Abstract
The purpose of this study is to evaluate the effect of ultraviolet UV photo functionalization on the antimicrobial properties in implants installed in patients with type 2 diabetes mellitus.
The study included 74 patients with diabetes with unilateral/bilateral missing teeth (aged 36 to 63 years). The participants were randomly divided into 2 groups; Group-A included 38 patients with UV photo functionalized 204 implants and Group-B included 36 patients with non UV photo functionalized 183 implants. The clinical parameters of the dental implants were evaluated by assessing PD, BOP, MBL 6 months, 12 months, and 3 years after implant installation, compared with values at last follow-up. Concentrations of interleukin IL-1β and TNF-α cytokines in blood serum were evaluated using enzyme-linked immunosorbent assay.
After implant surgery, the patients in Group-A had minimal postoperative reactions including swelling, discoloration, discomfort, hematomas. After implant surgery, the patients in Group-B had a mild postoperative reaction including swelling and discomfort. Preoperative serum IL-1β levels and TNF-α in Group-A and Group-B patients did not differ significantly.
After implant surgery indicators of cytokines markers show correlation with Group-A and Group-B, the rates of IL-1β levels and TNF-α are higher in patients of Group-B. Analysis of the results showed the greatest increase in the rates of IL-1β levels and TNF-α in peri-mucositis and peri-implantitis.
The survival rate of implants in Group A patients is 97.3% and in Group B patients is 94.2% after five-years. The results of the study showed that monitoring levels of the cytokines TNF-α and IL-1β may aid in the early diagnosis of peri-implantitis and prognosis in high-risk patients with type 2 diabetes mellitus.
Keywords
Type 2 diabetes, Ultraviolet photo functionalization, Surface modification, Implants, Cytokines, IL-1β, TNF-α
Abbreviations
T2DM: Type 2 Diabetes Mellitus; UV: Ultraviolet; CT: Computed Tomography; ISQ: Implant Stability Quotient; RFA: Resonance Frequency Analysis; PPD: Periodontal Probing Depth; BOP: Bleeding On Probing index; MBL: Marginal Bone Loss; IL-1β: InterLeukin-1beta; TNF-α: Tumor Necrosis Factor-alpha.
Introduction
Diabetes mellitus is a serious metabolic disease that often leads to dysfunction of many tissues and organs, including microvascular complications, retinopathy, nephropathy, impaired bone metabolism, delayed wound healing [1-3]. Long-term hyperglycemia characterized by decreased bone formation markers alters the function of osteoblasts, osteocytes including proliferation, migration and adhesion, to altered function [4-6].
Of the oral complications of diabetes mellitus, periodontal disease is reported, resulting in tooth loss, dysfunction of the salivary glands, taste disturbances, the risk of fungal and bacterial infections, damage to the soft tissues of the oral cavity, and impaired healing of oral wounds [7-9]. After the loss of teeth due to periodontitis in patients with diabetes mellitus due to bone resorption, the effectiveness of treatment with removable plate dentures is low and can lead to nutritional disorders and metabolic disorders [10,11].
Currently, with the development of implantology, new opportunities for effective prosthetic rehabilitation are opening up for patients with type 2 diabetes mellitus (T2DM). Dental implant treatment in patients with T2DM presents challenges due to the impaired wound healing and increased risk of infection associated with the condition [12,13]. Hyperglycemia has been reported to impair osseointegration, potentially compromising long-term implant survival [14].
Surface modification techniques, such as ultraviolet (UV) photo functionalization, have shown potential in improving osseointegration and antimicrobial properties of implants. Biomechanical testing of implants has shown that UV treatment accelerates osseointegration of implants [15,16]. Photo functionalized Implant in a high-glucose environment increased the hydrophilicity of the implant surface and thereby leading to increased osteoblast adhesion, cell proliferation, and mineralization [17,18]. There is an in vitro study that reported improved osseointegration of photo functionalized implants in diabetic rats [19]. The role of photo functionalization of dental implants placed in patients with diabetes to improve osseointegration strength is not so much [20-22]. Patients with diabetes are at risk of peri-implantitis [23].
