Abstract
The important immune cells in the brain are called microglia acting as the central junction between neuroinflammation and neurodegenerative diseases. In patients of cognitive disorders and Alzheimer’s disease (AD) animal models, amoebic morphology and inflammatory pathways are activated to release numerous cells in the inflammatory factors by active microglia. Dendrobium nobile Lindl. alkaloids (DNLA) are a kind of naturally extracted product that exhibits neuroprotective and anti-inflammatory activities. We previously reported that DNLA effectively inhibited NF-κB signal pathway in AD models. But how does DNLA exert antiinflammatory action is unknown. To study microglia-mediated inflammation, BV2 microglia cells were used to examine the effects of DNLA against LPS-induced inflammatory response. We found that DNLA reduced LPS-induced production of TNF-a and levels of Nlrp3 and IL-1ß mRNA, inhibited LPS-induced morphological changes, and decreased the protein expression of microglia marker Iba-1, inflammation-related factors iNOS in BV2 cells. Fortunately, DNLA significantly affected the expression of NF-κB p65, IκBa and their phosphorylative products in cellular nucleus and cytosol of LPS-induced BV2 microglia, and decreased TLR4, NLRP3, ASC, caspase-1, NEK7 and GSDMD expression of LPS-induced BV2 microglia. Taken together, the present study clearly demonstrated the protective effects of DNLA against LPS-induced inflammation in BV2 microglia, probably mediated through NF-κB and NLRP3 signaling pathways.
Keywords
Dendorbium Nobile Lindl. alkaloids, Lipopolysaccharide, Neurodegenerative diseases, BV2 microglia, NF-κB, NLRP3 inflammasome
Abbreviations
DNLA: Dendorbium Nobile Lindl. Alkaloids; LPS: Lipopolysaccharide; IL-1β: Interleukin-1β; TNF-α: Tumor Necrosis Factor; iNOS: Induced Nitric Oxide Synthase; TLR4: Toll-like Receptor 4; NLRP3: NLR family pyrin domain-containning protein 3; NF-κB: Nuclear Factor-κB; IκBα: Inhibitor of NF-κB; ASC: Apoptosis-associated Speck-like protein containing CARD; Iba- 1: Ionized calcium binding adaptor; GSDMD: Gasdermin D; NEK7: NIMA-related kinase-7
Introduction
Alzheimer’s disease (AD) is the most common brain disease with aging characterized by progressive memory loss and cognitive decline. The hallmark of AD is the formation of senile plaques composed of amyloid-β (Aβ) and neurofibrillary tangles (NFTs). Accumulating evidence indicates that neuroinflammation is also a critical hallmark of AD [1]. Microglia are the major regulators of neuroinflammation in mammalian brain and play a pathological role in AD development and progression [2]. Macroglia could degrade Aβ to resist its deposition, on the other hand, microglia can release proinflammatory mediators to cause neuroinflammation and aggravate Aβ and tau pathology [3-5].
Nuclear factor-kappa B (NF-κB) is one of the intracellular inflammatory signaling pathways, which can be activated by Aβ binding to TLR4 on the surface of microglia. The NF- κB activation can induce inflammatory gene transcription and further promote the production of inflammatory cytokines from microglia, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), inducible nitric oxide synthase (iNOS), and so on [6].
In general, neuroinflammation formation requires multiple steps. The transcription of NLR family pyrin domain-containing protein 3 (NLRP3) is activated via the NF-κB signaling pathway [7]. The activation of NLRP3 inflammasome is a critical event for AD neuroinflammatory processes in microglia [8]. Additionally, the increasing expression of NLRP3 inflammasome is a critical event for AD neuroinflammatory processes [8]. “Sensor” protein NLRP3 combined with the adapter apoptosis-associated speckled protein (ASC), then combined with inactive procaspase- 1 recruited by ASC to form NLRP3 inflammasome [9]. After inflammasome NLRP3 formation, pro-caspase-1 activates itself and generates cleaved-caspase-1. Cleavedcaspase- 1 can effectively promote the maturation and secretion of interleukin-1β (IL-1β) and interleukin-18 (IL- 18) [10]. Moreover, NIMA-related kinase 7 (NEK7), another important component of the NLRP3 inflammasome, is involved in the activation of NLRP3 inflammasome. The activation of NLRP3 inflammatory can be inhibited in the absence of NEK7 [11,12]. It has been reported that the transcriptional activity and expression of NEK7 can be increased by LPS-induced TLR4/NF-κB signaling pathway. The up-regulated expression of NEK7 can bind with NLRP3, finally make Gasdermin D (GSDMD) split into cleaved-GSDMD [13,14]. GSDMD belongs to the Gasdermin protein family and cleaved-GSDMD is formed with cleaved-caspase-1. Once it is formed, cleaved-GSDMD (an oligomeric p30 protein) anchors on the cell membrane to open the channel pore to induce pyroptosis [15-17].
Dendrobium Nobile Lindl. is a Chinese medicine for many disorders and Dendrobium Nobile Lindl. alkaloids (DNLA) has been shown to have protective effects against neurodegenerative diseases, such as to reduce the Aβ25-35-induced cytotoxicity and axonal degeneration in primary neurons [18,19], attenuate LPS-induced hyperphosphorylation of tau, Aβ-induced neuronal apoptosis and synaptic loss [20,21], reduce the formation and aggregation of toxic amyloid in SD rats [22], and also protect against aging-related learning and memory deficits, neuronal aging and loss in SAMP8 mice [23,24]. In addition, DNLA are effective in alleviating inflammation. DNLA is also effective in protecting against LPS-induced rat brain impairment via inhibition of MAPK and NF-κB signal pathway [25]. DNLA can activate the Nrf2 signaling pathway to protect against CCl4-induced hepatotoxicity in mice [25-27]. These results indicate that DNLA are a promising candidate for neurodegenerative diseases and neuroinflammation. Thus, the present study aimed to explore the protective effects and mechanisms of DNLA in neuroinflammation through NF-κB and NLRP3 signaling pathways.
