Introduction
This document is a commentary on the review entitled "Nanotechnology in the development of cardiac stents" [1] focusing on clinical applications of stents.
Summary of History of Stent Development
Early diagnosis and treatment of atherosclerosis are of vital importance in cardiology. Because of high risk and complexity of open-heart surgery, nowadays balloon angioplasty and stent implantation are common techniques to extend arterial vessels narrowed by atherosclerosis. Serious drawbacks of previous stents, such as complications induced by delayed healing and local hypersensitivity reactions and so re-narrowing and vascular reocclusion, have led to the development of stent designs, stent delivery systems, ultrasonic guidance of stent situation, and high-pressure dilatation post-stenting modifications [2-5].
Bare metal stents (BMSs) have been designed based on inert metals, mostly cobalt chromium or stainless steel [6]. In order to avoid defections in BMSs, drug-eluting stents (DES), as the second generation of stents, were developed that utilized biodegradable or non-biodegradable materials to incorporate pharmacologic agents [6,7]. Advances in the stent platform and polymer coating led to introduction of third-generation devices [8]. Covered stents (CSs), with thin layer coating on metallic scaffolds of stent, reduce the risk of metallic ion release, internal hemorrhage and decrease radial pressure of the stent, prevent tissue ingrowth and thromboembolic release and simultaneously act as drug delivery platforms [9].
Nanotechnology Applications in Stent Development
Modifications based on nanotechnology can improve all three categories’ properties from the perspectives of hemocompatibility, platelet and monocyte adhesion, inflammatory activation, late neointimal and consequently the risk of late restenosis, mechanical strength, flexibility, and corrosion resistance. These modifications also provide reservoir for drug and gene delivery, obtaining sustained release profile to promote reendothelialization and reducing smooth muscle cell proliferation; and generally improving biocompatibility and safety profile [10-23].
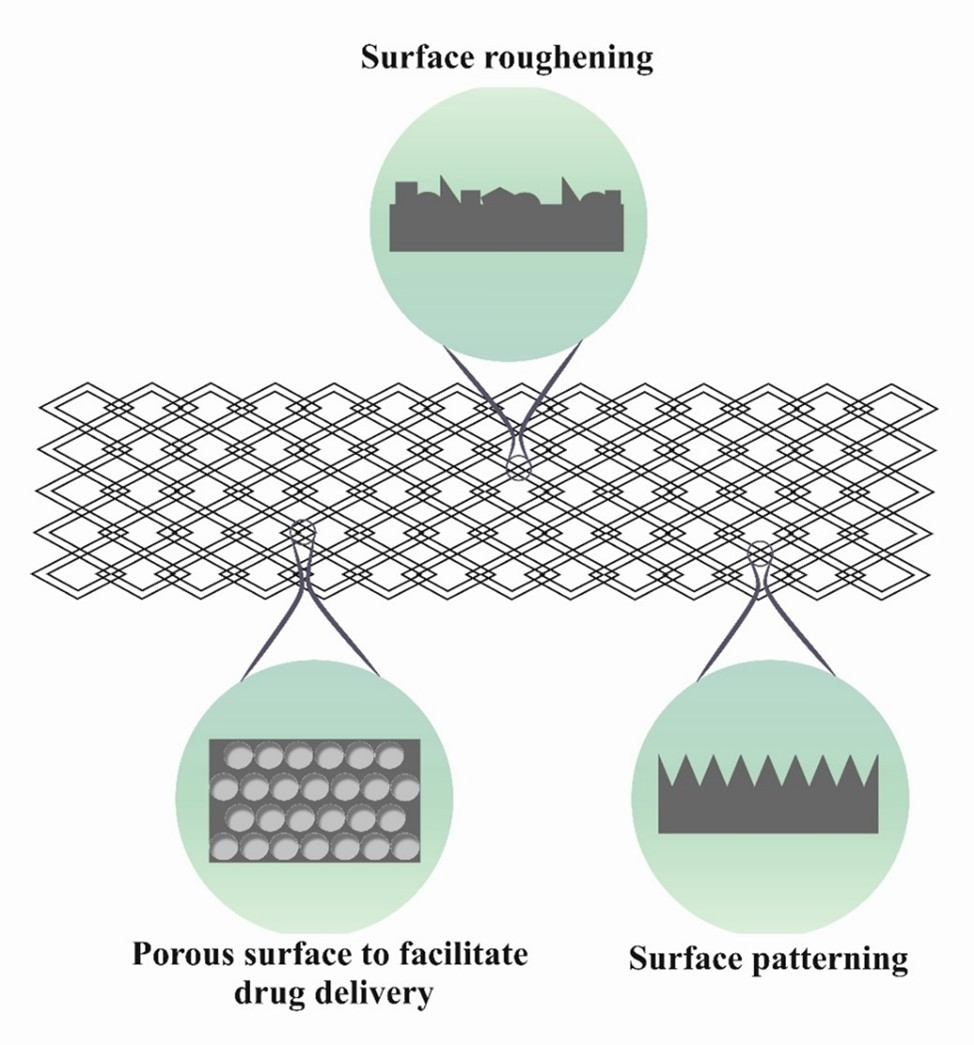
The biocompatibility and hemocompatibility of an implant are very important issues because implants are in direct contact with the blood and tissues. Physical modification is easy and economical way to improve the surface characteristics of implants. In physical modifications, controlled and oriented micro- and nanopatterns are created on stents (Figure 1), based on different techniques. This category of modifications, because of related sizes to extracellular matrix components and a huge surface area, controls and directs the cell behavior on implanted biomedical materials [24,25]. Significant effects of oriented physical modifications on stent bio- and hemocompatibility are: (1) More corrosion resistance because of less metal ion release from intravascular stents (2) improvement of reendothelialization rate induced by enhancing attachment, uniform distribution and proliferation of endothelial cells (ECs) and (3) avoiding thrombogenicity to enhance hemocompatibility [26,27]. Combination of micro and nano-patterns on the implant surface produces synergistic effects in improving bio- and hemocompatibility [26].