A study by Al-Askar et al. concluded that in patients with diabetes, glycemic status influenced the levels of inflammatory cytokines [24]. The results from the studies by Alqahtani et al., and Monje et al. showed that chronic hyperglycemia is a strong mediator of inflammation [23,25].
It is known that ultraviolet radiation also has an antimicrobial effect through photochemical reactions, affecting the bacteria [26]. UV modifying of Ti surfaces also used in the complex of prevention and treatment of peri-implantitis [16,27-30]. The role of UV photo functionalization in enhancing the antibacterial properties of implants and in the prevention of peri-implantitis has been little studied.
The inflammatory process and excessive synthesis of mediators of inflammation can increase the development dental implant complications [31]. In patients with type 2 diabetes elevated levels of TNF-α, IL-1β, and IL-6 are found [32]. The immune-mediated response activates the rise of cytokines and the secretion of growth factors, including inflammation, proliferation, regeneration, differentiation, and hemostasis [33-35]. IL-1α are vital mediators of immunity, inflammation, tissue destruction, and hemostasis and are important markers of bone turnover [36,37]. TNF-α has similar immunological effects to IL-1β and is an important mediator in the pathogenesis of peri-implant disease. Cytokines and immune cells perform extensive and varied functions in peri-implantitis [37,38].
The balance between pro- and anti-inflammatory cytokines and cells plays a critical role in implant prognosis. Imbalance between pro- and anti-inflammatory cytokines can create conditions for the destruction of soft and bone structures of the peri-implant zone, which weakens osseointegration [39]. TNF- α in conjunction with IL-1β initiates the main mediators of the inflammatory cascade [40,41].
Monitoring inflammatory markers in T2DM blood samples can serve as a valuable test for prognosis of implant treatment [42]. For this reason, the determination of the level of cytokines in patients with dental implants patients with type 2 diabetes is relevant. Based on this, the aim of this study is to monitor cytokine markers IL-1β and TNF-α in UV-photo functionalized and non UV-photo functionalized implants installed in patients with type 2 diabetes mellitus.
Materials and Methods
The study included 74 patients, with diabetes with unilateral/bilateral missing teeth (39 men and 35 women aged 36 to 63 years). The diagnosis of patients was based on clinical, laboratory, and radiographic criteria. Patients were given detailed information about the methods and concepts of treatment with short implants.
Inclusion criteria
Patients with type 2 diabetes, single or multiple posterior missing teeth of the mandible and maxilla upper jaw, availability of space for prosthetics; residual bone height from > 8 mm; horizontal residual bone of at least > 6 mm.
Exclusion criteria
Uncontrolled oral and general disease.
Study design
The participants were randomly divided into 2 groups (Figure 1):
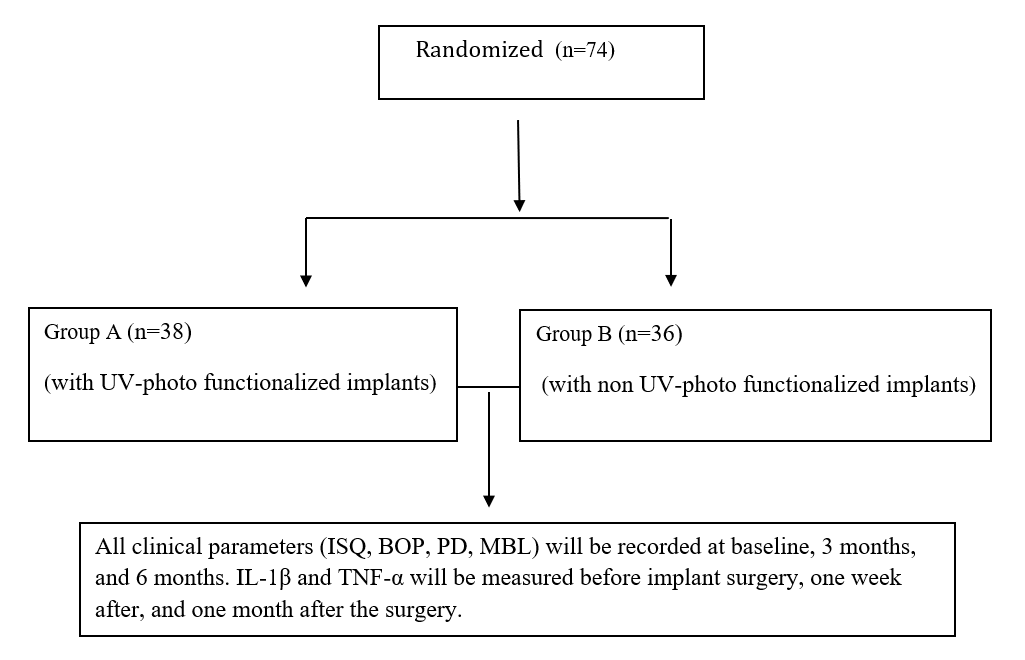
Figure 1. CONSORT FLOW CHART Study participants.