Materials and Methods
Materials
Dendrobium nobile was obtained from Xintian TCM Industry Development Co., LTD., of Guizhou Provinc, batch number: Qian YPBZ0045-2014 [21]. The DNLA were 79.8% after isolation and purification from the stem of Dendrobium nobile by our research group. Components included dendrobine (C16H25O2, 92.6%), dendrobine-Noxide (C16H25O3N, 3.3%), nobilonine (C17H27O3N, 2.0%), dendroxine (C17H25O3N, 0.9%), 6-hydroxy-nobilonine (C17H27O4N, 0.32%), and 13-hydroxy-14-oxodendrobine (C16H23O4N, 0.07%), which have been shown in detail in the previous articles of our research group [19,21]. LPS was obtained from Sigma (St. Loius, MO). MTT was supplied from Solarbio Technology (Beijing, China). The ELISA kit for mouse TNF-α was purchased from Thermo Fisher Scientific. Anti-β-actin antibody was purchased from Proteintech (Wuhan, China), anti-iNOS (ab15323), Anti- Ionized calcium binding adaptor (Iba-1, ab178847), anti-TLR4 (ab13556), anti-GSDMD (ab209845), anticleaved- GSDMD (ab215203) and anti-NEK7 (ab133514) antibodies were bought from Abcam (Cambridge, UK). Anti-NLRP3 antibody (NBP2-12446) was bought from Novus biologicals (Centennial, CO , USA). Anti-NF-κB p65 antibody was obtained from Cell Signaling Technology (CST-D14E12). Anti-ASC (SCBT -514414), anti-caspase 1 (SCBT -14F468), anti-cleaved-caspase 1 (SCBT -22166), anti-p-NF-κB p65 (Ser536, SCBT -136548), anti-inhibitor of NF-κB (IκBα, SCBT -1643), anti-p-IκBα (SCBT -8404) antibodies were bought from Santa Cruz Biotechnology (Santa Cruz, USA).
Cell culture
BV2 microglial cell line was purchased from China Center for Type Culture Collection (CCTCC). Dulbecco’s Modified Eagle Medium (DMEM) was supplemented with 10% Fetal Bovine Serum, 100 U/mL penicillin, and 100 μg/mL streptomycin for BV2 microglia cultures. Cells were kept at a constant temperature of 37°C and in a humidity environment (with 5% CO2). BV2 microglia were dissociated by trypsin, and 6/24 well plates were used to culture BV2 cells suspensions for 12 h. Then fresh DMEM without FBS was replaced for 24 h to synchronize cells. BV2 microglia were pretreated with DNLA for 1 h before LPS addition.
Cell viability by the MTT assay
96-well plates (5 × 103 cells/well) were used to culture BV2 microglia. After cultured in DMEM without FBS for 24 h, BV2 cells were treated with LPS (10, 100 and 1000 ng/mL) for 3 h and 6 h. Then, MTT was dissolved in PBS (5 mg/mL) and 20 μL was added to each well and were incubated for 4 h. Then we removed the supernatants and added 150 μL dimethyl sulfoxide (DMSO) to solve MTT. The plates were gently shaken for 10 min. After that, microplate reader (Bio-Rad, CA, USA) was used to detect the optical density (O.D.) values (at 490 nm).
Enzyme-linked immunosorbent assay (ELISA)
BV2 microglia were cultured in 24-well plates, then cultured in DMEM without FBS for 24 h. These cells were pretreated with DNLA with various concentrations (3.5, 35 and 350 ng/mL) for 1 h and followed stimulated with LPS (100 ng/mL) for additional times. After the culture period, culture supernatants were collected and centrifuged (1000 rpm, 5 min). The production of TNF-α in supernatant was detected by ELISA kit according to the manufacturer’s instructions. The O.D. values were measured using a microplate reader (at 450 nm).
Quantitative real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from microglia, and extracted RNA (1 μg) was reversely transcribed using a ReverTra Ace qPCR Reverse Transcription Kit (TAKARA, Otsu, Japan), and the mRNA level was quantified using an Eco Real- Time PCR System (Bio-Rad, CA, USA). All RT-PCRs were performed in a 20 μl reaction mixture in 96-well PCR plates (Bio-Rad, CA, USA) using a iQ SYBR Green Supermix (Bio-Rad, CA, USA) and the primer pairs (Sangon Biotech, Shanghai, China) listed in Table 1.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Nlrp3 | ATTACCCGCCCGAGAAAGG | TCGCAGCAAAGATCCACACAG |
| IL-1ß | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| ß-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Table 1: Primer sequences used in this study..
Western blot analysis
BV2 microglia were lysed with RIPA buffer (Solarbio, China) for total proteins, nuclear and cytoplasmic proteins were extracted separately by nuclear and cytoplasmic extraction reagents (Thermo scientific, MA, USA). BCA protein assay kit was used to determine protein concentrations. 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) was used to separate proteins. Then the proteins were electrotransferred to polyvinylidene fluoride (PVDF) membranes (Thermo, MA, USA). The membranes were sealed in 5% skim milk powder for 1 h and were incubated with corresponding primary antibodies in Tris-buffered saline- Tween 20 (TBST) with the addition of 5% bovine serum albumin (BSA) (4°C, 12 h). The unbound antibodies were removed by washing three times. Then appropriate peroxidase-conjugated secondary antibodies were used to incubate the membranes. Lastly, the membranes were detected by the enhanced chemiluminescence reagent.
