Abstract
Introduction: In the heart failure population, overweight individuals are associated with lower mortality, termed the obesity survival paradox. The influence of body mass index (BMI) on outcomes in cardiac amyloidosis has not been characterized. We sought to determine the association between BMI and mortality, and hematologic and cardiac response in patients with light chain (AL) amyloidosis.
Methods: We conducted a retrospective study of patients with cardiac AL amyloidosis referred between 1/1/2009 and 09/30/2018. We collected baseline demographics including BMI and recorded mortality and hematologic and cardiac response. Cox proportional hazards model and logistic regression models were constructed to examine the association between BMI and outcomes.
Results: Of the 78 patients, 17 patients had a BMI of 17-22.5, 19 a BMI of 22.6-25, 23 a BMI of 25.1-29.9, and 19 a BMI of ≥ 30 kg/ m2. The median follow-up was 51 months. There was no relationship between BMI as a continuous variable and mortality (adjusted HR 0.98, 95% CI 0.91-1.05, p=0.54). While there was no relationship between high BMI (≥ 25 kg/m2) and hematologic response (adjusted OR 0.97, 0.34-2.76, p=0.96), there was a relationship between high BMI and a lower likelihood of achieving cardiac response (adjusted OR 0.23, 0.07-0.71, p=0.011).
Conclusions: In this cohort of patients with AL cardiac amyloidosis, there was no significant relationship between BMI and mortality. Patients with a higher BMI were significantly less likely to achieve a cardiac response. These findings highlight the importance of a multidisciplinary approach involving oncologists, cardiologists, and nutritionists in the treatment of this very complex multi-organ disease.
Keywords
AL amyloidosis, BMI, Mortality, Hematologic response, Cardiac response
Introduction
Elevated body mass index (BMI) has been associated with an increased risk of cancer and has been shown to have a negative impact on survival in patients with breast, prostate, oral cancer, and leukemia [1-4]. In plasma cell dyscrasias, obesity has not only been shown to be a risk factor for the development of multiple myeloma, but also has been associated with a higher rate of progression from monoclonal gammopathy of unknown significance (MGUS) to multiple myeloma, and if intervened on, has been observed to prevent the transformation from MGUS to myeloma [5-10].
Light-chain amyloidosis (AL) is a clonal plasma cell disorder in which monoclonal light chains form fibrils that deposit in the tissues of vital organs. Incidence and prevalence are low and has been estimated to range from 3-12 cases per million persons/year and 30,000-45,000 patients in the United States and European Union, respectively [11].
Approximately half of AL amyloidosis patients present with cardiac involvement which impacts survival [12-16]. In contrast to the observation that higher BMI is associated with reduced mortality in cancer patients, in the heart failure population, mild/moderate obesity has been shown to be associated with a lower mortality termed the “obesity survival paradox” [17-19]. For every 1% rise in percent body fat, it was observed that there was a 13% reduction in major cardiovascular events [20]. The observation of improved outcomes in patients with higher BMI may be secondary to protection from cachexia, which has been associated with negative outcomes such as loss of cardiac muscle, increased risk of bacterial translocation from enterocyte atrophy, exacerbation of anemia, and increased neurohormonal activation [21].
To our knowledge, due to the rarity of the disease, there is minimal literature investigating the association of BMI on survival in AL amyloidosis. This clinical question is especially relevant in patients with AL amyloidosis who harbor both a plasma cell dyscrasia and a cardiomyopathy, conditions in which BMI has been shown to have conflicting prognostic implications. We sought to determine the impact of BMI on outcomes in patients with cardiac AL amyloidosis and hypothesized that patients would exhibit an obesity survival paradox similar to what has been described in heart failure populations.
Methods
Cohort selection
We conducted a single tertiary center retrospective study of consecutive ambulatory patients with cardiac AL amyloidosis, referred between 1/1/2009 and 09/30/2018. We collected baseline demographics, including age, race, gender, cardiac stage, height, and weight prior to treatment. Cardiac staging was classified by Mayo 2004 staging system [22]. BMI was calculated by dividing the weight in kilograms by height in meters squared. Patients were grouped into four BMI cohorts: BMI <22.5 kg/ m2, 22.6-25 kg/m2, 25.1-29.9 kg/m2, and ≥ 30 kg/m2. These cohorts were chosen based on the World Health Organization (WHO) BMI classification and based on a collaborative study of 900,000 adults in 57 prospective studies which demonstrated the optimal BMI to be 22.5- 25 kg/m2 [23].