Figure 1. Some examples of Physical modification on the surface of bare stent [1].
Chemical modifications, based on nanotechnology, is another solution to overcome drawbacks of primary stents. Bare stents (BS) with a covering film show the structural integrity and reduced radial pressure force to the vessel wall. A Covering film limits platelet adhesion and SMCs proliferation by providing a physical obstacle between the bloodstream and the endoluminal area of the vessel wall. It also offers a capable drug delivery system by enhancing mechanical properties and surface area [28-30]. Chemical modifications can be provided using polymeric, inorganic and carbon-based nanomaterials.
Biocompatible polymeric nanomaterial coatings prevent the release of ions from metallic alloy implants such as nitinol (NiTi), stainless steel, cobalt chromium and etc., in the human body, so they can reduce unwanted biological reactions and improve biocompatibility, mechanical properties and surface resistance [29,31-33].
Despite these advantages, release of acidic byproducts from some biodegradable polymers can lead to vigorous inflammatory reactions and so clinical failure. The composition of biodegradable polymers with nano-sized amorphous calcium phosphate (ACP) and magnesium hydroxide (Mg(OH)2) nanoparticles prevents the release of acidic byproducts, resulting from degradation of biodegradable polymers, and provide better biocompatibility and biomechanical properties [34-36]. Titanium oxides, with appropriate hemocompatibility, are used as coating for blood-contacting devices [37-39]. The appropriate surface free energy of TiO2 surfaces control the wettability, adsorption and as well as the interaction of the solid surface with proteins, cells, and microorganisms present in the surrounding liquid [40].
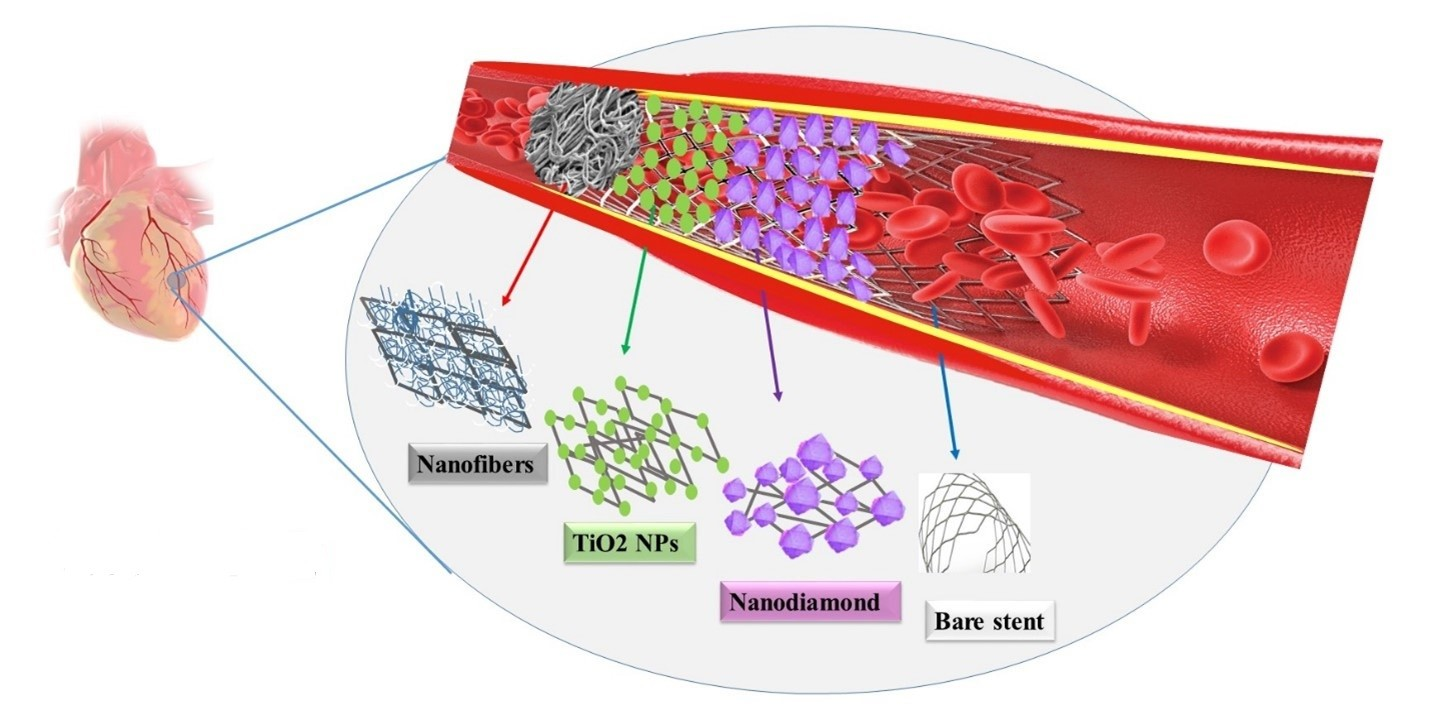
Coating with high oxidation resistant materials, as a barrier to oxygen, have increasingly attracted the attention of researchers for metal protection. Different carbon allotropes (i.e., carbon thin film, graphene (Gr), carbon nanotube (CNT), nanocrystalline diamond, etc.) and also carbon compounds such as graphene oxide (GO) have been applied for surface modification of cardiovascular stents. Gr with high oxidation resistance have increasingly attracted the attention of researchers, as a barrier to oxygen and new coating material for metal protection. Specifically, graphene coated nitinol (Gr–NiTi) induces better cell growth and proliferation and is widely used in the design of implants and stents. Moreover, excellent adsorption of serum albumin on Gr–NiTi leads to lower thrombosis rate. In general, various studies showed the modification using carbon nanomaterials and also composites of carbon nanomaterials and metal oxide nano-structures improves the reendothelialization, hemocompatibility, antithrombogenicity and biomechanical properties and reduces the restenosis and inflammation [18,41-48]. Obtained surfaces also affect the endothelial cell phenotype, diminish the endothelial-to-mesenchymal transition and thus reduce the risk of in-stent restenosis [42]. Figure 2 shows some examples of chemical modifications on stent surface.
