Abstract
Very early on clinicians around the world reported that in addition to aging and various heart pathologies, excess of body weight, especially obesity is a major risk factor for the severity of COVID-19 infection. The multitude of symptoms that have been described from human patients likely arises from the broad distribution of ACE2, a member of the angiotensin receptor family, the receptor for SARS-CoV-2, among which adipose tissue is a prominent one. We previously discussed some of the potential contributions of adipose tissue to the infection severity. While most of them have been supported by the recent literature novel hypothesis have also emerged, which will be discussed here. Moreover, recent pieces of evidence also support the notion that individuals with a high risk of severe disease are found at both extremes of the body mass index. Altogether these potential causes that lead to increased risk of severe forms of COVID-19 by deviation from standard BMI values open up for specific research and consideration of treatment of this population.
Keywords
ACE2, Body mass index, COVID-19, Obesity, Lipid, SARS-CoV-2
Abbreviations
ACE2: Angiotensin-Converting Enzyme II; BMI: Body Mass Index; COVID-19: Corona Virus Disease 2019; PAI-1: Plasminogen Activator Inhibitor-1; RAS: Renin Angiotensin System; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2; TNF-α: Tumor Necrosis Factor Alpha
Introduction
Coronaviruses are one of the viruses causing the common cold, a disease that has never had a cure nor any effective prevention [1,2]. They form are a large family of enveloped, positive-sense, single-stranded RNA viruses that infect a broad range of vertebrates, and for which bats are believed to be an important reservoir [3]. Since late 2019, the occurrence of a variant strain exhibiting stronger virulence and efficient cross-contamination in humans has been responsible for a severe pandemic crisis and has been called severe acute respiratory syndrome coronavirus (SARS-CoV-2).
The Angiotensin-Converting-Enzyme 2 (ACE2), a type I transmembrane metallocarboxypeptidase involved in the Renin-Angiotensin system (RAS) was undoubtedly identified as the major receptor used by viral spike proteins of SARS-CoV2 and thus for viral cell entry [4,5]. Supporting these observations anti-ACE2 and anti-spike antibodies block cellular entry of SARS-CoV-2 [6,7]. The large pattern of ACE2 expression likely explains the large number of cells that can be infected by SARS-CoV-2, which in turn explains the multitude of the organ affected and symptoms that have been found in COVID-19 patients [8]. These various clinical manifestations show that the infectious pathology COVID-19 is also a systemic disease, that is to say characterized by a diffuse attack simultaneously affecting many tissues and organs. Although most patients will naturally overcome the disease, 5-10% will need hospital care treatments and among those a subgroup of