. 2017 Oct 17;13(10):e1006676.
50. Ponterio E, Gnessi L. Adenovirus 36 and Obesity: An Overview. Viruses. 2015 July 8;7(7):3719-40.
51. Ayari A, Rosa-Calatrava M, Lancel S, Barthelemy J, Pizzorno A, Mayeuf-Louchart A, et al. Influenza infection rewires energy metabolism and induces browning features in adipose cells and tissues. Communications Biology. 2020 May 14;3(1):237-52.
52. Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2009 Mar 31;106(13):5300-5.
53. Matsui K, Ozawa M, Kiso M, Yamashita M, Maekawa T, Kubota M, et al. Stimulation of alpha2-adrenergic receptors impairs influenza virus infection. Scientific Reports. 2018 Mar 15;8(1):4631-41.
54. Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nature Immunology. 2019 June 18;20(7):793-801.
55. Thyagarajan B, Foster MT. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Hormone Molecular Biology and Clinical Investigation. 2017 June 23;31(2):1-13.
56. Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nature Reviews in Immunology. 2015 May 15;15(6):335-49.
57. Schieber AM, Ayres JS. Thermoregulation as a disease tolerance strategy. Pathogens and Disease. 2016 Nov;74(9):ftw106.
58. Wang S, Yang X. Inter-organ regulation of adipose tissue browning. Cellular and Molecular Life Sciences. 2017 May;74(10):1765-76.