- Group-A included 38 patients with UV-photo functionalized 204 implants.
- Group-B included 36 patients with non-UV-photo functionalized 183 implants.
The patients underwent a thorough clinical examination. The jaw bones were diagnosed using cone beam computed tomography (CT) and were analyzed in three dimensions to obtain the mean residual bone height.
Implant surgery was performed after periodontal therapy. Patients underwent implantation with HbA1c levels <7.2% or less than 154 mg/dL. Postoperative control CT image is taken after implantation.
Primary stability of implants ISQ (Implant Stability Quotient) was assessed uisng the resonance frequency analysis (RFA) method during implant placement, secondary stability after the submerged healing period (after 4-5 months) using Oss tell Mentor device (Oss tell AB, Goteborg, Sweden). Implant stability was assessed based on ISQ values, low implant stability with ISQ <60, medium with ISQ values of 60–70, and high with ISQ values >70.
Patients were advised to strictly follow the postoperative instructions. All patients were prescribed prophylactic antibiotics. Final dental prosthetics were performed after 3-5 months with bridge prosthetics or crowns. The clinical parameters of the dental implant sites were evaluated by assessing probing depth (PD), the bleeding on probing index (BOP), marginal bone level (MBL).
MBL was assessed by X-ray image taken immediately (base line for comparison), 1 year, 3 years, and 5 years after implant installation, and the values were compared at the last follow-up.
Control and postoperative radiographs were compared by analyzing the mesial and distal levels of crestal bone adjacent to the implant, and the mean values per implant were calculated.
Blood serum concentrations of proinflammatory cytokines IL-1β and TNF-α were determined using enzyme-linked immunosorbent assay (ELISA) (Duo set kit; R&D diagnostics Inc, MN, USA) before and after dental implantation according to the manufacturers’ protocols. The serum cytokine levels were compared between both groups.
Statistical analysis
Statistical analyses were performed using SPSS software. Descriptive analysis (Mean ± SD for continuous and frequencies/proportion for categorical variables) were computed for all variables of interest. Differences between groups were evaluated using “chi-square” or “Fisher’s exact” tests for categorical variables and “Wilcoxon signed rank test” for continuous variables. Spearmen correlation was performed for determination of relationships between continuous variables. P-value was considered significant at <0.05 and <0.001 for highly significant results. Analyses were conducted using Excel 2013 and R software.
Results
After implant surgery, the patients in Group-A had minimal postoperative reactions including swelling, discoloration, discomfort, while the patients in Group-b had a mild postoperative reaction including swelling, discoloration, and discomfort.
Clinical radiological results of dental implants in patients have shown satisfactory results and are encouraging. There were no clinical examinations of serious biological or prosthetic complications, and the functional and aesthetic outcome assessed for the patients was good.
A year after the functional load, 7 patients in Group-A and 16 patients in Group-B were found to be having peri-implant mucositis, which was stopped after local conservative therapy. Three years after the functional load, 2 patients in Group-A and 6 patients in Group-B had mild peri-implantitis and 2 moderate were revealed, which was stopped by local conservative therapy or bone graft therapy.