Figure 2. Chemical modifications on bare stent. Nanofibers, TiO2 and Nano diamond are illustrating of polymeric, inorganic and carbon-based nano materials, respectively [1].
Organic and inorganic coatings have provided advantages in terms of chemical resistance, biocompatibility, drug loading and delivery. But these chemicals have no biological activity. Immobilization of bioactive molecules, such as extracellular matrix (ECM) molecules, cell-adhesive peptides, VEGF, proteins, and cell recognition peptides, have been considered to stimulate a positive response on the stent surface. These biological molecules have been used as biomimetic agents to confer hemocompatibility, reproduce natural biological structures at the molecular level and have the potential for accelerating healing of vascular stent lesions [49-51].
The impact of physical, chemical and biological modifications on stents improvement is discussed in detail in our review article [1].
Nowadays, the design of nanotechnology-based reservoirs for drug loading and targeted delivery is one of the attractive research fields [52,53]. Drug eluting stents (DESs) developed based on physical modifications and chemical coatings in stents to provide a capacity for drug loading and delivery [54-61]. Nanotechnology introduces structures with capabilities such as drug solubility regulation, dug transport and targeted drug delivery and so, improves effectiveness and controls side effects of drugs. Chemical and biological agents can be loaded in biocompatible nano-reservoirs, such as nanoliposomes, polymeric nanofibers, mesoporous NPs and etc. in stent structure for local and controlled drug delivery [20,62-66]. The fiber and polymer network could function as a reservoir to support the immobilization of biomimetic coatings for anti-coagulant, anti-inflammatory and reendothelialization promotion properties [61,67-69]. Liposomes, as drug carriers, have attracted much attention due to biodegradability, ability to carry both hydrophobic and hydrophilic materials at the same time and also drug transportation ability through biological membranes [20]. The development of multi-agent drug delivery systems increases efficacy and reduces side effects through synergistic effect and reducing the amount of drug required [68]. Coupling shockwave therapy to stents can be applied to break down calcium in arteries using sonic waves [70].