Clinical endpoints
We recorded important clinical endpoints from the electronic medical record including all-cause mortality, and hematologic and cardiac response after first-line treatment. Data was collected until date of death or date of last follow up. Hematologic and cardiac responses were defined per consensus guidelines validated by the Roundtable on Clinical Research in Immunoglobulin Light Chain Amyloidosis [24-26]. Hematologic response was grouped into response (complete response, very good partial response, partial response) or no response (no response or progression) [24]. Cardiac response was defined as >30% and >300ng/l decrease in NT-proBNP in patients with baseline NT-proBNP ≥ 650 ng/l or NYHA class response (≥ 2 class decrease in subjects with baseline NYHA class 3 or 4), and progression defined as >30% and >300ng/l increase in NT-proBNP, increase in cardiac troponin ≥ 33%, or decrease in ejection fraction (≥ 10%) [24]. For analysis of cardiac response, patients were grouped into response or no response (progression was categorized as “no response”).
Statistical analysis
Patients were analyzed with BMI as a continuous variable with respect to mortality and among BMI cohorts with respect to cardiac response and hematologic response. We constructed a Cox proportional hazards model examining the association between BMI and mortality. Logistic regression models were constructed to examine the association between high BMI (defined as BMI ≥ 25 kg/ m2) and cardiac or hematological response. Models were adjusted for age, sex and cardiac stage. Requirement of full consent was waived due to retrospective nature of study. IRB approval was obtained prior to data collection.
Results
Of the 78 patients identified, 17 patients had a BMI of 17-22.5 kg/m2, 19 a BMI of 22.6-25 kg/m2, 23 a BMI of 25.1-29.9 kg/m2, and 19 a BMI of ≥ 30 kg/m2. Patient characteristics are summarized in Table 1. Forty-three (55.1%) patients were males and 35 (44.9%) were female. Median age for all patients was 63 years and most patients had cardiac stage 3 (78%). Of the 76 patients whose race was available, 63 (83%) were Caucasians and 13 (17%) non- Caucasians. Median number of organs involved was 2 in all subgroups of BMI. Sixty-six of the 78 patients received first-line treatment, most of whom received bortezomibbased therapy and 12 (15%) who received an autologous stem cell transplant. Of the twelve patients who did not have documented front-line therapy in the medical chart, 4 patients were treated locally, 3 patients were lost to follow-up, 4 patients died prior to starting treatment, and 1 patient refused therapy.
| BMI, kg/m2 | Total (N) | Male (N) | Female (N) | Mean Age (years) | Cardiac stage 1* (N) | Cardiac stage 2* (N) | Cardiac stage 3* (N) |
|---|---|---|---|---|---|---|---|
| ≤ 22.5 | 17 | 6 | 11 | 57.4 | 1 | 2 | 14 |
| 22.6-25 | 19 | 8 | 11 | 67.1 | 0 | 5 | 14 |
| 25.1-29.7 | 23 | 15 | 8 | 64 | 1 | 4 | 18 |
| ≥30 | 19 | 14 | 5 | 63.5 | 1 | 3 | 15 |
| *Mayo 2004 staging system | |||||||
The median follow-up period was 51 months. At the end of the study follow-up period, 30 of the 78 patients had died, with a crude mortality rate of 38%. There was no relationship between BMI as a continuous variable and mortality adjusted for age, sex, and cardiac stage at diagnosis (adjusted HR 0.98, 95% CI 0.91-1.05, p=0.54).
