Abstract
Zinc oxide nanoparticles (ZnO-NPs) are odorless, white, and insoluble in water and alcohol. There are various applications for ZnO due to its unique semiconducting, piezoelectric, and optical features. Biodegradability and low toxicity are important properties of ZnO nonmaterial. The OH groups on the ZnO surface allow different surface decorating molecules to easily functionalize it. Mineralized in muscle, brain, skin, bone, and other tissues, zinc is a necessary mineral. It is a major component of most enzyme systems and is involved in protein and macromolecule production, neurogenesis, and hematopoiesis, among other functions. Due to its small particle size, Nano-ZnO is easily absorbed by the body. So-called nano-ZnO is commonly utilized in food additives. Chemical surface properties, particle shape, size distribution, and particle reactivity in solution all influence the biological activity of nanoparticles. Determining the morphology, size, and functioning of nanoparticles with regulated structures is critical for biological applications. In this review article, biological and non-conventional synthesis and applications of ZnO-NPs are highlighted in terms of their biological activity. ZnO-NPs have shown therapeutic activity against cancer, diabetes, microbial infection, inflammation, and potential antioxidant activity, which can be used as drug carriers, imaging tools, and biosensors.
Keywords
Nanoparticle, Zinc oxide, Biological activity, Agriculture, Biomedical.
Introduction
In nature, nanoparticles can be found as natural or synthetic. Nanoparticles may form either physically or chemically. Nanoparticles are uniform types of common material whose dimensions are less than 100 nm [1]. Volcanic ash, ocean spray, fine sand, and dust are some examples of natural nanoparticles. Synthetic nanoparticles, also known as anthropogenic nanoparticles, are engineered nanoparticles that are specifically designed to study various physiochemical features for research purposes. According to various studies in synthetic nanoparticles, the ratio of amounts of atoms is not equal on the inside and outside of the nanoparticle, i.e., the high surface to volume ratio, which makes nanoparticle research an interesting branch of science in recent years. The maximum availability of atoms on the surface turns the nanoparticle into distinctive physical and chemical properties such as high surface reactivity, high damping property, high mechanical stability, and high potency with superior thermal conductivity. From the analytical research point of view, the nanoparticles can be classified into various categories, namely metal nanoparticles (e. g. gold and silver nanoparticles), metal oxide nanoparticles (e.g., zinc oxide, magnesium oxide), carbon nanoparticles and quantum dots (e.g., Cadmium Selenide) [2].
Nanoparticle research is a subset of nanotechnology, which has advanced into modern biological and medical implications and may soon transform our daily lives, from the size of our electronic devices to the path for disease diagnosis and treatment. is also a nanoparticle that belongs to the biomedical application of nanotechnology. Chemotherapy, radiation therapy, and surgery were once the first choices for cancer treatment. However, they all have deleterious effects on the cells and, due to their non-selective effects on the cells, may also impact normal cells. Considering the circumstances, manufactured nanoparticles may prove to be a viable alternative for treating a variety of ailments as they are efficient against disease cells as well as cancer cells without causing toxicological harm to the body system [3]. Metal-based drugs, which include inorganic nanomaterials in general, have been investigated as a potential next-generation nanomedicine, viz., iron oxide (Fe3O4 or -Fe2O3), titanium dioxide (TiO2), cerium dioxide (CeO2), copper oxide (Cu2O or CuO), silica (SiO2), gold, silver, platinum, and zinc oxide (ZnO) nanoparticles (NPs) [4]. The physicochemical properties of ZnO-NPs, including mechanical, electrical, optical, magnetic, and chemical sensing properties, distinguish ZnO-NPs as a versatile amphoteric semiconductor material that garners considerable attention among other nanoparticles [5]. ZnO is a white granular n-type semiconductor with no odor and has a band gap of about 3.37 eV at room temperature. The excitation binding energy is about 60 MeV. It is not soluble in water and alcohol but is soluble in all acids and alkalis. Due to their large surface area [6], ZnO-NPs present a notable achievement in catalytic activity. The morphological behavior of such materials allows them to open a wide new range of possibilities in the field of nanobiotechnology [6].
Because of their low toxicity and biodegradability [7], ZnO-NPs have unique properties in the treatment of some diseases. In comparison to other nanoparticles, ZnO-NPs have biocompatibility, bioavailability, and high solubility. These properties cause ZnO-NPs to retain an alternative biomolecular activity in a variety of body systems and can be localized to control cellular cycle activity [8]. Such nanoparticle interactions can be evaluated under several biological systems. In the present review, we summarized the studies related to biological and non-conventional synthesis of ZnO-NPs and the therapeutic strategies regarding uses of synthesized ZnO-NPs in various fields.