The results of the clinical parameters ISQ are shown in (Table 1), and PD, BOP, MBL are shown in (Table 2). The Survival rate of implants in Group-A patients during the 3 years of functional loading was 96,6%. The survival rate of implants in Group-B patients during the first year of functional loading was 94.8%. The reason for the loss of implants in Group-A patients was peri-implantitis, and in Group-B was peri-implantitis and failed osseointegration.
|
Group |
ISQ |
p-value |
|
|
After implant surgery |
After 4 months of implant surgery |
||
|
Group-A (n=38) |
69.2 ISQ |
73.6 ISQ |
<0.05 |
|
Group-B (n=36) |
68.7 ISQ |
71.3I SQ |
<0.05 |
|
Clinical index |
Time after implant surgery |
p-value |
|||
|
6 months |
12 months |
3 years |
|||
|
Group A |
|||||
|
BOP |
0.93 |
1.16 |
1.37 |
<0.05 |
|
|
PD |
1.21 mm |
1.67 mm |
1.98 mm |
<0.05 |
|
|
MBL |
0.65 mm |
0.87 mm |
1.23 mm |
<0.05 |
|
|
Group B |
|||||
|
BOP |
1.23 |
1.84 |
2.1 |
<0.05 |
|
|
PD |
0.56 mm |
1.67 mm |
2.34 mm |
<0.05 |
|
|
MBL |
0.84 mm |
1.23 mm |
1.46 mm |
<0.05 |
|
Preoperative serum IL-1β levels in Group-A and Group-B patients did not differ significantly. After implantation indicators of cytokines markers show correlation with Group-A and Group-B, the rates of IL-1β and TNF-α levels were higher in Group-B patients (Table 3, Figures 2 and 3). Analysis of the results showed the greatest increase in the levels of anti-inflammatory cytokines in peri-mucositis and peri-implantitis (Table 4, Figure 4).
|
Indicators |
Before implant surgery |
After implant surgery |
After 1 month of implant surgery
|
p-value |
|
Group A |
||||
|
IL-1β |
16.2 pg/mL |
18.3 pg/mL |
21.73 pg/mL |
<0.05 |
|
TNF-α |
4.31 pg/mL |
4.6 pg/mL |
5.1 pg/mL |
<0.05 |
|
Group B |
||||
|
IL-1β |
15.9 pg/mL |
23.2 pg/mL |
25.3 pg/mL |
<0.05 |
|
TNF-α |
4.2 pg/mL |
6.1 pg/mL |
6.5 pg/mL |
<0.05 |
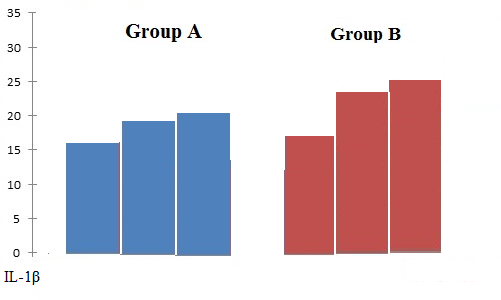
Figure 2. Average mean of IL-1β levels in patients of Group A and Group B.
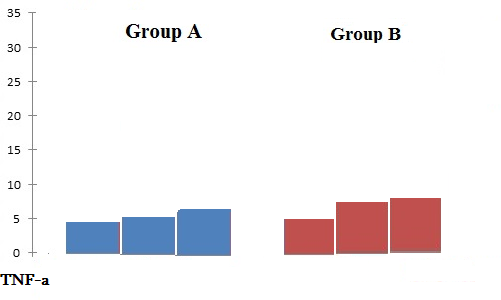
Figure 3. Average mean of TNF-α levels in patients of Group A and Group B.
|
Indicators |
Peri-mucositis |
Peri-implantitis |
p-value |
|
IL-1β |
24.3 pg/mL |
32.4 pg/mL |
<0.05 |
|
TNF-α |
6.8 pg/mL |
11.7 pg/mL |
<0.05 |
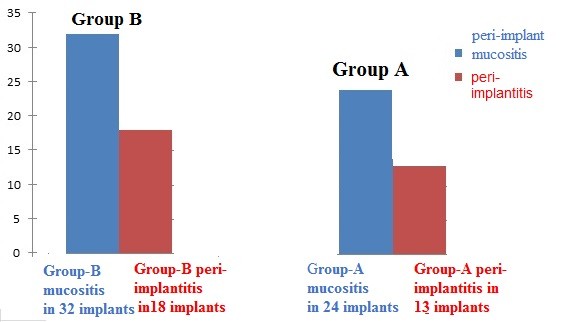
Figure 4. Incidence of peri-implant mucositis and peri-implantitis is higher in Group B compared to Group A after 3 months, 6 months, and 3 years.