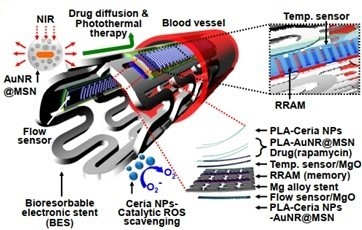
In the future, loading different therapeutic, imaging and targeting agents on stents will develop theranostic systems (Figure 3) [71-75]. Smart stents, equipped with different imaging, sensing, targeting and therapeutic agents can control post-implant conditions [76,77]. 3D-printing technology can provide personalized stents from biodegradable materials which will improve clinical consequences [78]. Using smart materials such as photo-crosslinked resins can provide high print resolution and so, sophisticated and smaller structures with intricate features [79].
Figure 3. A smart stent [80].
Conclusion
Our study shows that nowadays the most common stents in the clinic are those made based on cobalt chromium and coated with biodegradable polymers containing potent immunosuppressant and anti-proliferative agents. Stents, despite being used for decades, still have considerable research potential. It is hoped that nanotechnology, by introducing new materials, synthesis techniques, fabrication methods and structures, will be able to design and construct next-generation coronary stents, to address the challenges of present-day stents. Such systems will provide in- and post-operative monitoring in order to reduce the need for subsequent invasive interventions and so provide high efficiency and safety of treatments. Along with the development of nanotechnology applications in the treatment of cardiovascular diseases, it is necessary to carry out sufficient and accurate studies on the safety and potential toxic effects of nanostructures on the body, scale up of fabrication techniques to an industrial level and extensive validation studies in preclinical and clinical trials to approve the next generation nanotechnology-based devices.
References
2. Lin J, Ozan S, Li Y, Ping D, Tong X, Li G, et al. Novel Ti-Ta-Hf-Zr alloys with promising mechanical properties for prospective stent applications. Sci Rep. 2016 Nov 29; 6:37901.
3. Kim Y, Park JK, Seo JH, Ryu HS, Lim KS, Jeong MH, et al. A rapamycin derivative, biolimus, preferentially activates autophagy in vascular smooth muscle cells. Sci Rep. 2018 Nov 8;8(1):16551.
4. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987 Mar 19;316(12):701-6.
5. Haase KK, Karsch KR. Coronary stents--implantation of foreign bodies into stenotic human coronary arteries: dream or nightmare? Eur Heart J. 1997 Apr;18(4):552-53.
6. Wang J, Li Y, Gao L, Wang S, Mao A, Liu B. Preparation of the micro/nano structures of the biomimetic coating stent for loading MiRNA126 by four-beam laser interference. Optik. 2017 Jan 1; 128:247-52.
7. Saleh YE, Gepreel MA, Allam NK. Functional Nanoarchitectures For Enhanced Drug Eluting Stents. Sci Rep. 2017 Jan 12; 7:40291.
8. Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S, Fremes SE. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013 Nov 20;310(19):2086-95.
9. Farhatnia Y, Tan A, Motiwala A, Cousins BG, Seifalian AM. Evolution of covered stents in the contemporary era: clinical application, materials and manufacturing strategies using nanotechnology. Biotechnol Adv. 2013 Sep-Oct;31(5):524-42.
10. Styllou P, Silber S. A case report of the new Polyzene™-F COBRA PzF™ Nanocoated Coronary Stent System (NCS): Addressing an unmet clinical need. Cardiovasc Revasc Med. 2016 Apr-May;17(3):209-11.
11. Lee CH, Hsieh MJ, Chang SH, Chiang CL, Fan CL, Liu SJ, et al. Biodegradable Cable-Tie Rapamycin-eluting Stents. Sci Rep. 2017 Mar 8;7(1):111.
12. Misra SK, Ostadhossein F, Babu R, Kus J, Tankasala D, Sutrisno A, et al. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv Healthc Mater. 2017 Jun;6(11):1700008.
13. Varshosaz J, Javanmard SH, Soghrati S, Behdadfar B. Magnetic chondroitin targeted nanoparticles for dual targeting of montelukast in prevention of in-stent restenosis. Rsc Advances. 2016;6(15):12337-47.
14. Fan H, Ma J, Li C, Xing G, Han Y. Biodegradable coated stent in the treatment of coronary heart disease in the elderly. Applied Nanoscience. 2023 May;13(5):3543-50.
15. Castellino M, Stolojan V, Virga A, Rovere M, Cabiale K, Galloni MR, et al. Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents. Anal Bioanal Chem. 2013 Jan;405(1):321-29.
16. Paul A, Shao W, Shum-Tim D, Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNA(Ang1+Vegf) nanoparticles. Biomaterials. 2012 Oct;33(30):7655-64.
17. Muthiah M, Islam MA, Cho CS, Hwang JE, Chung IJ, Park IK. Substrate-mediated delivery of microRNA-145 through a polysorbitol-based osmotically active transporter suppresses smooth muscle cell proliferation: implications for restenosis treatment. J Biomed Nanotechnol. 2014 Apr;10(4):571-9.