Synthesis Techniques for ZnO-NPs
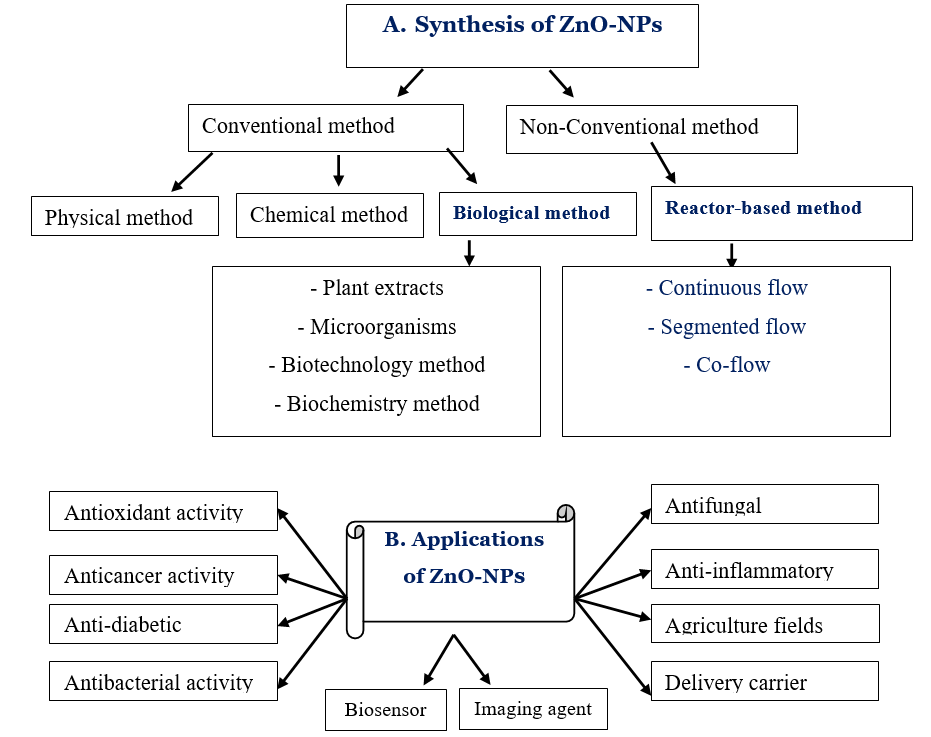
Conventional and unconventional methods can both be used to make zinc oxide nanoparticles (ZNPs) [9]. Conventional methods of synthesis include physical, chemical, and biological (green) synthesis, whereas non-conventional methods include microfluidic reactor-based synthesis. Biological approaches (green synthesis) and microfluidic reactor-based methods have prospective alternatives to physical and chemical procedures since they are environmentally friendly, non-toxic (safer), reproducible, and promising (Figure 1).
Figure 1. Schematic diagram of synthesis of ZnO-NPs with special reference to biological and non-conventional synthesis (A) and their potential applications (B).
Biological Methods for ZnO-NPs Synthesis
Biological approaches are promising non-toxic alternatives to physical and chemical synthesis [10]. The biological synthesis of ZnO-NPs has used microorganisms (bacteria, fungi, algae, and bacteriophage), plant extract DNA, and proteins [11]. The methods of biological synthesis of ZnO-NPs are still unknown.
Some bacteria utilizing specific enzymes can produce ZnO-NPs. The antibacterial agents ZnO-NPs (10-95 nm) have been synthesized by bacteria such as Bacillus megaterium, Sphingobacterium thalpophilum, Staphylococcus aureus, Halomonas elongata, Candida albicans, and Aspergillus niger [12–15]. The 61 nm and 25 nm ZnO-NPs produced by fungi can be employed as antibacterial agents and for steroidal pyrazoline production. They have been shown to synthesize ZnO-NPs in Pichia fermentans JA2 and Pichia kudriavzevii [16]. In yeast, the antibacterial agent’s hexagonal wurtzite and smooth/elongated ZnO-NPs (10-61 nm) were generated. Both Sargassum muticum and Chlamydomonas reinhardtii have been utilized to produce ZnO-NPs. Their nanoflowers (55-80 nm HR-SEM; 21 nmXRD) and hexagonal wurtzite NPs (FE-SEM; 42 nmXRD) show green chemistry is gaining popularity due to its environmental responsiveness and its usage in the biological synthesis of metal oxide NPs [17]. These approaches are claimed to be more eco-friendly, cost-effective, nontoxic, and biocompatible than chemical and physical methods. This method's ZnO-NPs have outstanding anticancer and antibacterial action and can be used in solar photocatalysis, dye photodegradation, and antimicrobial agents. Egg albumin was used to produce spherical and hexagonal wurtzite ZnO-NPs and the NPs were 16 nm (XRD), 10-20 nm (TEM), and 8-22 nm (TEM) (AFM) [18]. Gelatin was also employed to manufacture 20 nm ZnO-NPs (Zn, 59.10%; O, 28.93%) with antibacterial and anti-angiogenic properties [19].