Discussion
Peri-implantitis is a serious problem that reduces the lifespan of implants. Over the last decade, scientific literature has reported an increase in the prevalence of chronic peri-implantitis, with it being diagnosed in 16–28% of cases [43-45]. This is exacerbated by the unwarranted expansion of indications for dental implantation, as well as inadequately defined objectives for the comprehensive management of dental implant treatment. It can be suggested that the clinical manifestation of peri-implantitis depends not only on the initial state of immunity, but also on the level of functioning of other adaptive systems of the body. The main components of this system are the cytokine and antioxidant systems that interact in a single structural and functional unit and perform the basic functions of maintaining the constancy of internal homeostasis at a normal level in the body. It is known that disruption of free radical lipid oxidation contributes to the suppression of the function of immunocompetent cells and the synthesis of anti-inflammatory cytokines, which creates conditions for the formation of an inferior immune response and the development of a protracted chronic inflammatory process [33,46,47]. In turn, the lack of cytokines leads to disruption of the enzyme superoxide dismutase, which is one of the main enzymatic antioxidants [48].
Comprehensive studies of the cytokine system in individual patients, along with the identification of specific clinical and laboratory correlations in the development of active or low-grade complications during dental implantation, are particularly important for understanding the mechanisms underlying the inflammatory process in wound tissues after implant placement. Such studies can also aid in the development of new strategies for prevention.
The inflammatory process and excessive synthesis of inflammatory mediators can enhance the development of complications after dental implantation. In patients with early and late peri-implantitis elevated levels of IL-1β cytokines are found compared to the healthy group [49-51].
Studies have shown that UV radiation on titanium implant surfaces enhances the efficiency of osseointegration. However, there is limited research on the antimicrobial effects of UV radiation on implants in patients with type 2 diabetes mellitus (T2DM), particularly with the monitoring of cytokine markers [52,53]. For this reason, determining cytokine levels in such patients is highly relevant in the context of dental implants.
The prevention of peri-mucositis and peri-implantitis focuses on inhibiting biofilm formation by modifying the implant surface to reduce bacterial colonization [51]. Among various surface modifications, UV radiation is considered a promising alternative for enhancing the antibacterial properties of implant surfaces [54]. Many authors in their studies have demonstrated the positive effect of UV radiation on osteointegration and antimicrobial activity [30,55].
To find out the antimicrobial effect of UV-photo functionalized dental implants against oral pathogens, we conducted a study and compared the levels of IL-1β -and TNF-α in the blood serum of patients after the use of photo functionalized and non-photo functionalized dental implants, and implants with peri-mucositis’s and peri-implantitis.
After dental implantation, an increase in IL-1β and TNF-α levels was observed, which plays a crucial role in the rapid tissue response to the introduction of foreign implants or surgical trauma. An increased concentration of cytokine markers in patients with T2DM with early postoperative complications may reflect the activity of the inflammatory process, which indicates that a systemic inflammatory response develops against the background of activation of the local inflammatory process.
The study analyzed the treatment results of 74 patients with diabetes who had unilateral or bilateral missing teeth and underwent prosthetic rehabilitation with dental implants. Patients were randomly assigned to two groups. Group A included patients who received UV-photo-functionalized implants, while Group B included patients who received non-UV-photo-functionalized implants. Serum concentrations of IL-1β and TNF-α were measured before and after dental implantation, and cytokine levels were compared between the two groups. Analysis of the clinical indexes and cytokine markers showed a correlation with the groups. In particular, the levels were higher in patients in Group B. The results of the study showed that implant failure occurred mainly after the 1st stage of surgery and after 4-5 years of functional load it is associated with peri-implantitis.