Cellular immunofluorescence
BV2 microglia were cultured on cover glasses, then fixed with 4% paraformaldehyde for 60 min at 4°C or carbinol (60 min, 4°C). PBS with BSA (5%) and Triton X-100 (0.3%) were used to permeabilize BV2 cells. Then PBS, BSA (1%) and Triton X-100 (0.3%) were co-incubated with primary antibodies in against Iba-1, NLRP3 or NF-κB p65 BV2 microglia cells overnight (4°C). After washing with PBS three times, BV2 cells were incubated with corresponding secondary antibodies conjugated to Alexa Fluor (488 or 546), and then were incubated with BSA (5%) at room temperature for 1 h. After that, cells were counterstained with Hoechst 33258 (5 μg/mL, 10 min, 37°C) and washed with PBS three times. Positive fluorescence microscope (Olympus Corp., Tokyo, Japan) was used to acquire Immunofluorescent images.
Statistical analyses
SPSS Software 18.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the study data, and the data were analyzed by one-way ANOVA or Dunnet’s multi-comparison test. P<0.05 was considered statistically significant, and the results were all expressed as mean ± SEM.
Results
LPS stimulated BV2 microglia to establish a neuroinflammatory model
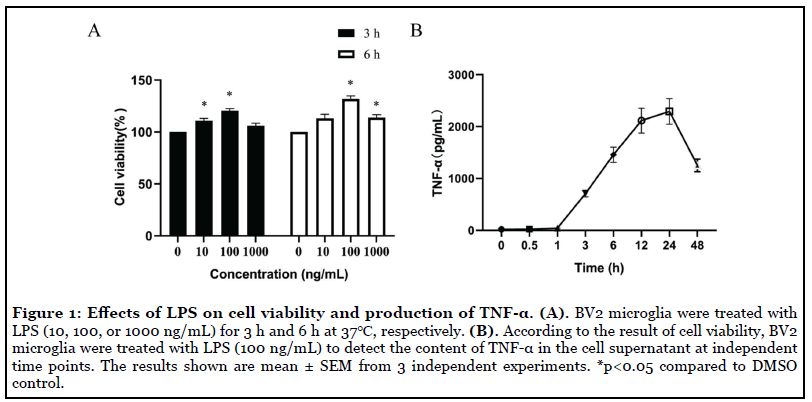
Effects of the LPS on the cell viability and TNF-α production of BV2 microglia: BV2 microglia produce stress responses and induce proliferation and viability after LPS stimulation. Effects of LPS on cell viability of BV2 microglia were evaluated by MTT assay. The obtained data showed that the cell activity was significantly increased after LPS (100 ng/mL) stimulation. Therefore, the concentration of LPS (100 ng/ml) was subsequently used in this study (Figure 1A). Then, temporal changes TNF-α level induced by LPS (100 ng/ml) in the supernatant was determined by ELISA. Data showed the level of TNF-α in the supernatant raised gradually, peaked at 24 h and then gradually decreased (Figure 1B). Thus, in this study, LPS (100 ng/mL) were used to deal with BV2 microglia cells for 24 h.
LPS affected the morphology of BV2 microglia: LPS-induced morphological changes of BV2 microglia were observed under the microscope. Images showed that most BV2 microglia had approximately 2-3 protrusions under normal state. Moreover, with the increase of concentration of LPS and the elongation of time, the protrusions and volume of BV2 cells increased gradually, and the cell morphology changed significantly. Cell protrusions decreased after 12 h of LPS stimulation (Figure 2).

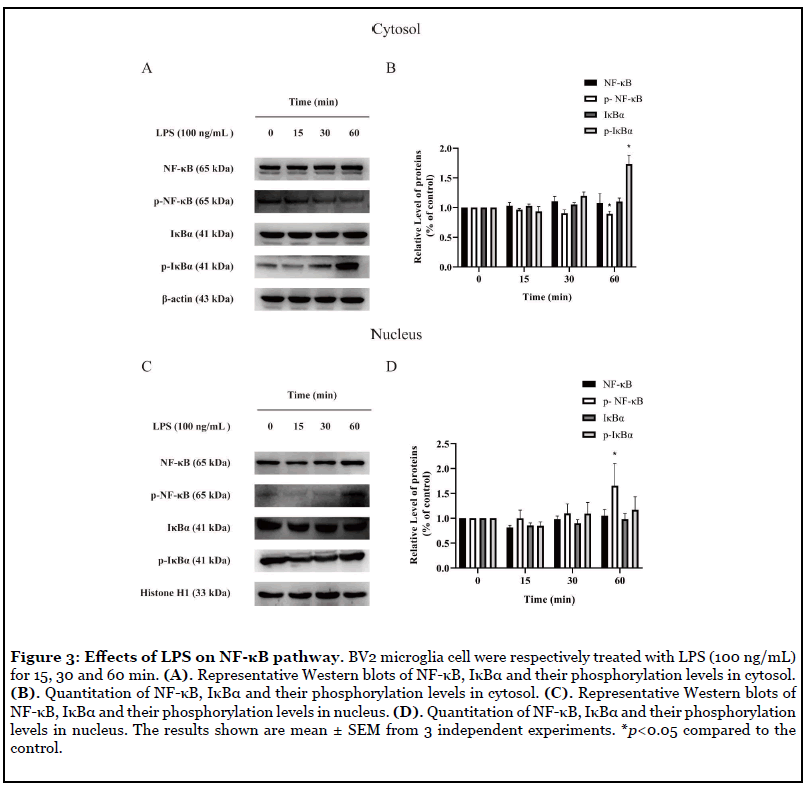
Effects of LPS on NF-κB signaling at different time points: Protein phosphorylation plays an important role in the maintenance of normal functions of organisms. Protein phosphorylation is a rapid reaction in cells, therefore, we examined the expressions of NF-κB p65, IκBα and the phosphorylation of NF-κB p65, IκBα at different time points. In the cytoplasm of BV2 microglia, phosphorylated IκBα increased significantly and phosphorylated NF-κB decreased at 60 min after LPS (100 ng/mL) administration (Figure 3B). However, in the nucleus of BV2 microglia, phosphorylated NF-κB increased obviously at 60 min after LPS (100 ng/mL) administration (Figure 3D).
