Abstract
Aim: The aim of the study was to assess the stability after early loading of nano coated hydroxyapatite implants in posterior maxilla.
Methodology: This study was conducted on nine patients with at least a missing one maxillary posterior tooth. Ten Nano-coated hydroxyapatite implants (ETIII NH implant by Hiossen) were inserted in nine patients, and then subjected to early loading according to the secondary stability readings taken by Osstell®. Implant stability was measured at the time of implant insertion (T0), 4 (T1), 6 (T1 modified), weeks, and 4 months (T2) post-operative. Cone-beam computed tomography (CBCT) was performed in all patients before starting the treatment. Nine implants healed well except for one implant that failed due to infection.
Results: Secondary stability results after 6 weeks of implant incretion were sufficient enough for implant loading, there was significant difference (F= 12.642, DF 3, P value <0.001) among T0, T1, T1 modified, and T2.
Conclusion: Nano-coated hydroxyapatite implants are of good choice in posterior maxilla and they can be early loaded.
Keywords
Dental Implants, Implant stability, Implants surface treatment, Implants surface coating
Introduction
Implant stability is a measurement of the clinical immobility of implants and an indicator of osseointegration. An implant's stability in the surrounding bone is essential to allow for continued healing and bone formation after insertion and to allow for the best possible stress distribution from masticatory and occlusal functional stresses through the implant tissue interface. While secondary stability is needed following osseointegration, which occurs throughout function, primary stability is needed at the time of implant placement. These prerequisites for healing, function, and stability differ slightly from one another [1].
A popular biomedical tool for assessing implant stability is Resonance Frequency Analysis. It is a non-invasive analytical technique that evaluates the stability of the implant and the density of the bone at different stages using vibration and the structural analysis principle. Implant Stability Quotient, or ISQ, is a unit used to measure implant stability. Its value reflects the implant stability in the bone regarding primary and secondary stability [2].
Numerous studies have been conducted on coatings made of calcium phosphate (CaP), such as Hydroxyapatite (HAP). Nanoscale alteration of implant surfaces was proven by studies to enhance the biomimicry of dental implants due to the interaction of extracellular matrix proteins, growth factors, and many osteogenic potential cells at this scale. It is interesting to note that the possibility of improving osseointegration has been studied by applying nanostructured CaP to implant surfaces. It has been noted that electro-polished surfaces with nanometer-sized HAP coatings can increase bone-to-implant contact by about 300% compared to surfaces without coatings [3].
To date only a few clinical studies have attempted to test the early loading of nano-coated hydroxyapatite implants in posterior maxilla and their effect on marginal bone loss. Therefore, the objective of this study is to demonstrate the ability of nano-coated hydroxyapatite implants to achieve rapid osteointegration in posterior maxilla and qualify for early loading and achieve minimal marginal bone loss.
Aim of Study
The aim of the study was to assess the stability after early loading of nano coated hydroxyapatite implants in posterior maxilla.
Materials and Methods
This study was conducted on a total of nine patients who had at least one missing maxillary posterior tooth seeking restoration. A total of ten implants were inserted.
Inclusion criteria
- Patients with missing upper posterior teeth and seeking implant placement.
- Patients who have adequate bone height and width that allows implant placement.
- Patients free from any condition that may compromise the final outcome of the dental implantation procedure (ex: bruxism, previous radiation).
Exclusion criteria
- Patients with systemic diseases that may hinder the normal healing process, for example Diabetes mellitus and peripheral vascular disease.
- Patients with intra-bony lesions or infections that may retard the healing.
Surgical approach
- Infiltration local anesthesia (Articaine HCL 4% with epinephrine 1/100000)[1] was injected intraorally on the site of implant placement and a paracrestal incision was made at the edentulous area extending one tooth mesial and distal to the edentulous area to create a full thickness mucoperiosteal flap.
- Sequential drilling under copious irrigation was done till the desired implant size was achieved.
- All implants used for this study were bone level ETIII NH implant with open thread and tapered body implant by Hiossen which are made of Ti6Al4V medical grade titanium alloy and coated with bioresorbable apatite, coating is 10 nanometers thick. Thickness of coating layer 5,000 : 1[2].
- The implant was placed according to the manufacturer’s instructions.
- Primary implant stability was measured immediately post-operatively (T0) using the Osstell ISQ system[3], one reading was taken for each of the four aspects of the implant.