18. Nie C, Ma L, Cheng C, Deng J, Zhao C. Nanofibrous heparin and heparin-mimicking multilayers as highly effective endothelialization and antithrombogenic coatings. Biomacromolecules. 2015 Mar 9;16(3):992-1001.
19. Rodriguez-Contreras A, Guadarrama Bello D, Flynn S, Variola F, Wuest JD, Nanci A. Chemical nanocavitation of surfaces to enhance the utility of stainless steel as a medical material. Colloids Surf B Biointerfaces. 2018 Jan 1; 161:677-87.
20. Salehi-Nik N, Malaie-Balasi Z, Amoabediny G, Banikarimi SP, Zandieh-Doulabi B, Klein-Nulend J. Sustained release of growth hormone and sodium nitrite from biomimetic collagen coating immobilized on silicone tubes improves endothelialization. Mater Sci Eng C Mater Biol Appl. 2017 Aug 1; 77:1204-15.
21. Sharipova A, Swain SK, Gotman I, Starosvetsky D, Psakhie SG, Unger R, et al. Mechanical, degradation and drug-release behaviour of nano-grained Fe-Ag composites for biomedical applications. J Mech Behav Biomed Mater. 2018 Oct; 86:240-9.
22. Sotoudehbagha P, Sheibani S, Khakbiz M, Ebrahimi-Barough S, Hermawan H. Novel antibacterial biodegradable Fe-Mn-Ag alloys produced by mechanical alloying. Mater Sci Eng C Mater Biol Appl. 2018 Jul 1; 88:88-94.
23. Laloy J, Haguet H, Alpan L, Raichman D, Dogné JM, Lellouche JP. Impact of functional inorganic nanotubes f-INTs-WS 2 on haemolysis, platelet function and coagulation. Nano Convergence. 2018 Dec; 5:1-10.
24. Zhou K, Li Y, Zhang L, Jin L, Yuan F, Tan J, et al. Nano-micrometer surface roughness gradients reveal topographical influences on differentiating responses of vascular cells on biodegradable magnesium. Bioact Mater. 2020 Aug 22;6(1):262-72.
25. Cherian AM, Joseph J, Nair MB, Nair SV, Vijayakumar M, Menon D. Coupled benefits of nanotopography and titania surface chemistry in fostering endothelialization and reducing in-stent restenosis in coronary stents. Biomater Adv. 2022 Nov; 142:213149.
26. Shen Y, Wang G, Chen L, Li H, Yu P, Bai M, et al. Investigation of surface endothelialization on biomedical nitinol (NiTi) alloy: Effects of surface micropatterning combined with plasma nanocoatings. Acta Biomater. 2009 Nov;5(9):3593-604.
27. Han ED, Kim BH, Seo YH. Anti-cell adhesion characteristics of nanotextured surface for implantable biomedical device. International Journal of Precision Engineering and Manufacturing. 2017 Feb; 18:239-44.
28. Farhatnia Y, Pang JH, Darbyshire A, Dee R, Tan A, Seifalian AM. Next generation covered stents made from nanocomposite materials: A complete assessment of uniformity, integrity and biomechanical properties. Nanomedicine. 2016 Jan;12(1):1-12.
29. Merkle VM, Tran PL, Hutchinson M, Ammann KR, DeCook K, Wu X, et al. Core-shell PVA/gelatin electrospun nanofibers promote human umbilical vein endothelial cell and smooth muscle cell proliferation and migration. Acta Biomater. 2015 Nov; 27:77-87.
30. Phan T, Jones JE, Liao Y, Yu Q, Chen M. The Mechanical and Electrochemical Stability of Trimethysilane Plasma Nanocoatings Deposited onto Cobalt Chromium Cardiovascular Stents. Materials (Basel). 2024 Jul 26;17(15):3699.
31. Bakhshi R, Darbyshire A, Evans JE, You Z, Lu J, Seifalian AM. Polymeric coating of surface modified nitinol stent with POSS-nanocomposite polymer. Colloids Surf B Biointerfaces. 2011 Aug 1;86(1):93-105.
32. Cho Y, Vu BQ, Bedair TM, Park BJ, Joung YK, Han DK. Crack prevention of biodegradable polymer coating on metal facilitated by a nano-coupled interlayer. Journal of Bioactive and Compatible Polymers. 2014 Sep;29(5):515-26.
33. Mutuk T, Gürbüz M. Surface Modification of Ni–Ti Stents by Biodegradable Binary PVA/Propolis Electrospun Nano Fibers. Arabian Journal for Science and Engineering. 2023 Mar;48(3):3391-402.
34. Kang EY, Choi B, Park W, Kim IH, Han DK. One step bulk modification of poly(L-lactic acid) composites with functional additives to improve mechanical and biological properties for cardiovascular implant applications. Colloids Surf B Biointerfaces. 2019 Jul 1;179:161-9.