DNLA inhibited LPS-induced inflammation in BV2 microglia
Effects of DNLA on TNF-α content, IL-1β, Nlrp3 mRNA levels, and Iba-1, iNOS protein levels in BV2 cells induced by LPS: BV2 microglia pretreated with DNLA for 1 h, and followed by LPS (100 ng/mL) treatment for 24 h. The content of TNF-α in the supernatant was determined by ELISA. As shown in Figure 4A, treatment with LPS presented a significant increase of TNF-α in the supernatant, and each dose of DNLA significantly prevented the increasing effect. After pretreatment with DNLA for 1 h, and followed by LPS (100 ng/mL) treatment for 3 h, the mRNA expression of IL-1β and Nlrp3 were detected. The levels of IL-1β and Nlrp3 mRNA increased significantly in BV2 microglia after exposure to LPS. Fortunately, Nlrp3 mRNA showed a downward trend and IL-1β mRNA was decreased obviously with DNLA (350 ng/mL) treatment (Figure 4B).
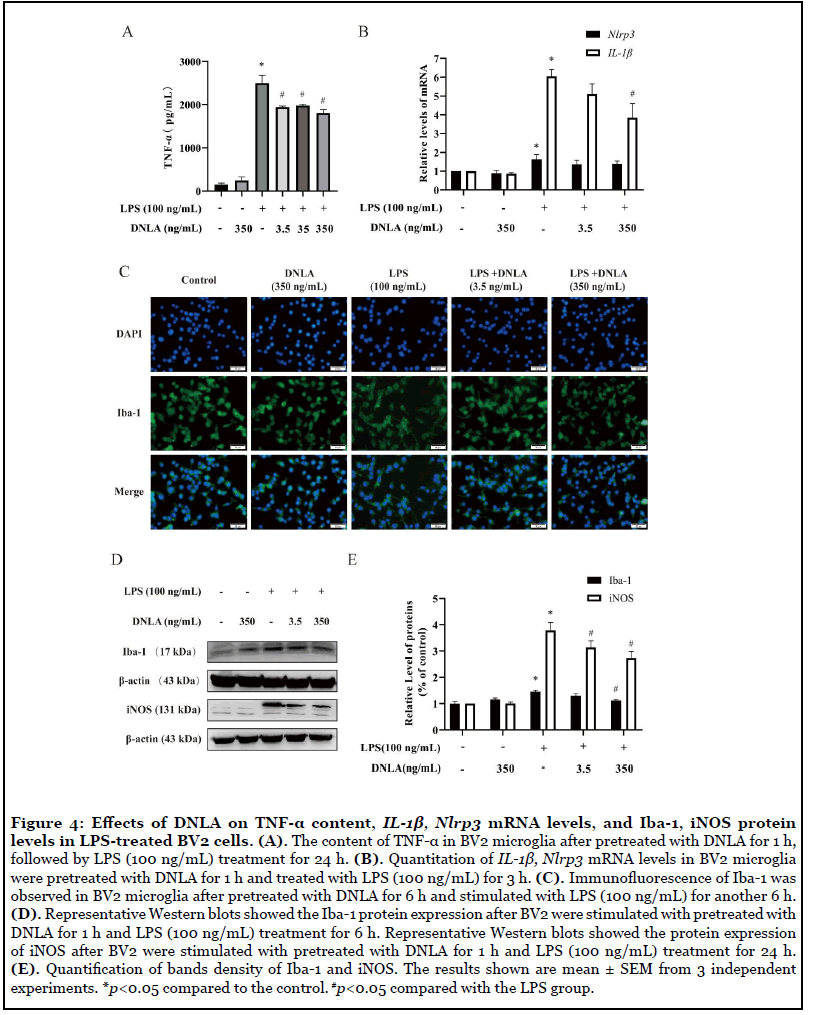
LPS can induce the activation of microglia and activated microglia can induce protease activation and release various pro-inflammatory cytokines [28]. To further assess whether DNLA inhibits LPS-stimulated BV2 cell activation, we analyzed the protein expression of Iba- 1by using immunofluorescence and western blot. As shown in Figure 4C, BV2 microglia cells in normal state are small and round with few protrusions. However, LPS-activated BV2 microglial cells became larger and had an amoeboid morphology. DNLA treatment could markedly reverse BV2 microglia morphological changes. Additionally, the expressions of microglial marker Iba-1 and iNOS were also increased by LPS stimulation. After pretreatment with DNLA, Iba-1 and iNOS protein levels decreased significantly. These results suggested that DNLA pretreatment alleviate nerve inflammation, which is closely related to inhibition of microglia activation (Figures 4D and 4E).
DNLA inhibited TLR4/NF-κB pathway in LPSinduced neuroinflammation: We measured TLR4 proteins expression because activation of TLR4 triggers intracellular signaling pathways that activate NF-κB and regulate the release of inflammatory cytokines. As expected, in the LPS group, TLR4 levels were significantly increased, indicating that LPS activates the NF-κB signaling pathway. Fortunately, TLR4 protein expression can be inhibited by DNLA administration (Figures 5B and 5C).