- Implant Stability Measurement:
The implant stability was measured at the time of insertion using the Resonance Frequency Analysis via the Osstell ISQ system.
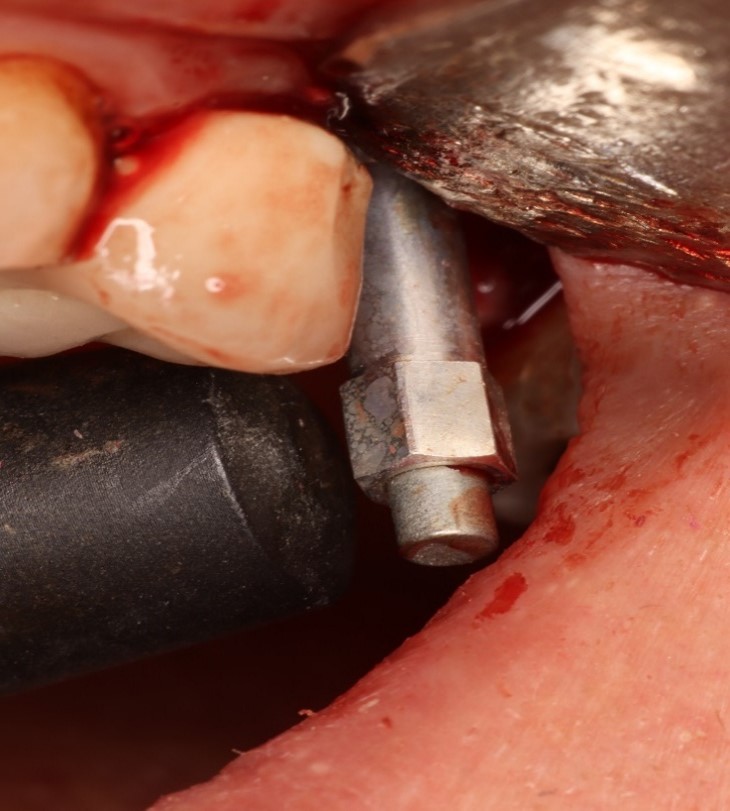
The system includes the use of a SmartPeg™ attached to the dental implant or abutment by means of an integrated screw.
The SmartPeg is excited by a magnetic pulse from the measurement probe on the handheld instrument (Figure 1).
The resonance frequency, which is the measure of implant stability, is calculated from the response signal and results are displayed on the device as the ISQ.
The SmartPeg probe was held close to the SmartPeg to measure the ISQ value from the buccal, palatal, mesial and distal aspects, 4 readings were taken from each aspect, then the mean value for each aspect was calculated. The final readings for the buccal, palatal, mesial, and distal aspects were added to each other, then the mean value for the whole implant was calculated.
Figure 1. SmartPeg and measurement probe.
- Closure of the mucoperiosteal flap with absorbable suture[4].
Post-operative care
- Post-operative medications:
Patients were prescribed:
- Antibiotics: Amoxicillin 875 mg and Clavulonic acid[5] 125mg tablets every 12 hours for 5 days post-surgically.
- Analgesic: Diclofenac Potassium[6] 50 mg analgesic tablets three times daily for 3-5 days post-surgically.
- Mouth rinsing with Chlorhexidine[7] 3 times daily starting one day postoperatively.
Follow up and evaluation
The first visit was after 1 week for suture removal and healing assessment.
Implant exposure was done after 4 weeks through a paracrestal incision at the implant site, then a healing abutment was put after stability measurements were taken until the delivery of the final restoration.
Implant exposure was done after 4 weeks through a paracrestal incision at the implant site, then a healing abutment was put after stability measurements were taken until the delivery of the final restoration.
Assessment of implant stability
- Secondary stability was measured 4 weeks (T1) which was the proposed time for loading according to Alabed Mela et al. [8], 6 weeks (T1 modified) and 4 month (T2) post-operatively.
- Final restoration was constructed after 6 weeks post-operatively.
- Final restoration was done after confirmation of adequate secondary stability for implant loading according to the evidence-based Osstell ISQ scale.
- Patients’ satisfaction survey was done throughout the treatment phases to assess the overall satisfaction from the procedure.