Non-Conventional Method: The Microfluidic Reactor-Based Synthesis
Microfluidic reactors are employed for controlled, non-conventional synthesis of nanoparticles, which may be exploited for bench-top material production [20]. Kang et al. [21] established a continuous and efficient microfluidic reactor-based synthetic process, which was implemented using a time-pulsed mixing approach that had been refined using numerical simulations and experimental methodologies. Since the microfluidic reactor mixes reactants on a microscale, viscosity is the dominant factor determining flow rather than inertial forces (low Reynolds number 102) [22]. The microfluidic ZnO-NP production provides improved control of the reaction [23]. In the microfluidic environment, mixing happens through diffusion and laminar flow. The reaction temperature should also be precisely regulated in a microfluidic reactor when manufacturing NPs (73/100). In the microfluidic reactor’s microchannel, the reactions are controlled by reducing agents and metal salts at low temperatures (15-20°C). Dynamic precursors are produced via reduction after the reactants are combined. Finally, NP nucleation and growth occur at a higher temperature (80-90°C). To prepare high-quality NPs with a high degree of crystallinity and limited size distribution, each step of nanoparticle formation should be controlled within a narrow time frame and terminated at the required stage. Microfluidic reactor technologies provide various advantages in NP synthesis [24]. Compared with conventional techniques of synthesis, a microfluidic reactor employs tiny reagent amounts and offers selectivity, environmental friendliness, short reaction time, a small footprint, and better safety [25]. Specifically, controlling the flow (e.g., continuous, or segmented flows) alters the reaction conditions (e.g., temperature, time, and reagent concentrations) in the microfluidic reactor to produce high-quality products with improved characteristics and enhanced performance [26]. Metal oxide NPs, semiconductors, and quantum dots (QDs) are common products of microfluidic reactor-based synthesis. Microfluidic reactor systems have also been used to manufacture ZnO-NPs. In a microfluidic reactor, ZnO-NPs/nanowires were produced using a hydrothermal technique [26]. Joo et al. [27] have revealed the bottom-up device fabrication for creating ZnO nanowires in a continuous flow from ZnO seeds. A segmented flow was used for the synthesis of ZnO-NPs, which formed microfluidic segments of droplet-like tiny reaction mixture sections at high flow rates [28]. Using a static micromixer, they boosted internal convection by boosting heat exchange between the reaction mixture and the channel environment. The microfluidic synthesized ZnO-NPs had strong preservability and stability in working solution, and the synthetic microfluidic system provides a low-cost, environmentally friendly method for the continuous [24].
Potential Applications oF ZnO-NPS
Therapeutic biological activity of ZnO-NPs
The Food and Drug Administration (FDA) has designated ZnO-NPs as a 'GRAS' (Generally Recognized As Safe) material and has been recognized as an effective anticancer therapy [29]. As ZnO-NPs are low in toxicity, they can be used in a variety of biological applications such as antioxidant, antibacterial, antifungal, anticancer, anti-diabetic, and agricultural applications [30].
Antioxidant activity: The electron contribution of an oxygen atom contributes to the antioxidative characteristics of ZnO-NPs. ZnO-NPs can be used as a powerful antioxidant and an important additional treatment for chemotherapeutic drugs that induce male reproductive failure [31]. This property enables ZnO-NPs to maximize doxorubicin's potential therapeutic efficacy while minimizing its gonadotoxic effects. Furthermore, numerous studies have revealed that ZnO-NPs can heal oxidative damage and glutathione depletion. Other studies have found zinc in the male reproductive system, which has the potential to increase reproductive function and seminal fluid. As a result, Zn is an essential component of the spermatogenesis process [32]. The ability of the produced ZnO-NPs can be very beneficial in the therapeutic treatment of many diseases caused by oxidative stress. Antioxidant molecules can eliminate excess free radicals in the body. When the body produces an overabundance of free radicals, ZnO-NPs can help to boost the body's antioxidant system. Because free radicals can harm biomolecules in the body and promote oxidative stress, they should be avoided [33]. ZnO-NPs can boost antioxidant enzyme levels while decreasing malondialdehyde (MDA) [34]. As a result, such nanoparticles have the potential to protect the cell membrane properties of the body system from oxidative stress damage. ZnO-NPs have strong antioxidant properties and can scavenge free radicals up to 91% in 90 minutes [35]. In the same context, ZnO-NPs can cause oxidative stress, apoptosis in the liver, and DNA damage if exposed to them orally [36]. Lower dosages of ZnO-NPs have cytoprotective effects, which lead to an increase in antioxidant activity and a reduction in excess free radicals caused by Aflatoxin B1 [37]. Through free radical scavenging, such nanoparticles can protect hepatic cells from the ravages of Aflatoxin B1. According to a study, there is a link between dietary aflatoxin penetration and liver cancer in humans [38].