The results of this study showed that levels of IL-1β and TNF-α are correlated with peri-implantitis and can be used as markers for the diagnosis of peri-implant mucositis and peri-implantitis. UV photo-functionalization is an effective tool for preventing peri-implantitis and biofilm formation in patients after dental implantation. Monitoring levels of the cytokines IL-1β and TNF-α may aid in the early diagnosis of peri-implantitis and prognosis in high-risk patients with T2DM.
However, to obtain more convincing evidence of the effectiveness of the cytokine markers IL-1β and TNF-α, a larger sample size and a longer follow-up period are required. Further studies may also include additional cytokine markers.
Conclusion
Modification of the implant surface using UV photo functionalization has an antimicrobial effect and is one of the measures to prevent peri-implantitis in patients with T2DM. IL-1β and TNF-α assays are non-invasive means of studying the host response to peri-implant disease and may be a marker for early detection of peri-implantitis.
Acknowledgements
None.
Competing Interest
The authors declare that they have no competing Interests. None of the authors have any relevant financial relationships with commercial interests.
Funding
This work was not funded.
Ethical Approval and Consent to Participate
The study was reviewed and approved by the University Ethics Committee and was conducted in accordance with the principles of the World Medical Association and the Helsinki Declaration.
Consent for Publication
Patients were informed about the study both verbally and in writing and provided written informed consent.
Availability of Data and Materials
All data generated or analyzed during this study are included in this article.
Author’s Contributions
HKh: Conceptualization, Methodology, Investigation, Validation, Funding acquisition, Writing − original draft, Writing – review & editing. LY,GH: Review & editing.
References
2. Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015 Sep;21 Suppl 2:S27-32.
3. Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008 Nov;88(11):1322-35.
4. Wongdee K, Charoenphandhu N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J Diabetes. 2011 Mar 15;2(3):41-8.
5. Picke AK, Campbell G, Napoli N, Hofbauer LC, Rauner M. Update on the impact of type 2 diabetes mellitus on bone metabolism and material properties. Endocr Connect. 2019 Mar 1;8(3):R55-R70.
6. Shahen VA, Gerbaix M, Koeppenkastrop S, Lim SF, McFarlane KE, Nguyen ANL, et al. Multifactorial effects of hyperglycemia, hyperinsulinemia and inflammation on bone remodeling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2020 Oct;55:109-18.
7. Khachatryan H. Oral Manifestations in Patients with Diabetes Mellitus. Review literature. Bulletin of Stomatology and Maxillofacial Surgery. 2024;20(2):86-97.
8. Al-Maskari AY, Al-Maskari MY, Al-Sudairy S. Oral Manifestations and Complications of Diabetes Mellitus: A review. Sultan Qaboos Univ Med J. 2011 May;11(2):179-86.
9. Rohani B. Oral manifestations in patients with diabetes mellitus. World J Diabetes. 2019 Sep 15;10(9):485-9.
10. Yang S, Li Y, Liu C, Wu Y, Wan Z, Shen D. Pathogenesis and treatment of wound healing in patients with diabetes after tooth extraction. Front Endocrinol (Lausanne). 2022 Sep 23;13:949535.
11. Katyayan PA, Katyayan M, Shah RJ. Rehabilitative considerations for dental implants in the diabetic patient. J Indian Prosthodont Soc. 2013 Sep;13(3):175-83.
12. Wagner J, Spille JH, Wiltfang J, Naujokat H. Systematic review on diabetes mellitus and dental implants: an update. Int J Implant Dent. 2022 Jan 3;8(1):1.
13. Dubey RK, Gupta DK, Singh AK. Dental implant survival in diabetic patients; review and recommendations. Natl J Maxillofac Surg. 2013 Jul;4(2):142-50.
14. Yamazaki S, Masaki C, Nodai T, Tsuka S, Tamura A, Mukaibo T, et al. The effects of hyperglycaemia on peri-implant tissues after osseointegration. J Prosthodont Res. 2020 Apr;64(2):217-23.
15. Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, et al. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014 Jul;10(7):2907-18.
16. Nakhaei K, Ishijima M, Ikeda T, Ghassemi A, Saruta J, Ogawa T. Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials (Basel). 2020 Dec 31;14(1):151.