[1] Art Pharma® Iodine and Potassium Iodide quality products
[2] Hiossen® ,OSSTEM IMPLANT, CO., LTD
[3] Osstell® ,integration Diagnostic AB, Goteborg,Sweden
[4] Isuture Isorb: AlDawlia ICO, Asyut, Egypt.
[5] Augmentin 1gm. tablets, Smithkline Beecham Pharmaceuticals Co., Brentford, England
[6] Cataflam 50mg. tablets, Novartis Pharma AG, Basle, Switzerland
[7] Oraldene; Chlorhexidine hydrochloride 125mg in each 100 ml solution. EDCO, Egypt
Results
In this study a total of 10 implants were inserted in 9 patients who had at least one missing maxillary posterior tooth and were selected from the Out Patient Clinic of Oral and Maxillofacial Surgery Department, Faculty of Oral and Dental Medicine, Cairo University.
The selected patients were 7 females and 3 males. Their age ranged from 22-57 years with a mean of 38.3 years (7 ± 5). The total implants placed were 10 implants.
Assessment of implant stability
Implant stability was measured at time of insertion (T0), 4 (T1), 6 (T1 modified), weeks, and 4 months (T2) post-operative as shown in (Tables 1 and 2), between 4 and 6 weeks a healing abutment was put.
|
Descriptive Statistics |
|||||
|
|
N |
Minimum |
Maximum |
Mean |
Std. Deviation |
|
T0 (at insertion) |
9 |
51 |
77 |
66.40 |
8.31 |
|
T1 (4weeks) |
9 |
24 |
61 |
41.72 |
18.95 |
|
T1 (modified - at 6 weeks) |
9 |
50 |
72 |
62.89 |
6.35 |
|
T2 (4 month after insertion) |
9 |
55 |
81 |
68.56 |
7.69 |
|
|
Mean |
Std. Deviation |
Std. Error Mean |
Lower |
Upper |
T |
P value |
|
|
Pair 1 |
T0 (at insertion) - T1 (4weeks) |
24.944 |
12.411 |
4.137 |
11.063 |
38.825 |
6.030 |
.000 |
|
Pair 2 |
T0 (at insertion) - T1 (modified - at 6 weeks) |
3.778 |
11.054 |
3.685 |
-8.586 |
16.141 |
1.025 |
.335 |
|
Pair 3 |
T0 (at insertion) - T2 (4 month after insertion) |
-1.889 |
10.706 |
3.569 |
-13.863 |
10.085 |
-.529 |
.611 |
|
Pair 4 |
T1 (4weeks) - T1 (modified - at 6 weeks) |
-21.167 |
21.665 |
7.222 |
-45.398 |
3.065 |
-2.931 |
.019 |
|
Pair 5 |
T1 (4weeks) - T2 (4 month after insertion) |
-26.833 |
20.955 |
6.985 |
-50.271 |
-3.396 |
-3.842 |
.005 |
|
Pair 6 |
T1 (modified - at 6 weeks) - T2 (4 month after insertion) |
-5.667 |
3.279 |
1.093 |
-9.334 |
-2.000 |
-5.185 |
.001 |
The decision to measure again after 6 weeks was made as the implant secondary stability results on 4 weeks did not qualify it for early loading, so the decision to wait for 2 more weeks was made, measurements were taken again after 6 weeks post-operatively.
One implant failed after 4 weeks due to infection and was excluded from the statistical analysis.
Shapiro-Wilk test was done to detect data normality, and because the distribution of data turned out to be normal and the data were paired in more than 2 groups. Repeated Measures ANOVA test was used to test the differences in implant stability score means among T0, T1, T1 modified, and T2. There was significant difference (F= 12.642, DF 3, P value <0.001), then Paired T-test was applied among the 6 pairs to assign difference among pairs at significance 0.008, there was significant difference among Pair 1 which was between T0 (at insertion) - T1 (4weeks), Pair 4 which was between T1 (4weeks) - T1 (modified - at 6 weeks), Pair 5 which was between T1 (4weeks) - T2 (4 month after insertion) , and Pair 6 which was between T1 (modified - at 6 weeks) - T2 (4 month after insertion) , and the P value was <0.001, 0.019, 0.005, and 0.001 respectively. (Tables 2 and 3).
|
Source |
Type III Sum of Squares |
df |
Mean Square |
F |
Sig. |
|
|
Implant_stability |
Sphericity Assumed |
4140.521 |
3 |
1380.174 |
12.642 |
.000 |
Patient satisfaction
Patients’ satisfaction survey was done for the process, the results of overall patient’s satisfaction showed that 91% of patients were very satisfied and 63% of patients were satisfied. Figure 2 shows the statistical data regarding patients’ satisfaction rate.