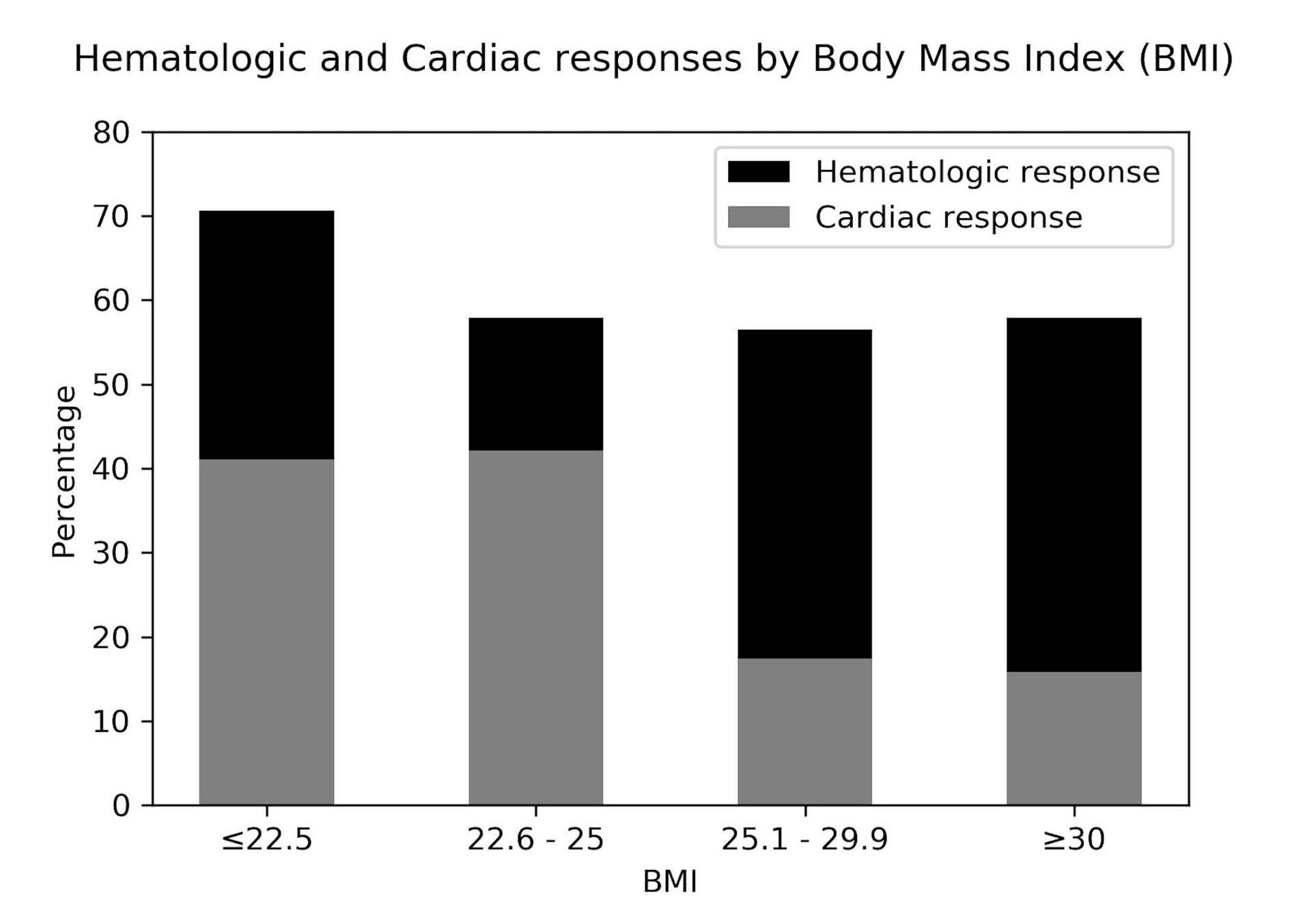
Twelve of 17 patients (71%) in the BMI ≤ 22.5 group, 11 of 19 patients (58%) in the 22.6 ≤ BMI ≤ 25 group, 13 of 23 patients (57%) in the 25.1 ≤ BMI ≤ 29.9 group, and 11 of 19 patients (58%) in the BMI ≥ 30 group achieved a hematologic response. There was no relationship between higher BMI (≥ 25 kg/ m2) and hematologic response to first line therapy (adjusted OR 0.97, 0.34-2.76, p=0.96).