17. Kheur S, Kheur M, Madiwal V, Sandhu R, Lakha T, Rajwade J, et al. In-Vitro Evaluation of Photofunctionalized Implant Surfaces in a High-Glucose Microenvironment Simulating Diabetics. J Funct Biomater. 2023 Feb 26;14(3):130.
18. Pacicca DM, Brown T, Watkins D, Kover K, Yan Y, Prideaux M, et al. Elevated glucose acts directly on osteocytes to increase sclerostin expression in diabetes. Sci Rep. 2019 Nov 22;9(1):17353.
19. Moraschini V, Barboza ES, Peixoto GA. The impact of diabetes on dental implant failure: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016 Oct;45(10):1237-45.
20. Sugita Y, Honda Y, Kato I, Kubo K, Maeda H, Ogawa T. Role of photofunctionalization in mitigating impaired osseointegration associated with type 2 diabetes in rats. Int J Oral Maxillofac Implants. 2014 Nov-Dec;29(6):1293-300.
21. Al Ansari Y, Shahwan H, Chrcanovic BR. Diabetes Mellitus and Dental Implants: A Systematic Review and Meta-Analysis. Materials (Basel). 2022 Apr 29;15(9):3227.
22. Khachatryan H, Aghajanova E, Gagik Hakobyan D Use of Dental Implants in Diabetic Patients. Review of Literature. JSM Diabetol Manag. 2024;6(1):1008.
23. Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycemia and peri-implant diseases: Systematic review and meta-analysis. J Clin Periodontol. 2017 Jun;44(6):636-48.
24. Al-Askar M, Ajlan S, Alomar N, Al-Daghri N.M. Clinical and Radiographic Peri-Implant Parameters and Whole Salivary Interleukin-1β and Interleukin-6 Levels among Type-2 Diabetic and Nondiabetic Patients with and without Peri-Implantitis. Med. Princ. Pract. 2018;27:133-8.
25. Alqahtani F, Alqhtani N, Alkhtani F, Divakar D.D, Al-Kheraif A.A, Javed F. Clinicoradiographic markers of peri-implantitis in cigarette-smokers and never-smokers with type 2 diabetes mellitus at 7-years follow-up. J. Periodontol. 2020;91:1132-8.
26. Areid N, Söderling E, Tanner J, Kangasniemi I, Närhi TO. Early Biofilm Formation on UV Light Activated Nanoporous TiO2 Surfaces In Vivo. Int J Biomater. 2018 Nov 22;2018:7275617.
27. Flanagan D. Photofunctionalization of Dental Implants. J Oral Implantol. 2016 Oct;42(5):445-50.
28. de Avila ED, Lima BP, Sekiya T, Torii Y, Ogawa T, Shi W, et al. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials. 2015 Oct;67:84-92.
29. Abdullatif FA, Al-Askar M. Does Ultraviolet Radiation Exhibit Antimicrobial Effect against Oral Pathogens Attached on Various Dental Implant Surfaces? A Systematic Review. Dent J (Basel). 2022 May 31;10(6):93.
30. Ishii K, Matsuo M, Hoshi N, Takahashi SS, Kawamata R, Kimoto K. Effect of ultraviolet irradiation of the implant surface on progression of periimplantitis—a pilot study in dogs. Implant Dent. 2016;25:47-53.
31. Sbricoli L, Bazzi E, Stellini E, Bacci C. Systemic Diseases and Biological Dental Implant Complications: A Narrative Review. Dent J (Basel). 2022 Dec 29;11(1):10.
32. Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012 Jan;57(1):136-42.
33. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007 Spring;45(2):27-37.
34. Van Dyke TE, Kornman KS. Inflammation and factors that may regulate inflammatory response. J Periodontol. 2008 Aug;79(8 Suppl):1503-7.
35. Ott LW, Resing KA, Sizemore AW, Heyen JW, Cocklin RR, Pedrick NM, et al. Tumor Necrosis Factor-alpha- and interleukin-1-induced cellular responses: coupling proteomic and genomic information. J Proteome Res. 2007 Jun;6(6):2176-85.
36. Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003 Mar;74(3):391-401.