35. Lih E, Kum CH, Park W, Chun SY, Cho Y, Joung YK, et al. Modified Magnesium Hydroxide Nanoparticles Inhibit the Inflammatory Response to Biodegradable Poly(lactide- co-glycolide) Implants. ACS Nano. 2018 Jul 24;12(7):6917-25.
36. Zheng X, Wang Y, Lan Z, Lyu Y, Feng G, Zhang Y, et al. Improved biocompatibility of poly(lactic-co-glycolic acid) orv and poly-L-lactic acid blended with nanoparticulate amorphous calcium phosphate in vascular stent applications. J Biomed Nanotechnol. 2014 Jun;10(6):900-10.
37. Foruzanmehr M, Hosainalipour SM, Tehrani SM, Aghaeipour M. Nano-structure TiO2 film coating on 316L stainless steel via sol-gel technique for blood compatibility improvement. Nanomed. J. 2014 Apr 1;1(128):10-7508.
38. Liu H, Zhou S, Wu Q, Pan C, Huang N, Liu Y. Investigation on endothelialization of Ti-O film modified vascular stent in vivo. Integrated Ferroelectrics. 2018 May 4;189(1):78-86.
39. Mohan CC, Cherian AM, Kurup S, Joseph J, Nair MB, Vijayakumar M, et al. Stable Titania Nanostructures on Stainless Steel Coronary Stent Surface for Enhanced Corrosion Resistance and Endothelialization. Adv Healthc Mater. 2017 Jun;6(11):1601353.
40. Schvezov CE, Vera ML, Schuster JM, Rosenberger MR. Production and characterization of TiO2 nanofilms for hemocompatible and photocatalytic applications. Jom. 2017 Oct;69(10):2038-44.
41. Skoog SA, Lu Q, Malinauskas RA, Sumant AV, Zheng J, Goering PL, et al. Effects of nanotopography on the in vitro hemocompatibility of nanocrystalline diamond coatings. J Biomed Mater Res A. 2017 Jan;105(1):253-64.
42. Wawrzyńska M, Bil-Lula I, Krzywonos-Zawadzka A, Arkowski J, Łukaszewicz M, Hreniak D, et al. Biocompatible Carbon-Based Coating as Potential Endovascular Material for Stent Surface. Biomed Res Int. 2018 Oct 4;2018:2758347.
43. Chen H, Tang N, Chen M, Chen D. Endothelialization of TiO2 Nanorods Coated with Ultrathin Amorphous Carbon Films. Nanoscale Res Lett. 2016 Dec;11(1):145.
44. Podila R, Moore T, Alexis F, Rao AM. Graphene coatings for enhanced hemo-compatibility of nitinol stents. RSC Advances. 2013;3(6):1660-5.
45. Bito K, Hasebe T, Maegawa S, Kitagawa T, Matsumoto T, Suzuki T, et al. Micropatterning of a 2-methacryloyloxyethyl phosphorylcholine polymer surface by hydrogenated amorphous carbon thin films for endothelialization and antithrombogenicity. Acta Biomater. 2019 Mar 15;87:187-96.
46. Zhang K, Zhang H, Liu P, Zhang C, Li W, Chen X, et al. Electrophoretic deposition of graphene oxide on NiTi alloy for corrosion prevention. Vacuum. 2019 Mar 1;161:276-82.
47. ElSawy AM, Attia NF, Mohamed HI, Mohsen M, Talaat MH. Innovative coating based on graphene and their decorated nanoparticles for medical stent applications. Mater Sci Eng C Mater Biol Appl. 2019 Mar;96:708-15.
48. Grundsteins K, Diedkova K, Korniienko V, Stoppel A, Balakin S, Jekabsons K, et al. Nanodiamond Decorated PEO Oxide Coatings on NiTi Alloy. Nanomaterials (Basel). 2023 Sep 20;13(18):2601.
49. Yang Z , Zhong S , Yang Y , Maitz MF , Li X , Tu Q , et al . Polydopamine-mediated long-term elution of the direct thrombin inhibitor bivalirudin from TiO2 nanotubes for improved vascular biocompatibility. J Mater Chem B. 2014 Oct 21;2(39):6767-78.
50. Liu T, Wang X, Tang X, Gong T, Ye W, Pan C, et al. Surface Modification with ECM-Inspired SDF-1α/Laminin-Loaded Nanocoating for Vascular Wound Healing. ACS Appl Mater Interfaces. 2017 Sep 13;9(36):30373-86.
51. Shim G, Kim D, Kim J, Suh MS, Kim YK, Oh YK. Bacteriomimetic poly-γ-glutamic acid surface coating for hemocompatibility and safety of nanomaterials. Nanotoxicology. 2017 Aug;11(6):762-70.