patients with severe COVID-19 will experience “cytokine storm syndrome” referring to the overproduction of immune cells and cytokines, which are associated with a surge of activated immune cells into the lungs, within one to two weeks following the onset of symptoms. This cytokine storm likely dampens innate adaptive immunity against SARS-CoV-2 infection [9]. Among those patients, overweight and obese people are by far over-represented, which prompted us to examine the role of adipose tissue excess in disease outcomes [10].
Body Mass Index and COVID-19: A Link in Disease Severity
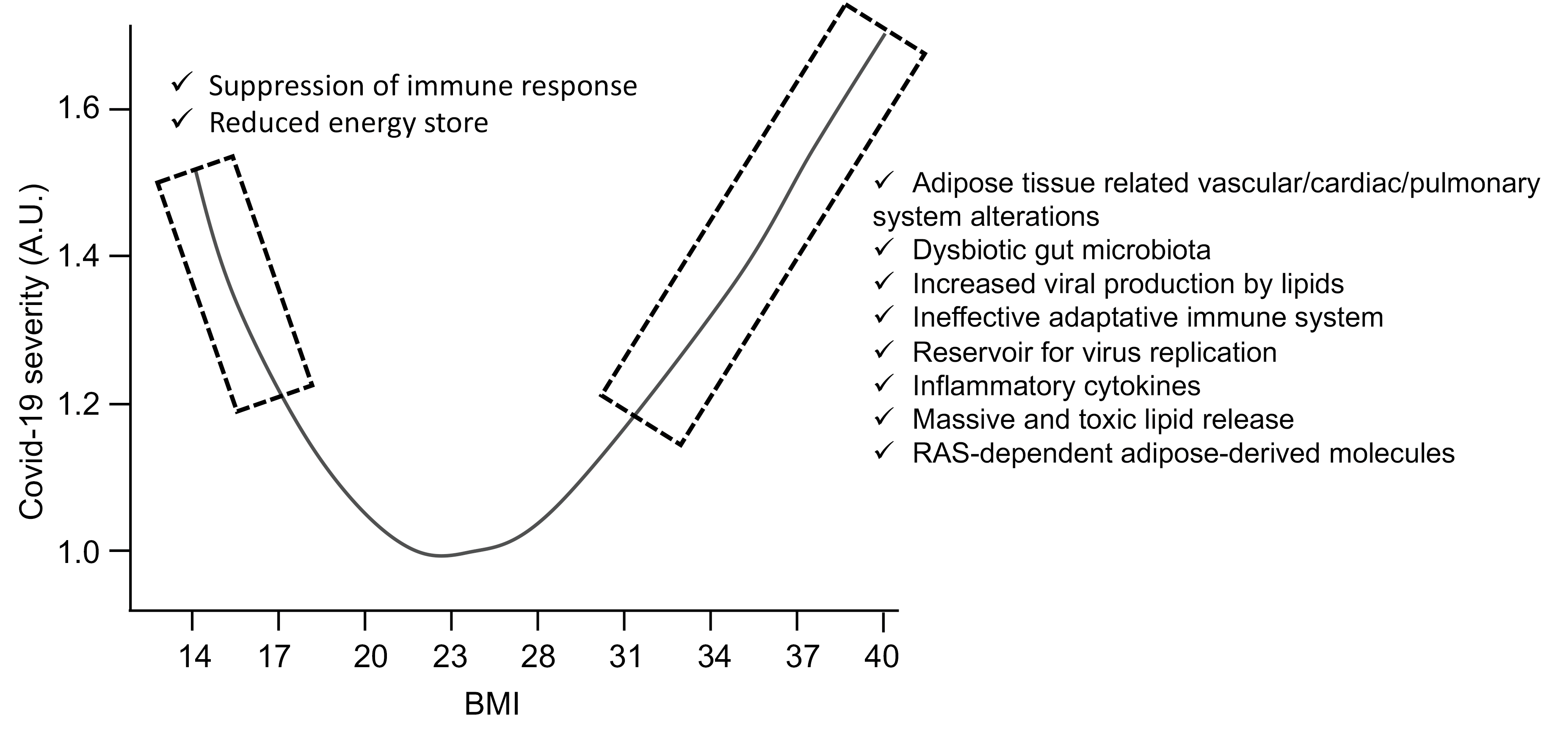
Indeed as of March 2020, clinicians around the world were reporting an unusually high number of obese patients with severe forms of COVID-19. Very quickly, studies were able to show that the prevalence of obesity, or adipositybased chronic disease, was high, between 40% and 50% in intensive care, and that obesity also increased the risk of needing to be placed under mechanical ventilation. The crucial importance of body mass index (BMI, defined as the ratio of kg body weight to square height in meter) was reported worldwide and obesity is now recognized as the second strongest independent predictor for hospitalization after old age [10]. The frequent co-occurrence of obesity with either diabetes mellitus, cardiovascular disease or kidney disease can clearly confound or at least make more difficult the identification of the independent role of obesity in the severity of SARS-CoV-2 infection. Systematic reviews and meta-analysis confirmed an independent contribution of obesity in severity and mortality, but also pointed out that risk stratification and effective control strategies for the COVID-19 should also be done according to comorbidities, age, and gender differences specific to geographical location [11,12]. Altogether these data pointed to the adipose tissue as a potential key player in the severity of COVID-19. It must be also pointed that at the other end of the spectrum, very low BMIs are also associated with an increased risk of a severe form of COVID-19 [13]. Through the analysis of nearly 7 million patients in the UK, it was found that a curve representing the severity over BMI presented a U-shape form, with a BMI of 23 kg/m2 being the lowest part of the curve (Figure 1). In fact, for hospital admissions, risk began to increase linearly above a BMI of 23, whereas the increase in the risk of death began to increase linearly at a slightly higher BMI of 28 [13]. Another important aspect of these findings is that BMI influence is negligeable for the elderly, but important for the young [13]. Hence, among 18-39-yearold, each BMI point increases by 9% the risk of being hospitalized. It would thus be wise that the vaccination policies around the world consider young obese as priority targets when there is a shortage of vaccines.
What are the Connections between Extremes BMI and COVID-19 Severity?