37. Jamshidy L, Tadakamadla SK, Choubsaz P, Sadeghi M, Tadakamadla J. Association of IL-10 and TNF-α Polymorphisms with Dental Peri-Implant Disease Risk: A Meta-Analysis, Meta-Regression, and Trial Sequential Analysis. Int J Environ Res Public Health. 2021 Jul 20;18(14):7697.
38. Jin Q, Teng F, Cheng Z. Association between common polymorphisms in IL-1 and TNFα and risk of peri-implant disease: A meta-analysis. PLoS One. 2021 Oct 5;16(10):e0258138.
39. Baseri M, Radmand F, Hamedi R, Yousefi M, Kafil HS. Immunological Aspects of Dental Implant Rejection. Biomed Res Int. 2020 Dec 9;2020:7279509.
40. Kligman S, Ren Z, Chung CH, Perillo MA, Chang YC, Koo H, et al. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J Clin Med. 2021 Apr 12;10(8):1641.
41. Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009 Apr;60(1):57-64.
42. Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011 Aug;22(4):189-95.
43. Acharya AB, Thakur S, Muddapur MV, Kulkarni RD. Cytokine ratios in chronic periodontitis and type 2 diabetes mellitus. Diabetes Metab Syndr. 2017 Oct-Dec;11(4):277-8.
44. Astolfi V, Ríos-Carrasco B, Gil-Mur FJ, Ríos-Santos JV, Bullón B, Herrero-Climent M, et al. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int J Environ Res Public Health. 2022 Mar 31;19(7):4147.
45. Schwartzenberg AV, Liu CC, Sahrmann P, Schmidlin PR, Jung RE, Naenni N. Risk Characteristics of Peri-Implant Infections: A Retrospective Evaluation in a University Consultation Setting. Dent J (Basel). 2022 Aug 29;10(9):159.
46. Roccuzzo A, Stähli A, Monje A, Sculean A, Salvi GE. Peri-Implantitis: A Clinical Update on Prevalence and Surgical Treatment Outcomes. J Clin Med. 2021 Mar 6;10(5):1107.
47. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014 Oct 7;5:491.
48. Chen X, Andresen1 BT, Hill M, Zhang J, Booth F, Zhang C. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Curr Hypertens Rev. 2008 Nov;4(4):245-55.
49. Faot F, Nascimento GG, Bielemann AM, Campão TD, Leite FR, Quirynen M. Can Peri-Implant Crevicular Fluid Assist in the Diagnosis of Peri-Implantitis? A Systematic Review and Meta-Analysis. J Periodontol. 2015;631-45.
50. Khatri M, Bansal M, Puri K, Mehrotra S, Kumar A, Rehan M. Evaluation of the correlation between interleukin 1β levels in peri-implant crevicular fluid as an adjunctive diagnostic marker with clinical and radiographic parameters for assessing the peri-implant health status. Natl J Maxillofac Surg. 2022 Sep-Dec;13(3):421-9.
51. Kajale AM, Mehta DS. Interleukin-1β level in peri-implant crevicular fluid and its correlation with the clinical and radiographic parameters. J Indian Soc Periodontol. 2014 Mar;18(2):220-5.
52. Kao RT, Curtis DA, Richards DW, Preble J. Increased interleukin-1 beta in the crevicular fluid of diseased implants. Int J Oral Maxillofac Implants. 1995 Nov-Dec;10(6):696-701.
53. Panagakos FS, Aboyousef H, Dondero R, Jandinski JJ. Detection and measurement of inflammatory cytokines in implant crevicular fluid: A pilot study. Int J Oral Maxillofac Implants. 1996;11:794-9.
54. Hirota M, Ozawa T, Iwai T, Mitsudo K, Ogawa T. UV-Mediated Photofunctionalization of Dental Implant: A Seven-Year Results of a Prospective Study. J Clin Med. 2020;9:2733.
55. Ishijima M, De Avila E.D, Nakhaei K, Shi W, Lux R, Ogawa T. Ultraviolet Light Treatment of Titanium Suppresses Human Oral Bacterial Attachment and Biofilm Formation: A Short-Term In Vitro Study. Int J Oral Maxillofac Implant. 2019;34:1105-13.