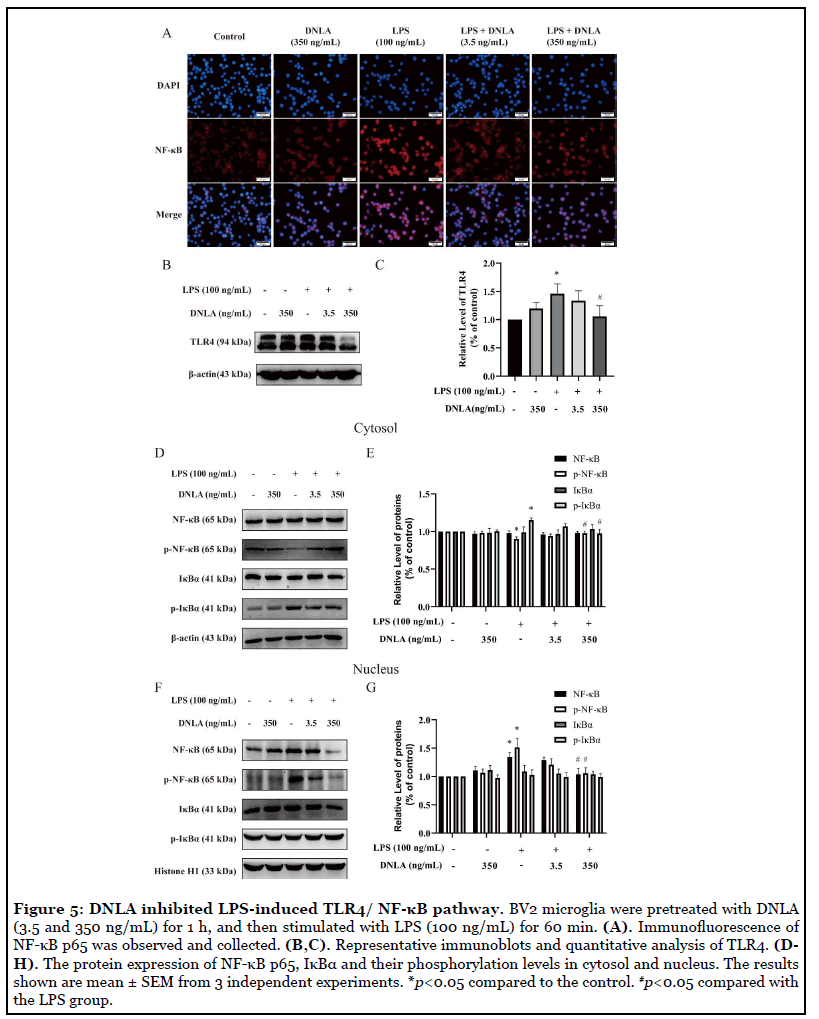
We further conducted immunofluorescent and Western blot staining analysis to examine the protein expression of NF-κB pathway. Our data showed that comparison with the control group, after 24 h of exposure to 100 ng/mL LPS, p-NF-κB were decreased and p-IκBα were increased in the cytosol. Moreover, NF-κB p65 and p-NF-κB p65 levels were increased in nucleus, suggesting that the NF-κB signaling pathway was activated by LPS. DNLA administration downregulated the protein expression of NF-κB p65 and p-IκBα levels in cytosol. Above results of protein expression were consistent with the data from immunofluorescent staining assay (Figures 5A-5G). Therefore, these results indicated that the LPS-induced TLR4/NF-κB signaling pathway was inhibited by DNLA in BV2 microglia.
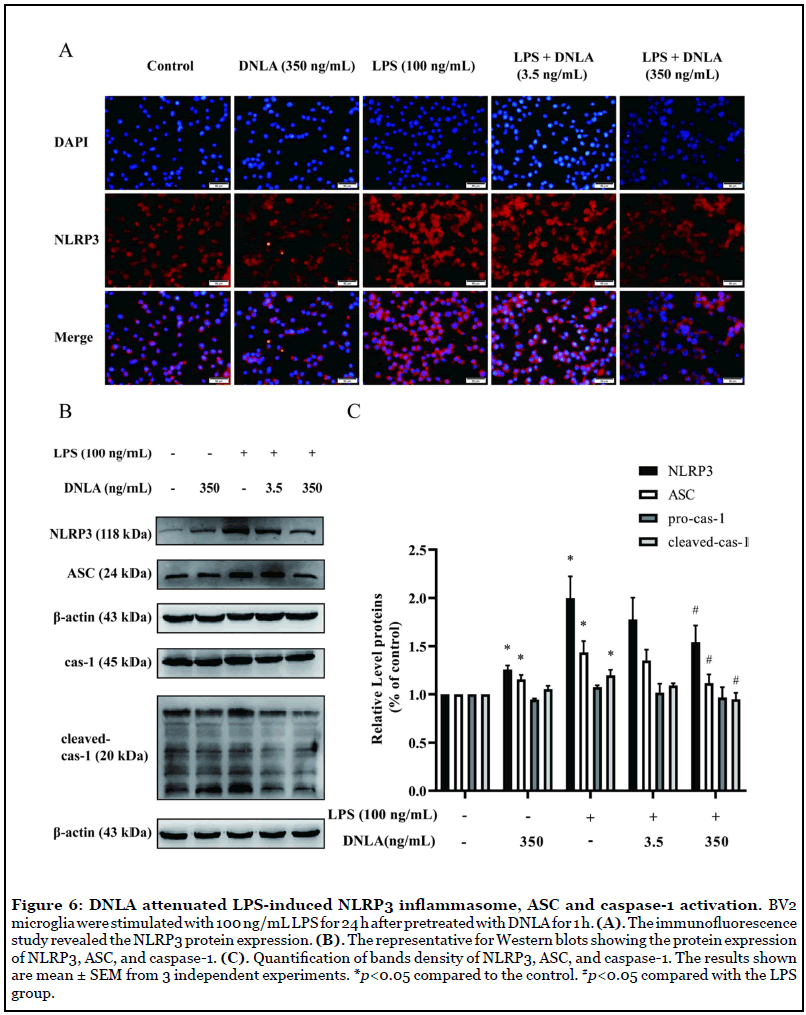
DNLA attenuated NLRP3 inflammasomes activation and reduced the expression of cleavedcaspase- 1 and ASC in LPS-induced BV2 cells: In the process of proinflammatory cytokines transcriptional regulation, NLRP3 inflammasome plays a significant role. We used immunofluorescent staining and Western blot to reveal the activation and expression of NLRP3 inflammasomes. BV2 microglia pretreated with DNLA for 1 h, then followed by LPS (100 ng/mL) treatment for 24 h. Immunofluorescent staining and Western blot analysis showed NLRP3 protein expression was significantly increased in LPS-induced BV2 microglia. Fortunately, DNLA treatment significantly decreased NLRP3 expression (Figures 6A-6C). Moreover, Cleaved-caspase-1 and ASC were upregulated in the LPS-induced BV2 microglia compared to the control. Fortunately, DNLA (350 ng/ mL) treatment significantly diminished cleaved-caspase-1 and ASC upregulation in BV2 microglia (Figures 6B and 6C). These results indicated that DNLA reduced NLRP3 inflammasomes activation in LPS-induced BV2 microglia.