Bodyweight excess is a well-established respiratory disease risk factor and is very often associated with other comorbidity factors in multifactorial pathologies often associated with metabolic inflexibility. On one side, alterations in respiratory mechanisms found in overweighted persons include increased airway resistance, impaired gas exchange, reduced respiratory volume and lower lung muscle strength. This is of particular importance as respiratory failure is one of the most severe symptoms of COVID-19. In the other side, high BMI is closely associated with increased cardiac pathologies, coronaropathy, as well as diabetes, all also at higher risk of severe forms of COVID-19 [10].
Serum levels of ACE2 receptors are higher in the obese patient [14] and are abundantly expressed in the adipose tissue that was thus suggested to represent an additional reservoir of the virus as many other pathogens including several viruses are found in the adipose tissue [15]. The fact that adipose tissues are often located in close vicinity of virus entry sites (respiratory and alimentary tracks) further supports this hypothesis. Another interesting observation is that viruses like Hepatitis C Virus were shown to use the extremely abundant lipid droplets in liver adipocytes adipose as a platform for virus replication and assembly. Although data establishing that viral loads are indeed proportional to adipose tissue mass in patients, these observations suggest that this model is plausible and worth investigating. Of interest, it was recently reported that in a group of COVID-19 patients, the elderly showed a positive linear correlation between BMI and levels of SARS-CoV-2 virus in exhaled aerosol, which may suggest a higher viral load, at least in the lungs of these patients [16].
Lipids generally found in excess in the obese patient can also provide an important source of energy needed for viral replication and are known to play a key role in the various membrane trafficking steps necessary for the completion of the viral cycle [17]. Of note among these lipids, cholesterol and phosphatidic acid (PA) seem to play a particularly important role for numerous viruses. Accordingly, it was recently found that SARS-CoV-2 requires cholesterol for viral entry and the formation of syncytia, a specific structure required for viral fusion after interaction of the viral spike protein and the cellular ACE receptor [18]. In support of the role of cholesterol in severe COVID-19 cases, an association of serum HDL-cholesterol with the risk of severe SARS-CoV-2 infection was recently reported [19]. Furthermore, preprint data also suggest that PA metabolism is vital for the formation of the doublemembrane vesicle replication organelles used by SARSCoV- 2 for viral production [20]. It is, therefore, possible that lipid bioavailability and lipid metabolism alterations occurring in the obese patient may improve key steps of the SARS-CoV-2 life cycle and thus leading to the severity of the disease.
Hypertrophic adipocytes, in particular in the visceral white adipose tissue, recruit polarized macrophages that induce low-grade systemic inflammation through the production of excessive amounts of inflammatory cytokines including several interleukins and TNF-α. Adipose tissue can thus act as a reservoir for the production and secretion of IL-6 which may amplify the cytokine storm and contribute to higher mortality in COVID-19. In addition, the high cytokine levels secreted from hypertrophic fat cells into the bloodstream, including IL-6, can reach the lungs and counteract the termination of the anti-viral immune response and thereby promote a cytokine storm in COVID-19 patients with severe disease development.
Cytokines stimulate lipid mobilization through activated lipolysis by adipocytes triggering the release of large amounts of free fatty acids [21]. This massive cytokinemediated lipolysis could induce a burst of unbuffered circulating free fatty acids in the blood, with detergent-like properties further aggravating virus-mediated cytolysis. In agreement with an alteration of lipid metabolism severity of the disease in COVID-19 patients was positively correlated with a decrease in cholesterol levels [22-23]. Of note the development of this hypolipidemic profile and the severity of COVID-19 that could reflect metabolic deficiency in the liver, the most active lipoprotein producer could also explain why lower BMI was also found to be at higher risk [13]. It is of note that the lowest BMI in the general population has also been associated with reduced life expectancy presumably through greater vulnerability to diseases resulting from reduced energy storage. Indeed, the fat tissue represents the most abundant stock of energy available once the short-lived glycogen has been consumed.
The lipid-derived lipokines that include the eicosanoid family of inflammatory mediators are also recognized as a major player in inflammation and are massively produced by the adipose tissue. They are generated from 20-carbon polyunsaturated fatty acids mostly released from cell membrane phospholipids by the action of phospholipases, especially phospholipase A2 [24]. The adipose tissue uses these lipokines to communicate with distant organs and depending on the type of diet, these lipokines might be anti- or pro-inflammatory.