Seven of 17 (41%) in the BMI ≤ 22.5 group, 8 of 19 (42%) in the 22.6 ≤ BMI ≤ 25 group, 4 of 23 (17%) in the 25.1 ≤ BMI ≤ 29.9 group, and 3 of 19 (16%) in the BMI ≥ 30 group achieved a cardiac response. There was a statistically significant relationship between higher BMI (≥ 25 kg/ m2) and a lower likelihood of achieving a cardiac response (adjusted OR 0.23, 0.07-0.71, p=0.011), Figure 1.
Discussion
In this small cohort of patients with AL cardiac amyloidosis, there was no relationship between BMI and mortality, but patients with a higher BMI were significantly less likely to achieve a cardiac response. Patients with very advanced cardiac amyloidosis have been reported to have a poor prognosis, with median survival of approximately 6 months and thus, risk stratification remains important for management [27]. In a prior study of 165 patients with AL amyloidosis, increased cardiac wall thickness, older age, higher NYHA class, BNP, and CRP levels were prognostic indicators for increased mortality [28]. Progressive cachexia has been associated with increased morbidity and mortality in cancer patients and in heart failure patients and thus it was hypothesized that patients with increased BMI would be associated with lower mortality as fat reserve may permit a patient to better withstand the toxic side effects of chemotherapy and the metabolic stress of the disease [29-31]. In this study, it was observed there was no association between BMI (≥ 25 kg/m2) and mortality. This is in contrary to the general heart failure population. This suggests that in cardiac AL amyloidosis patients, BMI may play a less significant role in overall long-term mortality due to the complex nature of the disease, whereby mortality may be impacted by other factors such as additional organs involved [17-19]. The lack of association between BMI and mortality in AL amyloidosis may also be a factor of the limited power due to the small cohort sample in our study.
While there was no association observed between BMI and mortality, patients with a BMI ≥ 25 kg/m2 had a statistically lower likelihood of achieving a cardiac response following first-line therapy. One may hypothesize that patients with increased BMI have other cardiovascular risk factors such as hypertension, diabetes, chronic kidney disease, and coronary artery disease that also may impact overall cardiac response or the ability to administer standard of care treatment regimens independent of cardiac amyloidosis.
As amyloidosis is a rare entity, a limitation of the study was the retrospective nature of the data and small number of patients analyzed as large prospective studies remain a challenge in this patient population. Due to small number of clinical endpoints, we were also limited in the number of covariates we could adjust for in the regression models, meaning that residual confounding could remain. Furthermore, there was a very high proportion of patients with cardiac Mayo stage III (78%) in this study. Since we looked only at the AL cardiac amyloidosis population at our institution, stage III patients were likely over-represented as they are the cohort most likely to be referred to a tertiary care center for care, representing a referral bias. The high proportion of patients with advanced stage cardiac amyloidosis may impact outcomes (i.e., organ response). Overall, though there was no association between BMI and mortality in cardiac AL amyloidosis patients, the observation that higher BMI patients was associated with worse cardiac response in a disease where cardiac involvement impacts mortality, suggests that larger studies are necessary to better understand the impact of BMI on survival and also suggests that early involvement of a nutritionist in a patient’s care may be valuable.
Conclusion
We report a tertiary center experience of the relationships between BMI and mortality, and cardiac and hematologic response amongst patients with cardiac AL amyloidosis, which has not previously been studied in this patient population. While there was no relationship between BMI and mortality, it was observed that patients with higher BMI had a lower likelihood of achieving cardiac response. In a disease that is progressive and incurable, nutritional status and body habitus are some of the only modifiable factors that a patient can control and thus, every effort should be made to fully understand their impact on this disease so that early interventions can be adopted, highlighting the importance of a multidisciplinary approach involving oncologists, cardiologists, and nutritionists.
Disclosures
The authors of this study report no relevant disclosures.
References
2. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine. 2003 Apr 24;348(17):1625-38.
3. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. The lancet oncology. 2002 Sep 1;3(9):565-74.
4. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. New England Journal of Medicine. 2016 Aug 25;375(8):794-8.
5. Birmann BM, Andreotti G, De Roos AJ, Camp NJ, Chiu BC, Spinelli JJ, et al. Young adult and usual adult body mass index and multiple myeloma risk: a pooled analysis in the International Multiple Myeloma Consortium (IMMC). Cancer Epidemiology and Prevention Biomarkers. 2017 Jun 1;26(6):876-85.