Anticancer activity: Cancer is an uncontrolled division of malignant cells that is traditionally treated by radiotherapy, surgery, and chemotherapy. These traditional methods are effective against malignant cells, but they cause serious side effects on the nearby non-malignant cells as these methods are non-selective [39]. To overcome the relative side effects, we need to explore nanomaterial-based nanomedicine since they have high surface functionality, high biocompatibility, targeted drug delivery capacity, and cancer targeting. Taking the advantages into account, ZnO-NPs can be chosen as a promising nanomaterial platform to cure or treat malignant cells [40]. In vivo, Zn2+ is an essential trace element for humans, and it was considered safe in vivo [41]. ZnO-NPs have selective cytotoxicity among malignant cells compared to non-malignant cells. They have the ability to destroy malignant cells selectively in vitro and in vivo as well through arguing selective localization [42]. The nanosize and high surface reactivity characteristics of ZnO-NPs allow them to penetrate through the blood stream towards the malignant cells, hence acting as cytotoxicity against these cells. Due to nanosized, they can localize specifically inside the malignant cells [43]. ZnO-NPs show antiproliferative effects and this nanoparticle can treat human gastric adenocarcinoma cell lines, resulting in the use of ZnO-NPs as a promising anticancer agent in comparison to traditional therapy [44]. Such research findings suggested that a zinc deficiency could lead to cancer and make healthy cells unstable. ZnO-NPs modified with Polyethylene glycol (PEG) to treat different breast cancer cells and found such nanoparticles are very effective against different breast cancers [45]. These nanoparticles have the ability to destroy cancer cells by inducing reactive oxygen species and activating p53-dependent apoptosis, causing cell death. The anticancer properties of ZnO-NPs against human lung cancer showed that by generating reactive oxygen species, lung cancer proliferation can be inhibited, and indicated that ZnO-NPs also show genotoxic effects against lung cancer, which can be concluded that ZnO-NPs are a novel therapeutic anticancer agent [46]. The cytotoxicity effect of ZnO-NPs against ovarian cancer cells shows significant apoptosis and cytotoxicity of ovarian malignant cell SKOV3 through inducing reactive oxygen species and oxidative stress [47]. Sharma et al. [48] reported the pharmacological mechanism of ZnO-NPs on HepG2 cells, which is a human liver cancer cell. They found high cytotoxicity and genotoxicity against HepG2 cells when exposed to ZnO-NPs. The anticancer ability of ZnO-NPs shows inhibition of proliferation of fibrosarcoma HT1080 cells and suggests the phenomenon of autophagy by inducing reactive oxygen species in cancer cells [49]. In relation to autophagy and the regulatory mechanisms involved in autophagy, studies have shown that a decrease in autophagic effluent in A549 cells can result in an accumulation of autophagosomes [50]. The dissolution of ZnO-NPs correlated with autophagy generation in lysosomes and removing zinc ions from ZnO-NPs increased their capacity to destroy lysosomes, impairing autophagy flux and mitochondria, and excess production of reactive oxygen species causing cell death [50]. Overall, the ZnO-NPs contain the potency of anticancer activity.