52. Yuan W, Lu Z, Wang H, Li CM. Stimuli‐Free Reversible and Controllable Loading and Release of Proteins under Physiological Conditions by Exponentially Growing Nanoporous Multilayered Structure. Advanced Functional Materials. 2012 May 9;22(9):1932-9.
53. Yuan W, Lu Z, Liu J, Wang H, Li CM. ZnO nanowire array-templated LbL self-assembled polyelectrolyte nanotube arrays and application for charged drug delivery. Nanotechnology. 2013 Feb 1;24(4):045605.
54. Saleh YE, Gepreel MA-H, Allam NK. Titanium-based functional nano-architectures for drug eluting stents. 2020, Google Patents.
55. Zhang B, Qin Y, Yang L, Wu Y, Chen N, Li M, et al. A Polyphenol-Network-Mediated Coating Modulates Inflammation and Vascular Healing on Vascular Stents. ACS Nano. 2022 Apr 26;16(4):6585-97.
56. Dai Y, Wang R, Chen F, Zhang Y, Liu Y, Huang H, et al. Clinical outcomes in 2481 unselected real-world patients treated with a polymer-free sirolimus-eluting stent: 3 years results from the NANO multicenter Registry. BMC Cardiovasc Disord. 2021 Nov 12;21(1):537.
57. Liu Y, Zhang Y, Li Y, Qi T, Pan D, Wang H, et al. One-year clinical results of the NANO registry: A multicenter, prospective all-comers registry study in patients receiving implantation of a polymer-free sirolimus-eluting stent. Catheter Cardiovasc Interv. 2020 Feb;95 Suppl 1:658-64.
58. Oishi Y, Tsujita H, Arai T, Sakai R, Sato S, Tanaka H, et al. The vascular response to ultrathin biodegradable polymer sirolimus-eluting stent at 2-weeks and 1-year follow up in patients with ST-elevation myocardial infarction. European Heart Journal. 2022 Oct 1;43(Supplement_2):ehac544-2011.
59. Liu Y, Liu P, Song Y, Li S, Shi Y, Quan K, et al. A heparin-rosuvastatin-loaded P(LLA-CL) nanofiber-covered stent inhibits inflammatory smooth-muscle cell viability to reduce in-stent stenosis and thrombosis. J Nanobiotechnology. 2021 Apr 29;19(1):123.
60. Liang C, Tian Y, Zou X, Hu Y, Zhou H, Yang L, et al. Improve endothelialization of metallic cardiovascular stent via femtosecond laser induced micro/nanostructure dependent cells proliferation and drug delivery control. Colloids Surf B Biointerfaces. 2022 Apr;212:112376.
61. Moradi MR, Salahinejad E, Sharifi E, Tayebi L. Controlled drug delivery from chitosan-coated heparin-loaded nanopores anodically grown on nitinol shape-memory alloy. Carbohydr Polym. 2023 Aug 15;314:120961.
62. Jiang T, Xie Z, Wu F, Chen J, Liao Y, Liu L, et al. Hyaluronic Acid Nanoparticle Composite Films Confer Favorable Time-Dependent Biofunctions for Vascular Wound Healing. ACS Biomater Sci Eng. 2019 Apr 8;5(4):1833-48.
63. Lu Q, Ye F, Yang X, Gu Q, Wang P, Zhu J, et al. Accelerated Recovery of Endothelium Function after Stent Implantation with the Use of a Novel Systemic Nanoparticle Curcumin. Biomed Res Int. 2015;2015:291871.
64. Oh B, Lee CH. Advanced cardiovascular stent coated with nanofiber. Mol Pharm. 2013 Dec 2;10(12):4432-42.
65. Alexander GC, Hwang PTJ, Chen J, Kim J, Brott BC, Yoon YS, et al. Nanomatrix Coated Stent Enhances Endothelialization but Reduces Platelet, Smooth Muscle Cell, and Monocyte Adhesion under Physiologic Conditions. ACS Biomater Sci Eng. 2018 Jan 8;4(1):107-15.
66. Tan A, Goh D, Farhatnia Y, G N, Lim J, Teoh SH, et al. An anti-CD34 antibody-functionalized clinical-grade POSS-PCU nanocomposite polymer for cardiovascular stent coating applications: a preliminary assessment of endothelial progenitor cell capture and hemocompatibility. PLoS One. 2013 Oct 8;8(10):e77112.
67. Chen N, Li M, Wu H, Qin Y, Wang J, Xu K, et al. An extracellular matrix-mimetic coating with dual bionics for cardiovascular stents. Regen Biomater. 2023 May 30;10:rbad055.
68. Kawai K, Rahman MT, Nowicki R, Kolodgie FD, Sakamoto A, Kawakami R, et al. Efficacy and Safety of Dual Paclitaxel and Sirolimus Nanoparticle-Coated Balloon. JACC Basic Transl Sci. 2024 May 1;9(6):774-89.
