Keywords
ICAM-1, Efferocytosis, Wound healing, Inflammation, Migration, Adhesion
Introduction
ICAM-1 is a transmembrane, cell surface glycoprotein expressed by a variety of cells, but has been best-studied in vascular endothelium. Structurally, ICAM-1 is composed of five extracellular IgG- like domains to help facilitate cell-cell interactions and has a short cytoplasmic tail anchored to the cytoskeleton to facilitate intracellular signal transduction. ICAM-1 expressed by endothelial cells binds β2-integrins, CD11b/CD18 (Mac1), and CD11a/CD18 (LFA1) to mediate the adhesion of circulating leukocytes to the vessel wall and to facilitate transendothelial migration [1,2]. ICAM-1 can also regulate endothelial cell shape and vascular barrier function in response to inflammatory stimulation [3,4].
In addition to endothelial cells, ICAM-1 expression has been detected in vascular smooth muscle cells, pericytes, fibroblasts, keratinocytes, intestinal epithelial cells, and more recently in some subsets of immune cells [5-7]. For example, ICAM-1 expressed by T-cells helps deliver costimulatory signals for T-cell activation [8]. In dendritic cells, ICAM-1 helps bind T-cells and form immune synapses [9]. It has also been demonstrated that neutrophils can express ICAM-1 and increased neutrophilic ICAM-1 expression correlated with improved phagocytosis [10]. Macrophages were also found to express ICAM-1 [11], however, the role of ICAM-1 in macrophage function is an evolving area of investigation. This commentary specifically focuses on ICAM-1 in macrophages and its recently discovered role in facilitating efferocytosis.
ICAM-1 and Efferocytosis
Efferocytosis is a specialized process for the clearance of apoptotic cells (ACs) by tissue macrophages. It is essential for maintaining tissue homeostasis and when impaired can lead to non-resolving pathologic inflammation and tissue injury. Effective efferocytosis involves recognition of ACassociated ligands by macrophages via specialized surface receptors, reorganization of the macrophage cytoskeleton during AC engulfment, as well as phagolysosome fusion within the macrophages to degrade the internalized ACs.
Macrophages express a number of efferocytotic receptors including the TAM family tyrosine kinases (Tyro3, Axl, and Mer) [12]. The active process of efferocytosis also results in cellular reprogramming of the macrophage and acquisition of pro-resolution phenotype. After AC ingestion, macrophages reduce proinflammatory cytokine production and concurrently increase the production of cytokines that dampen inflammation such as IL-10, transforming growth factor β (TGF-β), and prostaglandin E2 [13-15]. This signaling switch from pro-inflammatory state to pro-resolution state is key for mediating tissue repair. Therefore, defective efferocytosis both exacerbates inflammation due to accumulation of dead cells and cellular debris, as well as impairs the ability of macrophages to facilitate wound repair.
ICAM-1 expression has been previously shown to be induced in macrophages and to contribute to macrophage polarization [11,16-18]. Intriguingly, the way in which ICAM-1 regulates macrophage polarization appears to be context-dependent. For example, in the tumor microenvironment macrophage ICAM-1 was associated with a pro-inflammatory phenotype. However, in acute lung injury ICAM-1 was associated with a pro-resolution phenotype [17].
Given the important role of macrophage efferocytosis in injury resolution and the emerging role of ICAM-1 in macrophage effector function, our group recently examined macrophage ICAM-1 functionality in macrophages in the context of inflammatory bowel disease (IBD) [7]. IBD is a symptomatic, debilitating disease driven by injury to the intestinal epithelium and dysregulated immune responses [19,20]. As such, macrophages play an important role in both initiation and resolution of colon inflammation [21].