The adipose tissue has been more recently appreciated as an endocrine organ secreting hormones such as Plasminogen Activator Inhibitor-1 (PAI-1) a serine protease inhibitor inactivating urokinase and plasminogen activators [25]. Interestingly, PAI-1 adipocyte production is higher in visceral adipose depots [26], which may favor thrombosis often associated with COVID-19 severity. Leptin is another hormone secreted predominantly by the adipose tissue. In addition to its role in energy regulation, leptin promotes inflammatory reactions by acting on the leptin receptor present on immune cells of both the innate and adaptive immune system. Leptin increases monocyte and macrophage proliferation and thus increases the level of immune factors and cytokines such as TNF-α and some interleukins. Increased leptin levels of patients with obesity thus also contribute to chronic low-grade inflammation associated with obesity. Conversely low levels of leptin in malnourished people, like often found for elderly people, suppresses the immune response and makes these people more susceptible to infections.
SARS-CoV-2 infection also causes a significant reduction in circulating lymphocytes and T-cells. The activation and function of CD4+ and CD8+ T-cells are reduced in people with overweight or obesity. Metabolic changes in T-cells associated with an impaired memory T-cell response were observed, which makes people with obesity more prone to reinfection suggesting that an alteration of the adaptative immune system is also likely to explain some part of the increased vulnerability of overweight people to the disease. Obesity is accompanied by low-level inflammation starting from the adipose tissue but progressing to many other metabolic organs. Hence as discussed previously obese patients start with an already challenged immune system, which could explain the over-amplified cytokine storm response of the immune system [10].
The microbiota is another aspect that has more recently been suspected to be linked with severe forms of COVID-19. Some people have a pathological microbiota and a more permeable intestinal barrier, which promotes the passage of the virus through the intestine, through a digestive gateway. Although this is only one hypothesis, it is likely that obese patients display more frequently an alteration of their intestinal barrier. Furthermore, it is now well established that obesity is associated with gut microbiota dysbiosis, associated with reduced diversity in bacterial genes [27]. As gut microbes are also active producers of host metabolites, an appealing hypothesis could be that some of them participate in inappropriate viral responses to SARS-CoV-2 in obesity.
Finally, the adipose tissue is also a potential link between obesity and hypertension as it expresses all the proteins of the RAS system. Furthermore, the adipose tissue RAS regulates the expression of adipose tissue-derived endocrine factors including prostacyclin, nitric oxide, PAI- 1, and leptin [28].
Conclusion and Perspectives
Although vaccination remains the most effective strategy to reduce contamination among the population and the load on intensive care units, the current shortage of vaccines worldwide imposes alternative options in health care politics. For instance, the development of effective treatments is still a major goal and this includes specific treatments for high-risk patients among the obese population. As reviewed here and elsewhere [10,29-32], deviation from a BMI value of 23 increases the risk of severe forms of COVID-19 either through the metabolic consequences of undernutrition or through the many consequences of excess adipose tissue. Mediterranean diet has been proposed to be the base for nutrition to reduce the severity of severe COVID-19 [33]. Other lifestyle interventions, including physical activity, may also prove to be beneficial, not only by improving the general health status of the population, but also indirectly by reducing the deleterious effect of the fat tissue listed here. Unfortunately, the succession of confinements and restrictions associated with anxious communication by the media have worsened the situation as the general population has gained weight. The current reduction in restriction opens for some hope on this side too and we believe that the health authorities should now take advantage of the situation to address the obesity issue with non-anxious messages.
Key future research priorities will be to establish whether BMI affects vaccine efficacy, and to understand whether people outside the BMI range considered to be healthy (18–25 kg/m2) are at increased risk of post-COVID-19 sequelae. Further careful epidemiological study of these and other emerging questions, including vaccine efficiency among obesity will inform the ongoing public health response to this new disease that is likely here to stay.
Acknowledgments
This work was supported by grants from the Fondation pour la Recherche Médicale (DEI20151234424) and the Agence Nationale pour la Recherche (ANR-19-CE44-0019) to N.V.
Conflict of Interest
The author declares no conflict of interest.
References
2. Harrison EL, Hinson RE, McKee SA (2009)Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav 34: 484-486.
3. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015:1-23.
4. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 Nov;426(6965):450-4.
5. Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome biology. 2003 Aug;4(8):1-5.
6. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell. 2020 Apr 16;181(2):271- 80.
7. Niu L, Wittrock KN, Clabaugh GC, Srivastava V, Cho MW. A Structural Landscape of Neutralizing Antibodies Against SARS-CoV-2 Receptor Binding Domain. Frontiers in Immunology. 2021;12.