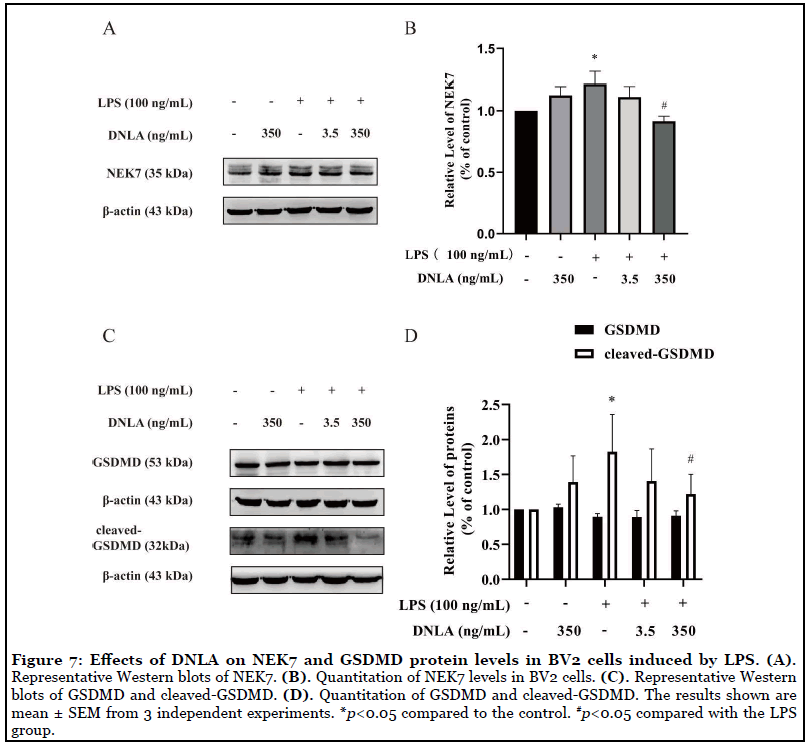
DNLA decreased the expressions of LPS-induced Gasdermin D and NEK7: It has been reported that the interaction between NEK7 and NLRP3 modulated GSDMD to be cleaved-GSDMD [14]. In order to verify the interaction between NEK7 and NLRP3 can be affected by DNLA, we measured the expressions of NEK7 and GSDMD. The obtained data showed that NEK7 and cleaved GSDMD increased significantly after LPS treatment, and DNLA treatment reversed the trend (Figures 7A-7D).
Discussion
The present study revealed that the neuroprotective effects of DNLA against LPS-induced neuroinflammation in BV2 microglia. It is observed that DNLA reduced LPSinduced production of TNF-α and level of Nlrp3, IL-1β mRNA, inhibited LPS-induced morphological changes, and decreased the protein expression of microglia marker Iba-1, inflammation-related factors iNOS in BV2 cells. Fortunately, DNLA significantly affected the expression of NF-κB p65, IκBα and their phosphorylative products in cellular nucleus and cytosol of LPS-induced BV2 microglia, and declined the expression of TLR4, NLRP3, ASC, caspase-1, GSDMD and NEK7 in total proteins of LPS-induced BV2 microglia. Taken together, these results suggested that DNLA presented as a potential therapeutic candidate for neuroinflammation, which was associated with suppressing NF-κB and NLRP3 signaling pathways induced by LPS.
Microglia plays a critical role in inflammatory responses and immune defense. LPS has the ability to induce microglia activation and caused a round amoeboid shape in BV-2 cells, which lead to the release of microglial M1 phenotype markers (such as IL-1β and TNF-α), as well as increased the expression of Nlrp3. Meanwhile, microglial markers Iba-1 and inflammation-related factors iNOS were also evaluated after LPS stimulation. Our experimental results provide further support to the notion. Fortunately, we found that DNLA in combined treatment with LPS was able to reverse these alterations. Specifically, in this study, we found DNLA can effectively reduce the secretion of TNF-α, decrease IL-1β, Nlrp3 mRNA expression, and decrease Iba-1 and iNOS protein expression.
In the central nervous system, TLR4 is widely expressed on the surface of microglia and is involved in the recognition and acceptance of LPS. After binding of LPS to TLR4, microglia are activated to produce inflammatory factors NLRP3 and pro- IL-1β [29,30].Under normal conditions, NF-κB usually forms a complex in the form of homologous dimer or heterodimer with its inhibiting protein IκBα, which exists in the cytoplasm in an inactive form. LPS-TLR4 signaling causes IκBα phosphorylation and ubiquitination, NF-κB p65 subunit translocation to the nucleus, and subsequently binds to NF-κB, activating NF-κB-dependent gene transcription. These processes can ultimately lead to the enhancement of inflammationrelated protein transcription [31]. Our previous study has found that DNLA is effective in protecting against brain damage caused by LPS in rats, and this effect is due to the suppression of NF-κB activity [25]. Therefore, in this study we used immunofluorescence and Western blot assays to determine whether DNLA affect TLR4/NF-κB signaling in vitro. The results showed that TLR4 was activated by LPS, then phosphorylated IκBα increased and phosphorylated NF-κB decreased in cytosol, and phosphorylated NF-κB increased in the nucleus of BV2 microglia. These results are consistent with previous studies [32]. DNLA treatment suppressed the expression of TLR4, the phosphorylation and degradation of IκBα, and nuclear translocation of NF- κB p65. Based on these findings, we found that DNLA can downregulate the activity of the TLR4/NF-κB signaling pathway.