6. Friedman GD, Herrinton LJ. Obesity and multiple myeloma. Cancer Causes Control. 1994 Sep;5(5):479-83.
7. Marinac CR, Birmann BM, Lee IM, Rosner BA, Townsend MK, Giovannucci E, et al. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: a prospective analysis in three large cohorts. British Journal of Cancer. 2018 Apr;118(7):1013- 9.
8. Chang SH, Luo S, Thomas TS, O’Brian KK, Colditz GA, Carlsson NP, et al. Obesity and the transformation of monoclonal gammopathy of undetermined significance to multiple myeloma: a population-based cohort study. JNCI: Journal of the National Cancer Institute. 2017 May 1;109(5).
9. Thompson MA, Kyle RA, Melton LJ, Plevak MF, Rajkumar SV. Effect of statins, smoking and obesity on progression of monoclonal gammopathy of undetermined significance: a case-control study. Haematologica. 2004 Jan 1;89(5):626-8.
10. Carson KR, Bates ML, Tomasson MH. The skinny on obesity and plasma cell myeloma: a review of the literature. Bone Marrow Transplantation. 2014 Aug;49(8):1009-15.
11. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Advances. 2018 May 22;2(10):1046- 53.
12. Dispenzieri A, Lacy MQ, Kyle RA, Therneau TM, Larson DR, Rajkumar SV, et al. Eligibility for hematopoietic stemcell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. Journal of Clinical Oncology. 2001 Jul 15;19(14):3350-6.
13. D’Aguanno V, Ralli M, Artico M, Russo FY, Scarpa A, Fiore M, et al. Systemic amyloidosis: a contemporary overview. Clinical Reviews in Allergy & Immunology. 2020 Dec;59(3):304-22.
14. Kyle RA, Greipp P. Amyloidosis (AL). Clinical and laboratory features in 229 cases. In: Mayo Clinic Proceedings 1983; 58(10): 665-683.
15. Pellikka PA, Holmes DR, Edwards WD, Nishimura RA, Tajik AJ, Kyle RA. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Archives of Internal Medicine. 1988 Mar 1;148(3):662-6.
16. Tahir UA, Doros G, Kim JS, Connors LH, Seldin DC, Sam F. Predictors of mortality in light chain cardiac amyloidosis with heart failure. Scientific Reports. 2019 Jun 12;9(1):1-9.
17. Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. JACC: Heart Failure. 2015 Nov;3(11):917- 26.
18. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology. 2009 May 26;53(21):1925-32.
19. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. Journal of the American College of Cardiology. 2001 Sep;38(3):789-95.
20. Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. American Journal of Cardiology. 2003 Apr 1;91(7):891-4.
21. Okoshi MP, Romeiro FG, Paiva SA, Okoshi K. Heart failure-induced cachexia. Arquivos Brasileiros de Cardiologia. 2013 May;100(5):476-82.
22. Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. Amyloid and Amyloidosis. 2004 Jan 1:67-77.
23. Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. The Lancet. 2009 Mar 28;373(9669):1083-96.
24. Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012 Nov;26(11):2317- 25.
25. Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. Journal of Clinical Oncology. 2012 Dec 20;30(36):4541-9.
26. Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. American Journal of Hematology. 2005 Aug;79(4):319-28.
27. Witteles W, Witteles R, Arai S, Lafayette R, Schrier S. AL Amyloidosis: Prognosis of Cardiac Involvement Reconsidered. Blood. 2009;114:4872-4872.
28. Tahir UA, Doros G, Kim JS, Connors LH, Seldin DC, Sam F. Predictors of mortality in light chain cardiac amyloidosis with heart failure. Scientific Reports. 2019 Jun 12;9(1):1-9.
29. Anker SD, Negassa A, Coats AJ, Afzal R, Poole- Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. The Lancet. 2003 Mar 29;361(9363):1077-83.
30. Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. Journal of the American College of Cardiology. 2004 Apr 21;43(8):1439- 44.
31. Penet MF, Bhujwalla ZM. Cancer cachexia, recent advances, and future directions. Cancer Journal. 2015 Mar;21(2):117.