8. Delorey TM, Ziegler CG, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021 Apr 29:1-8.
9. Vaninov N. In the eye of the COVID-19 cytokine storm. Nature Reviews Immunology. 2020 May;20(5):277.
10. Dugail I, Amri EZ, Vitale N. High prevalence for obesity in severe COVID-19: Possible links and perspectives towards patient stratification. Biochimie. 2020 Dec 1;179:257-65.
11. Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta-analysis of obesity and COVID-19 outcomes. Scientific reports. 2021 Mar 30;11(1):1-1.
12. Thakur B, Dubey P, Benitez J, Torres JP, Reddy S, Shokar N, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Scientific reports. 2021 Apr 20;11(1):1-3.
13. Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, et al. Associations between bodymass index and COVID-19 severity in 6· 9 million people in England: a prospective, community-based, cohort study. The Lancet Diabetes & Endocrinology. 2021 Jun 1;9(6):350-9.
14. Emilsson V, Gudmundsson EF, Aspelund T, Jonsson BG, Gudjonsson A, Launer LJ, et al. Serum levels of ACE2 are higher in patients with obesity and diabetes. Obesity Science & Practice. 2021 Apr;7(2):239-43.
15. Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, et al. Specific biological features of adipose tissue, and their impact on HIV persistence. Frontiers in microbiology. 2019 Dec 17;10:2837.
16. Edwards DA, Ausiello D, Salzman J, Devlin T, Langer R, Beddingfield BJ, et al. Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proceedings of the National Academy of Sciences. 2021 Feb 23;118(8).
17. Ammar MR, Kassas N, Chasserot-Golaz S, Bader MF, Vitale N. Lipids in regulated exocytosis: what are they doing?. Frontiers in Endocrinology. 2013 Sep 17;4:125.
18. Sanders DW, Jumper CC, Ackerman PJ, Bracha D, Donlic A, Kim H, et al. SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation. ELife. 2021 Apr 23;10:e65962.
19. Hilser JR, Han Y, Biswas S, Gukasyan J, Cai Z, Zhu R, et al. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. Journal of Lipid Research. 2021 Jan 1;62.
20. Tabata K, Prasad V, Paul D, Lee JY, Pham MT, Twu WI, et al. Convergent use of phosphatidic acid for Hepatitis C virus and SARS-CoV-2 replication organelle formation. bioRxiv. 2021 Jan 1.
21. Grant RW, Stephens JM. Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. American Journal of Physiology-Endocrinology and Metabolism. 2015 Aug 1;309(3):E205-13.
22. Fan J, Wang H, Ye G, Cao X, Xu X, Tan W, et al. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020 Jun 1;107:154243.
23. Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID-19. Journal of Clinical Lipidology. 2020 May 1;14(3):297-304.
24. Thomas MH, Pelleieux S, Vitale N, Olivier JL. Dietary arachidonic acid as a risk factor for age-associated neurodegenerative diseases: potential mechanisms. Biochimie. 2016 Nov 1;130:168-77.
25. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. The Journal of Clinical Endocrinology & Metabolism. 2004 Jun 1;89(6):2548-56.
26. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004 May 1;145(5):2273-82.
27. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013 Aug;500(7464):585- 8.
28. Goossens GH, Blaak EE, Van Baak MA. Possible involvement of the adipose tissue renin-angiotensin system in the pathophysiology of obesity and obesityrelated disorders. Obesity Reviews. 2003 Feb;4(1):43-55.
29. de Leeuw AJ, Luttikhuis MA, Wellen AC, Müller C, Calkhoven CF. Obesity and its impact on COVID-19. Journal of Molecular Medicine. 2021 Apr 6:1-7.
30. Demeulemeester F, de Punder K, van Heijningen M, van Doesburg F. Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review. Cells. 2021 Apr;10(4):933.
31. Helvaci N, Eyupoglu ND, Karabulut E, Yildiz BO. Prevalence of obesity and its impact on outcome in patients with COVID-19: a systematic review and meta-analysis. Frontiers in Endocrinology. 2021;12:82.
32. Mohammad S, Aziz R, Al Mahri S, Malik SS, Haji E, Khan AH, et al. Obesity and COVID-19: what makes obese host so vulnerable?. Immunity & Ageing. 2021 Dec;18(1):1.
33. Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020 May;12(5):1466.