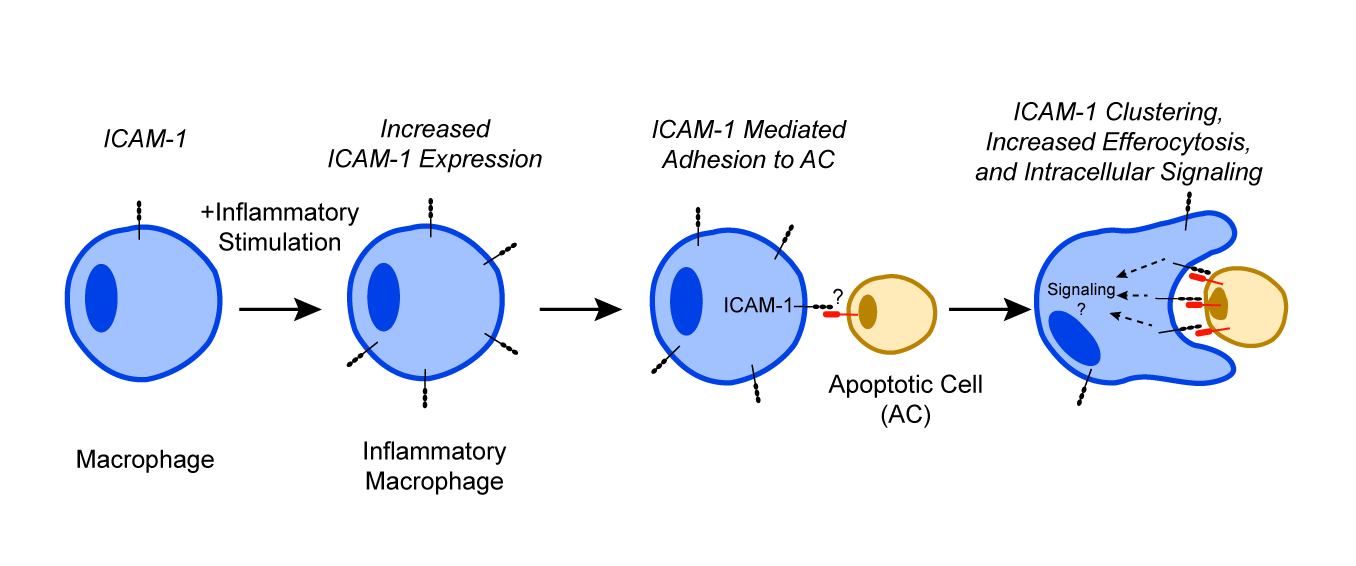
We found that ICAM-1 was indeed upregulated on inflammatory macrophages in the gut. Importantly, we identified a new function for ICAM-1 in mediating macrophage-AC binding and facilitating efferocytosis [7]. Whereas stimulation of bone-marrow derived macrophages by IL-4 to resemble the resident/pro-repair phenotype in culture did not induce ICAM-1 expression, macrophage differentiation towards the inflammatory phenotype by interferon γ (IFNγ) and lipopolysaccharide (LPS) stimulation robustly increased ICAM-1 expression. Similarly, in a model of murine colitis, inflammatory macrophages isolated from the colon showed increased ICAM-1 expression. This was additionally supported by data from human IBD patients. Tissue sections of colon with active IBD showed more macrophages that costained positive for ICAM- 1 compared to noninflamed colon sections. Macrophages lacking functional ICAM-1 receptor were unable to effectively perform efferocytosis in vitro and in vivo thus confirming the role of ICAM- 1 in efferocytosis. Furthermore, antibody-mediated inhibition or knockdown of ICAM-1 both in vitro and in vivo significantly decreased the ability of macrophages to engulf and uptake ACs. We also established that ICAM-1 clustered at the site of the engulfed ACs mediating their binding to macrophages.
These data demonstrate that ICAM-1 when induced in inflammatory macrophages plays an important role in binding of ACs during efferocytosis (summarized by the schematic, Figure 1).
These findings also raise additional important questions that should be considered in future investigation. For example, it is still unclear how ICAM-1 expression is regulated in macrophages. Increased iNOS and reactive oxygen species (ROS) have been suggested to promote ICAM-1 upregulation but given the diversity of macrophage phenotypes and their plasticity it remains to be seen whether there is a particular subset of inflammatory macrophages that undergo this change [18]. Similarly, given the variety of cells that macrophages can clear in various tissues, ICAM-1 ligands on ACs should be investigated in more detail. While in the case of immune cells, these interactions may be facilitated by ICAM-1 binding to β2-integrins, efferocytosis of epithelial cells or fibroblasts, which do not express β2-integrins will be mediated by other yet undefined ligands.