Figure 2. Bar chart showing patients’ satisfaction rates.
Discussion
Early loading was done 6 weeks after measurement of secondary stability and finding that the ISQ value is suitable for implant loading according to the evidence-based Osstell ISQ scale. Implant loading at six weeks was formerly regarded as an early loading according to Krawiec et al. [4]. The third and fourth weeks appear to be crucial for the process of secondary stability and osteointegration, though. The primary osseous tissue begins to mineralize during that time, and as a result, the bone tissue that surrounds an implant develops the mechanical properties that permit loading [4].
Hiossen ET III implants with HAP nano-coating and hydrophilic surface was used in this study, the advantages of HAP nano-coating and surface hydrophilicity was discussed by Tallarico et al. [5]. In their study which concluded that implants with the new hydrophilic Nano-Hydroyapatite (NH) surface of the Hiossen ET III implants appear to avoid the ISQ drop during the remodeling phase, allowing for benefits in immediate loading, poor bone quality, post extractive, smoking, and immunosuppression disease [5].
The hydrophilic surface of Hiossen ET III implant have greater surface energies, which accelerates the transition from the mechanical primary bone stability to the structural/biological secondary bone stability. This was supported by Velloso et al. which in their study reported increasing ISQ values during the follow-up period of hydrophilic SAE implant surface for 12 months after implant installation and suggested that hydrophilicity of the implant surface optimized the healing process and bone repair. It also stimulates cellular responses, enhances gene expression, osteoblastic stimulation, helps in early osseointegration, and bone mineralization [6].
The results of our study in the measurement of implant stability were similar to the results of a clinical trial by Körmöczi et al. [7] who compared the primary stability and secondary stability of NH (bioabsorbable apatite nanocoating) and SLA (large-grit sandblasted and acid-etched) surface implants, SA (alumina sandblasted and acid-etched), and SA (alumina sandblasted and acid-etched) surface implants after 6 weeks. The results showed that all three groups could be used safely in case of early loading protocol after 6 weeks. In the SLA group, the increase in implant stability over the course of six weeks was the lowest, while in the NH group, it was the highest [7].
The results of Alabed Mela et al. [8] clinical study also coincided with the results of our study, they evaluated the outcomes of 12 early-loaded implants in the posterior maxilla after 1 month of loading and found that the nanohydroxyapatite coating had a positive impact. They also found that the success rate achieved was 100% after 1 year post-loading and that the results had been encouraging in terms of stronger and more favorable bone regeneration, better Osseointegration, higher/better quality bone production, and improved secondary implant stability of implants, which coincides with our results [8].
Tallarico et al. [9] revealed similar results to our study from their Split-Mouth, Randomized Controlled Trial on 29 patients (mean age 59.9 ± 11.3 years) who were treated and followed up to 1 year after loading. Fifty-eight implants were placed, 29 of which were Hiossen ET III implants with the sandblasted and acid-etched (SA) surface and 29 of which were Hiossen ET III implants with the new hydrophilic (NH) surface. The researchers concluded that NH implants are a viable alternative to SA surfaces because they appear to avoid the ISQ drop during the implant remodeling phase. This allows it to be used in immediate loading and early loading protocols, individuals with poor bone quality, smokers, and cases of immunosuppression [9].
The results of a prospective case series study on early loading of titanium dental implants with a hydrophilic implant surface by Hicklin et al. confirmed that early functional loading of such implants is applicable, as the hydrophilic implant surfaces had increased wettability which enhanced the process of osseointegration, which goes in line with our results, however the loading in that study was done after 21 days in the posterior mandible of partially edentulous patients [10].
Lang et al. also studied the early healing and osseointegration of the hydrophilic SLActive implants and found that when compared to the hydrophobic SLA surface, the degree of osseointegration for the hydrophilic SLActive was greater after 4 weeks, which proves that surface hydrophilicity of the implant can positively affect the process of osseointegration, which agrees with the results of our study [11].
Conclusion
Within the context of this study, the following conclusions can be listed:
1. The measurement of the implant stability using a resonance frequency analysis is a good and easy method to predict the implant osteointegration.