LPS-induced TLR4/NF-κB activation causes an increase in NIMA-related kinase 7 (NEK7) expression. NEK7 is involved in NLRP3 activation process. NLRP3 gets a leucine-rich repeat domain, to which NEK7 is easy to bind [14]. In this study we found DNLA can effectively reduce the NEK high expression induced by LPS. The NLRP3 inflammasome contains NLRP3, ASC, and caspase-1, and mediates Aβ/Tau-triggered microglial inflammatory activation [33,34]. NLRP3 cleaves inactive pro-IL-1β into active IL-1β to release from microglia [35]. The release of proinflammatory cytokines further exacerbate the pathological processes of AD [36]. Evidence suggested that deletion of NLRP3 significantly alleviated neuroinflammation and improves AD-like pathology [37]. Our previous study has reported that DNLA reduced NF- κB phosphorylation and IL-1β production in the cortex and hippocampus of APP/PS1 mice [25].
The results of this study revealed that DNLA downregulated TNF-α production and microglial activation after LPS-treatment. It is also observed that DNLA could block the effects of increased cytokine levels including IL-1β and Nlrp3 mRNA. Also, LPS-induced NLRP3 inflammasome, including NLRP3, caspase-1, and the adaptor protein ASC, were inhibited by the treatment of DNLA. Moreover, the LPS-induced expression of NF-κB p65, IκBα and their phosphorylation levels were decreased by DNLA treatment. In summary, DNLA attenuate LPSinduced neuroinflammation through suppressing NF-κB and NLRP3 signaling pathway (Figure 8).
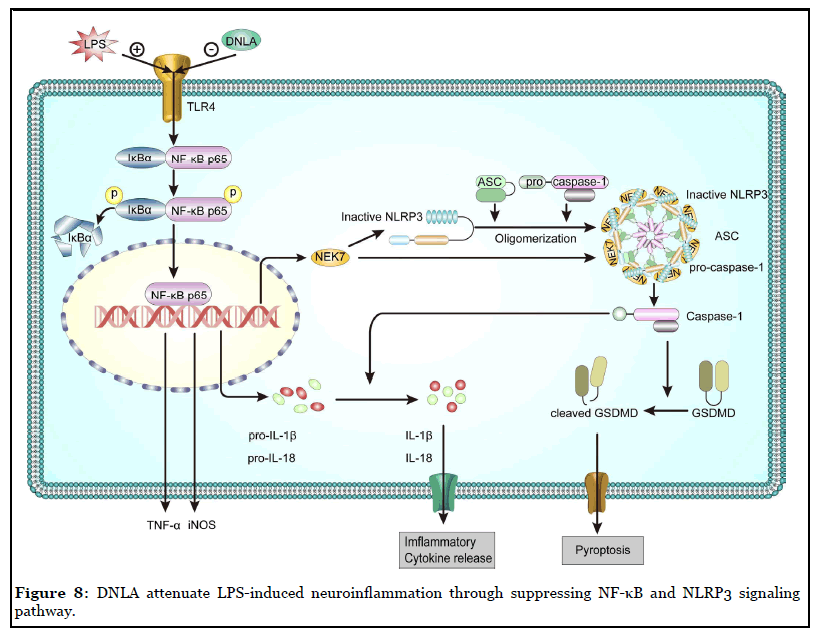
In conclusion, DNLA are beneficial in alleviating LPSinduced neuroinflammation in BV2 microglia, attenuating LPS-induced production of TNF-α and levels of Nlrp3 and IL-1β mRNA, inhibiting LPS-induced morphological changes, and decreasing the protein expression of microglia marker Iba-1, inflammation-related factors iNOS in BV2 cells. These beneficial effects appear to be mediated through the regulation of NF-κB and NLRP3 signaling pathways.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The National Natural Science Foundation of China (No. 81773739, No. 1812403), Guizhou Province First- Class Disciplines Project (Yiliu Xueke Jianshe Xiangmu) [GNYL(2017)006], and Shijingshan’s Tutor Studio of Pharmacology [GZS-536 2016(07)] supported this work.
Author Contributions
Jingshan Shi and Jiaojiao Liu designed the experiments; Jiaojiao Liu and Xi He performed the experiments; Bo Liu and Xi He performed data analysis; Bo Liu and Jing Shan Shi wrote and revised the manuscript. All the authors approved the submitted manuscript.
References
2. Yao YY, Ling EA, Lu D. Microglia mediated neuroinflammation-signaling regulation and therapeutic considerations with special reference to some natural compounds. Histology and Histopathology. 2020 Jul 14:18239.
3. Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microgliamediated neuroinflammation in neurodegenerative diseases. InSeminars in Cell & Developmental Biology 2019 Oct; 94: 112-120.
4. Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nature Neuroscience. 2018 Oct;21(10):1359-69.
5. Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annual Review of Immunology. 2017 Apr 26;35:441-68.
6. Ikram M, Muhammad T, Rehman SU, Khan A, Jo MG, Ali T, Kim MO. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aß mouse model. Molecular Neurobiology. 2019 Sep 15;56(9):6293- 309.
7. Qi Y, Shang L, Liao Z, Su H, Jing H, Wu B, Bi K, Jia Y. Intracerebroventricular injection of resveratrol ameliorated Aß-induced learning and cognitive decline in mice. Metabolic Brain Disease. 2019 Feb 1;34(1):257-66.