69. Du H, Li W, Li X, Qiu Z, Ding J, Zhang Y. Optimizing the Biocompatibility of PLLA Stent Materials: Strategy with Biomimetic Coating. Int J Nanomedicine. 2024 Jun 4;19:5157-72.
70. Tassone EJ, Tripolino C, Morabito G, Grillo P, Missiroli B. Shockwave coronary lithoplasty for the treatment of under-expanded stent. Journal of Cardiology and Cardiovascular Sciences. 2020 Jul 17;4(3).
71. Wang J, An Q, Li D, Wu T, Chen W, Sun B, et al. Heparin and vascular endothelial growth factor loaded poly (L-lactide-co-caprolactone) nanofiber covered stent-graft for aneurysm treatment. Journal of Biomedical Nanotechnology. 2015 Oct 1;11(11):1947-60.
72. Zohra FT, Medved M, Lazareva N, Polyak B. Functional behavior and gene expression of magnetic nanoparticle-loaded primary endothelial cells for targeting vascular stents. Nanomedicine (Lond). 2015 May;10(9):1391-406.
73. Polyak B, Medved M, Lazareva N, Steele L, Patel T, Rai A, et al. Magnetic Nanoparticle-Mediated Targeting of Cell Therapy Reduces In-Stent Stenosis in Injured Arteries. ACS Nano. 2016 Oct 25;10(10):9559-69.
74. Keum DH, Mun JH, Hwang BW, Kim J, Kim H, Jo W, et al. Smart Microbubble Eluting Theranostic Stent for Noninvasive Ultrasound Imaging and Prevention of Restenosis. Small. 2017 Mar;13(10).
75. Lee JS, Han P, Song E, Kim D, Lee H, Labowsky M, et al. Magnetically Coated Bioabsorbable Stents for Renormalization of Arterial Vessel Walls after Stent Implantation. Nano Lett. 2018 Jan 10;18(1):272-81.
76. Vishnu J, Manivasagam G. Perspectives on smart stents with sensors: From conventional permanent to novel bioabsorbable smart stent technologies. Medical Devices & Sensors. 2020 Dec;3(6):e10116.
77. Chaparro-Rico BD, Sebastiano F, Cafolla D. A smart stent for monitoring eventual restenosis: Computational fluid dynamic and finite element analysis in descending thoracic aorta. Machines. 2020 Nov 24;8(4):81.
78. Shen Y, Tang C, Sun B, Zhang Y, Sun X, Mohamed EN, et al. 3D printed personalized, heparinized and biodegradable coronary artery stents for rabbit abdominal aorta implantation. Chemical Engineering Journal. 2022 Dec 15;450:138202.
79. Zong J, He Q, Liu Y, Qiu M, Wu J, Hu B. Advances in the development of biodegradable coronary stents: A translational perspective. Mater Today Bio. 2022 Jul 19;16:100368.
80. Son D, Lee J, Lee DJ, Ghaffari R, Yun S, Kim SJ, et al. Bioresorbable Electronic Stent Integrated with Therapeutic Nanoparticles for Endovascular Diseases. ACS Nano. 2015 Jun 23;9(6):5937-46.
81. Maillard L, de Labriolle A, Brasselet C, Faurie B, Durel N, de Poli Fet al. Evaluation of the safety and efficacy of the Cobra PzF NanoCoated coronary stent in routine, consecutive, prospective, and high-risk patients: The e-Cobra study. Catheter Cardiovasc Interv. 2021 Jul 1;98(1):45-54.
82. FDA Clears Nano-coated Coronary Stent to Reduce DAPT. 2017. Available from: https://www.dicardiology.com/product/fda-clears-nano-coated-coronary-stent-reduce-dapt
83. Promus PREMIER™. Everolimus-Eluting Platinum Chromium Coronary Stent System. Boston Scientific Corporation. Available from: https://www.bostonscientific.com/en-US/products/stents--coronary/promus-premier-stent-system
84. XIENCE Drug-Eluting Stents. Abbott. Available from: https://www.cardiovascular.abbott/us/en/hcp/products/percutaneous-coronary intervention/xience-family.html
85. Ultimaster™: Sirolimus Eluting Coronary Stent System. Terumo. Available from: https://www.terumo-europe.com/en-emea/Products/Ultimaster%E2%84%A2-Sirolimus-Eluting-Coronary-Stent-System.
86. Yoon CH, Choi YJ, Park JJ, Kang SH, Kim SH, Suh JW, et al. BioMatrix versus Orsiro biodegradable polymer stents in all-comer patients with coronary artery disease: the multicentre, randomised BIODEGRADE trial. EuroIntervention. 2021 Apr 20;16(17):1404-12.