2. Nano-coated hydroxyapatite implants are of good choice in posterior maxilla, and our preliminary results supports success of early loading at 6 weeks.
Summary box
What is known on the topic:
1- Implant’s primary and secondary stability affects implants loading protocol
2- Type of bone affects the implant stability and loading protocol
3- Implant’s surface coating and modification helps in improving implant stability
What the submitted study adds:
1- Nano- coating of implants with hydroxyapatite improves implant 2ry stability and allows for early loading of the implants in posterior maxilla.
2- Nano coated hydroxyapatite implants with hydrophilic surface improves osseointegration with the surrounding bone and allows for early loading after 6 weeks in posterior maxilla.
List of Abbreviations
ISQ: Implant Stability Quotient; HAP: Hydroxyapatite; Ti6Al4V: Alpha-beta titanium alloy with a high specific strength and excellent corrosion resistance; CaP: Calcium Phosphate; CBCT: Cone Beam Computed Tomography; NH: Nano-Hydroxyapatite; SA: Alumina Sandblasted and Acid-etched; SLA: Large Grit Sandblasted and Acid-etched
Conflict of Interest Statement
No conflict of interest.
Author Contribution Statement
Conception and design of study/review/case series – OAA, NNE; Acquisition of data: laboratory or clinical/literature search – OAA, IMC, HMK, NNE; Analysis and interpretation of data collected – OAA; Drafting of article and/or critical revision – OAA, IMC, HMK, NNE; Final approval and guarantor of manuscript – OAA, IMC, HMK, NNE.
References
2. Kittur N, Oak R, Dekate D, Jadhav S, Dhatrak P. Dental implant stability and its measurements to improve osseointegration at the bone-implant interface: A review. Mater Today Proc. 2021;43:1064-70. doi:10.1016/J.MATPR.2020.08.243
3. Bryington MS, Hayashi M, Kozai Y, Vandeweghe S, Andersson M, Wennerberg A, et al. The influence of nano hydroxyapatite coating on osseointegration after extended healing periods. Dental Materials. 2013;29(5):514-20. doi:10.1016/j.dental.2013.02.004
4. Krawiec M, Hadzik J, Dominiak M, Grzebieluch W, Błaszczyszyn A, Kubasiewicz-Ross P. Early loading of titanium dental implants with hydroxyl ion modified surface: a 12-month prospective clinical trial. Applied Sciences (Switzerland). 2021;11(7):2958.
5. Tallarico M, Baldini N, Martinolli M, Xhanari E, Kim YJ, Cervino G, et al. Do the New Hydrophilic Surface Have Any Influence on Early Success Rate and Implant Stability during Osseointegration Period? Four-Month Preliminary Results from a Split-Mouth, Randomized Controlled Trial. Eur J Dent. 2019;13(1):95-101.
6. Velloso G, Moraschini V, dos Santos Porto Barboza E. Hydrophilic modification of sandblasted and acid-etched implants improves stability during early healing: a human double-blind randomized controlled trial. Int J Oral Maxillofac Surg. 2019;48(5):684-90.
7. Körmöczi K, Komlós G, Papócsi P, Horváth F, Joób-Fancsaly Á. The early loading of different surface-modified implants: a randomized clinical trial. BMC Oral Health. 2021;21(1):207.
8. Alabed Mela O, Abdel-Monem Tawfik M, Mohamed Ahmed Said Ahmed W, El Din Hassan Abdel-Rahman F. EFFICACY OF NANO-HYDROXYAPATITE COATING ON OSSEOINTEGRATION OF EARLY LOADED DENTAL IMPLANTS. Int J Adv Res (Indore). 2022;10(01):564-75.
9. Tallarico M, Baldini N, Gatti F, Martinolli M, Xhanari E, Meloni SM, et al. Role of New Hydrophilic Surfaces on Early Success Rate and Implant Stability: 1-Year Post-loading Results of a Multicenter, Split-Mouth, Randomized Controlled Trial. Eur J Dent. 2021;15(1):1-7.
10. Hicklin S, Janner S, Schnider N, Chappuis V, Buser D, Brägger U. Early Loading of Titanium Dental Implants with an Intraoperatively Conditioned Hydrophilic Implant Surface: 3-Year Results of a Prospective Case Series Study. Int J Oral Maxillofac Implants. 2020;35(5):1013-20.
11. Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011;22(4):349-56.