ICAM-1 and Wound Healing
Wound healing is a complex, multi-step cascade of events that requires careful spatial and temporal synchronization of a variety of different cells and processes. Key hallmarks of the wound healing process include hemostasis, inflammation, cellular proliferation, and tissue remodeling [22]. ICAM-1 has been previously implicated in facilitating wound repair via several implied but not well-defined mechanisms. For example, skin wound healing in ICAM Figure 1 KO mice was significantly delayed [23], a phenotype that was associated with reduced wound infiltration of neutrophils and macrophages and reduced granulation tissue formation [24]. These observations are consistent with the role of endothelial cell ICAM-1 in mediating leukocyte transendothelial migration and the wellrecognized contributions of both cell types to initiation of inflammation, host defense and injury resolution.
Similar to skin, repair after colonic injury was also significantly delayed in the absence of functional ICAM-1. Following injury to the colonic mucosa, ICAM-1 expression was highly induced in colon epithelial cells, facilitating neutrophil apical retention and promoting epithelial cell proliferation and wound closure [5,25]. This work has clearly demonstrated that the impairment in mucosal healing under these conditions was driven by ICAM-1 expression by epithelial cells. However, our recent findings demonstrating ICAM-1 expression on macrophages suggest that this may also be involved to the observed phenotype. We found that macrophage ICAM-1 is essential for facilitating efferocytosis and without efferocytosis, the resolution of inflammation and restoration of tissue homeostasis cannot occur. Furthermore, efferocytosis drives macrophage reprogramming and polarization towards a pro-resolution phenotype and promotes crucial healing effector functions of macrophages. Particularly in the gut, where the majority of tissue resident macrophages are derived from circulating monocytes, ICAM-1 expressed by various cell types appears to coordinate several important aspects of wound healing including monocyte recruitment into the wounded tissue (endothelial ICAM- 1), wound debridement and resolution of inflammation (macrophage ICAM-1) and wound reepithelialization (epithelial cell ICAM-1).
In addition to mediating cellular adhesion, ICAM-1 also serves as a signaling receptor. ICAM-1 engagement is associated with calcium signaling, Rho activation, Akt/β- catenin signaling, and is also coupled with cytoskeletal remodeling [3,25,26]. However, whether ICAM-1 signals in macrophages and whether this impacts efferocytosis remains unknown. This should be examined in future studies, as calcium influx, Rho activation, and cytoskeletal changes are all critical elements for AC engulfment and for macrophage effector function.
Finally, ICAM-1 has been previously considered as a therapeutic target by several clinical trials however, without apparent success. For example, ICAM-1 inhibition was tested in clinical trial to treat ischemic stroke [27]. Given the emerging pro-resolution and pro-healing functions of ICAM- 1, this is perhaps not surprising. Current emerging identification of its roles in wound healing in specific cell types provides a better opportunity for targeted therapy and rekindles interest in this important molecule. Improperly healing wounds in the context of many organs including skin, lung, and colon represent a significant healthcare burden. Thus, developing effective therapies to improve wound healing remains an active research focus and exploring the role of macrophage ICAM-1 can significantly improve our current understanding of this process.
Funding
This work was supported by grants from the National Institutes of Health (NIH) DK124199, NIH HL134202, American Cancer Society Research Scholar Award, and Crohn’s & Colitis Foundation Senior Research Award.
Conflicts of Interest Declaration
Authors declare no conflicts of interest.
References
2. Long EO. ICAM-1: getting a grip on leukocyte adhesion. The Journal of Immunology. 2011 May 1;186(9):5021-3.
3. Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1- coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. The Journal of Immunology. 2000 Sep 15;165(6):3375-83.
4. Sumagin R, Lomakina E, Sarelius IH. Leukocyteendothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. American Journal of Physiology-Heart and Circulatory Physiology. 2008 Sep;295(3):H969-77.