8. Chen DB, Gao HW, Peng C, Pei SQ, Dai AR, Yu XT, Zhou P, Wang Y, Cai B. Quinones as preventive agents in Alzheimer’s diseases: focus on NLRP3 inflammasomes. Journal of Pharmacy and Pharmacology. 2020 Jul 15.
9. Sušjan P, Lainšcek D, Strmšek Ž, Hodnik V, Anderluh G, Hafner-Bratkovic I. Selective inhibition of NLRP3 inflammasome by designed peptide originating from ASC. The FASEB Journal. 2020 Aug;34(8):11068-86.
10. He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences. 2016 Dec 1;41(12):1012-21.
11. Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nature Immunology. 2016 Mar;17(3):250-8.
12. Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019 Jun;570(7761):338-43.
13. He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016 Feb;530(7590):354-7.
14. Chen X, Liu G, Yuan Y, Wu G, Wang S, Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death & Disease. 2019 Dec 2;10(12):1-2.
15. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1ß secretion. Cell Research. 2015 Dec;25(12):1285-98.
16. Pandeya A, Li L, Li Z, Wei Y. Gasdermin D (GSDMD) as a new target for the treatment of infection. MedChemComm. 2019;10(5):660-7.
17. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015 Oct;526(7575):660-5.
18. Zhang W, Wu Q, Lu YL, Gong QH, Zhang F, Shi JS. Protective effects of Dendrobium nobile Lindl. alkaloids on amyloid beta (25–35)-induced neuronal injury. Neural Regeneration Research. 2017 Jul;12(7):1131.
19. Li LS, Lu YL, Nie J, Xu YY, Zhang W, Yang WJ, et al. Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Aß25-35 in hippocampus neurons in vitro. CNS Neuroscience & Therapeutics. 2017 Apr;23(4):329-40.
20. Yang S, Gong Q, Wu Q, Li F, Lu Y, Shi J. Alkaloids enriched extract from Dendrobium nobile Lindl. attenuates tau protein hyperphosphorylation and apoptosis induced by lipopolysaccharide in rat brain. Phytomedicine. 2014 Apr 15;21(5):712-6.
21. Nie J, Tian Y, Zhang Y, Lu YL, Li LS, Shi JS. Dendrobium alkaloids prevent Aß25–35-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ. 2016 Dec 13;4:e2739.
22. Huang J, Huang N, Zhang M, Nie J, Xu Y, Wu Q, et al. Dendrobium alkaloids decrease Aß by regulating a-and ß-secretases in hippocampal neurons of SD rats. PeerJ. 2019 Sep 6;7:e7627.
23. Liu B, Huang B, Liu J, Shi JS. Dendrobium nobile Lindl alkaloid and metformin ameliorate cognitive dysfunction in senescence-accelerated mice via suppression of endoplasmic reticulum stress. Brain Research. 2020 May 4:146871.
24. Lv LL, Liu B, Liu J, Li LS, Jin F, Xu YY, et al. Dendrobium nobile Lindl Alkaloids Ameliorate Cognitive Dysfunction in Senescence Accelerated SAMP8 Mice by Decreasing Amyloid-ß Aggregation and Enhancing Autophagy Activity. Journal of Alzheimer’s Disease. 2020.
25. Li Y, Li F, Gong Q, Wu Q, Shi J. Inhibitory effects of Dendrobium alkaloids on memory impairment induced by lipopolysaccharide in rats. Planta Medica. 2011 Jan;77(02):117-21.
26. Li S, Zhou J, Xu S, Li J, Liu J, Lu Y, et al. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomedicine & Pharmacotherapy. 2019 Sep 1;117:109073.
27. Zhou J, Zhang Y, Li S, Zhou Q, Lu Y, Shi J, et al. Dendrobium nobile Lindl. alkaloids-mediated protection against CCl4-induced liver mitochondrial oxidative damage is dependent on the activation of Nrf2 signaling pathway. Biomedicine & Pharmacotherapy. 2020 Sep 1;129:110351.
28. Spangenberg EE, Green KN. Inflammation in Alzheimer’s disease: lessons learned from microgliadepletion models. Brain, Behavior, and Immunity. 2017 Mar 1;61:1-1.
29. Steele JM. Carfilzomib: a new proteasome inhibitor for relapsed or refractory multiple myeloma. Journal of oncology pharmacy practice. 2013 Dec; 19(4):348-54.
30. Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR, McGeough MD. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016 Feb 25;164(5):896-910.
31. Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine. 2007 Nov 1;13(11):460-9.
32. An J, Chen B, Kang X, Zhang R, Guo Y, Zhao J, et al. Neuroprotective effects of natural compounds on LPSinduced inflammatory responses in microglia. American Journal of Translational Research. 2020;12(6):2353.
33. Tejera D, Mercan D, Sanchez-Caro JM, Hanan M, Greenberg D, Soreq H, et al. Systemic inflammation impairs microglial Aß clearance through NLRP 3 inflammasome. The EMBO Journal. 2019 Sep 2;38(17):e101064.
34. Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, Lodder C, Brône B, Huaux F, Octave JN, Terwel D. Aggregated Tau activates NLRP3– ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathologica. 2019 Apr 1;137(4):599-617.
35. Zhang SJ, Xu TT, Li L, Xu YM, Qu ZL, Wang XC, Huang SQ, Luo Y, Luo NC, Lu P, Shi YF. Bushen-Yizhi formula ameliorates cognitive dysfunction through SIRT1/ ER stress pathway in SAMP8 mice. Oncotarget. 2017 Jul 25;8(30):49338-49350.
36. Voet S, Srinivasan S, Lamkanfi M, van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Molecular Medicine. 2019 Jun;11(6):e10248.
37. Abbott A. Is ‘friendly fire’ in the brain provoking Alzheimer’s disease? Nature. 2018 Apr 1;556(7700):426-9.