5. Sumagin R, Robin AZ, Nusrat A, Parkos CA. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunology. 2014 Jul;7(4):905.
6. Xia YF, Liu LP, Zhong CP, Geng JG. NF-?B activation for constitutive expression of VCAM-1 and ICAM-1 on B lymphocytes and plasma cells. Biochemical and Biophysical Research Communications. 2001 Dec 14;289(4):851-6.
7. Wiesolek HL, Bui TM, Lee JJ, Dalal P, Finkielsztein A, Batra A, et al. Intercellular Adhesion Molecule 1 Functions as an Efferocytosis Receptor in Inflammatory Macrophages. The American Journal of Pathology. 2020 Feb 6.
8. Chirathaworn C, Kohlmeier JE, Tibbetts SA, Rumsey LM, Chan MA, Benedict SH. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. The Journal of Immunology. 2002 Jun 1;168(11):5530-7.
9. Feigelson SW, Grabovsky V, Manevich-Mendelson E, Pasvolsky R, Shulman Z, Shinder V, et al. Kindlin-3 is required for the stabilization of TCR-stimulated LFA-1: ICAM-1 bonds critical for lymphocyte arrest and spreading on dendritic cells. Blood, The Journal of the American Society of Hematology. 2011 Jun 30;117(26):7042-52.
10. Woodfin A, Beyrau M, Voisin MB, Ma B, Whiteford JR, Hordijk PL, et al. ICAM-1–expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia. Blood, The Journal of the American Society of Hematology. 2016 Feb 18;127(7):898-907.
11. Bernatchez SF, Atkinson MR, Parks PJ. Expression of intercellular adhesion molecule-1 on macrophages in vitro as a marker of activation. Biomaterials. 1997 Oct 1;18(20):1371-8.
12. Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. The Journal of Immunology. 2017 Feb 15;198(4):1387-94.
13. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of Clinical Investigation. 1998 Feb 15;101(4):890-8.
14. Xiao YQ, Freire-de-Lima CG, Schiemann WP, Bratton DL, Vandivier RW, Henson PM. Transcriptional and translational regulation of TGF-ß production in response to apoptotic cells. The Journal of Immunology. 2008 Sep 1;181(5):3575-85.
15. Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PloS One. 2010 Mar 4;5(3):e9539.
16. Yang M, Liu J, Piao C, Shao J, Du J. ICAM-1 suppresses tumor metastasis by inhibiting macrophage M2 polarization through blockade of efferocytosis. Cell Death & Disease. 2015 Jun;6(6):e1780.
17. Gu W, Yao L, Li L, Zhang J, Place AT, Minshall RD, et al. ICAM-1 regulates macrophage polarization by suppressing MCP-1 expression via miR-124 upregulation. Oncotarget. 2017 Dec 19;8(67):111882.
18. Hubbard AK, Giardina C. Regulation of ICAM-1 expression in mouse macrophages. Inflammation. 2000 Apr 1;24(2):115-25.
19. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature Reviews Immunology. 2003 Jul;3(7):521-33.
20. Silva FA, Rodrigues BL, Ayrizono MD, Leal RF. The immunological basis of inflammatory bowel disease. Gastroenterology Research and Practice. 2016 Oct;2016:2097274.
21. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nature Reviews Gastroenterology & Hepatology. 2019 Jul 16(9):531-43.
22. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiological Reviews. 2019 Jan 1;99(1):665-706.
23. Gay AN, Mushin OP, Lazar DA, Naik-Mathuria BJ, Yu L, Gobin A, et al. Wound healing characteristics of ICAM- 1 null mice devoid of all isoforms of ICAM-1. Journal of Surgical Research. 2011 Nov 1;171(1):e1-7.
24. Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. The American Journal of Pathology. 2000 Jul 1;157(1):237-47.
25. Sumagin R, Brazil JC, Nava P, Nishio H, Alam A, Luissint AC, et al. Neutrophil interactions with epithelialexpressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunology. 2016 Sep;9(5):1151-62.
26. Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. The Journal of Immunology. 1998 Nov 15;161(10):5755-61.
27. Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001 Oct 23;57(8):1428-34.