Anti-diabetic activity: Diabetes affects people all over the world. According to reports, type-II diabetes patients outnumber type-I diabetes patients [51]. Zinc, an essential trace micronutrient, plays a role in insulin storage, secretion, and biosynthesis [52]. Zn is essential for the release of insulin from pancreatic cells [53]. As a result, ZnO-NPs have the potential to operate as an anti-diabetic drug in the treatment of diabetes mellitus. The anti-diabetic effects of ZnO-NPs and zinc sulphate on diabetic rats with high dosages outperform ZnSO4 in anti-diabetic effects [54]. Hussain et al. [55] employed HEC to make ZnO-NPs for diabetic complications research. These nanoparticles lower blood sugar, malondialdehyde, and asymmetric dimethylarginine (ADMA). Bayrami et al. [56] produced ZnO-NPs and evaluated their anti-diabetic efficacy, finding a large increase in high lipoprotein levels as well as a rapid decrease in blood sugar. Amiri et al. [57] produced ZnO-NPs containing thiamine, and the results demonstrate that diabetes therapy is enhanced. ZnO-NPs were very effective to reversing diabetes-induced pancreatic damage [58]. ZnO-NPs can improve glucose mitigation, serum insulin production, and blood sugar control [59]. ZnO-NPs mediated diabetic groups enhance insulin levels in serum and that these nanoparticles did not influence hyglycemia in living cells, implying that it might be employed as an insulin secretor [59]. Vilagliptin and ZnO-NPs had a significant effect on both type 1 and type 2 diabetes therapy, showing that such nanoparticles are significant anti-diabetic drugs [60]. Since ZnO-NPs aid in the lowering of high blood sugar levels, they have been identified as major potent anti-diabetic agents, and in the future, they may aid in the discovery of a suitable promising treatment against diabetes mellitus.
Antibacterial activity: The "American Heritage Medical Dictionary, 2007" defines antibacterial activity as "the action through which the rate of expansion of a bacterial colony is killed or inhibited." Antibacterial activity on microbes is essentially a surface effective function that is not toxic to the host [61]. Antibacterial drugs with proper dosages have the capacity to harm bacterial growth. As a result, antibacterial drugs can be used to prevent bacterial infection as well as to act as chemotherapeutic agents. If these chemicals can kill bacteria, they are said to be bactericidal [62]. ZnO-NPs are effective against the growth of both gram-positive and gram-negative bacteria, and ZnO-NPs can reduce the growth rate of bacteria, making ZnO-NPs potent antibacterial agents [63]. The high sensitivity type of bacteria with a lipid bilayer permits ZnO-NPs to be an efficient antibacterial agent [64].
ZnO-NPs are suitable antibacterial materials due to their great properties, such as their strong ability to inhibit pathogenic agents and their high surface reactive area [65]. ZnO-NPs have an antibacterial toxicity mechanism and have the ability to stimulate the development of excess reactive oxygen species such as hydroxyl radicals, superoxide anion, and hydrogen peroxide [66]. ZnO-NPs have antibacterial properties against E. coli and an average size of 30 nm can disrupt membrane integrity by interacting with the phospholipid bilayer of the bacterial membrane [67]. The antibacterial activity of ZnO-NPs can be inhibited by the augmentation of radical scavengers such as vitamin E, mannitol, and glutathione. It demonstrates that the generation of reactive oxygen species plays a significant role in the antibacterial activities of ZnO-NPs [67]. While studies on ZnO-NPs against Vibrio cholerae using two biotypes of cholera bacteria, classical and El Tor, reported that ZnO-NPs are more effective in resisting the rate of growth of the El Tor biotype of Vibrio cholerae, which is closely associated with the creation of reactive oxygen species. ZnO-NPs can increase permeability, damage bacterial membranes, and quickly affect bacterial conformational shape [68]. ZnO-NPs have a photocatalytic property, which makes them good antibacterial agents. It is employed in the food sector because of its feature that provides antibacterial activity for food packaging [62]. ZnO-NPs can be utilized as a coating material to prevent germs from spreading, adhering, and reproducing on a variety of surfaces, including medical devices.
Fungicidal activity: Plant pathogen growth is the primary cause of economic loss during crop and fruit harvesting [69]. It is difficult to control the rate of growth of a fungal pathogen. To solve this challenge, antifungal agents must be investigated using appropriate methodologies. Nanomaterials have developed physical and chemical properties in recent years, resulting in relative equivalents [70]. The preparation of various nanoparticles contributes significantly to the development of antifungal activity against various plant diseases or infections. Nanoparticles have a unique ability to boost antifungal effectiveness against plant pathogens [71]. Nanoparticles such as Zn and Cu can exhibit a wide variety of antibacterial actions against fungal [72] and bacterial species [73]. In comparison to other organic nanoparticles, inorganic nanoparticles such as ZnO-NPs have superior heat resistance, selectivity, and durability [74]. He et al. [75] studied the antifungal activity of ZnO-NPs against two postharvest pathogenic fungi, Botrytis cinerea and Penicillium expansum. At a low dosage of 3 mM, the antifungal activity against both B. cinerea and P. expansum is substantial. Increasing the concentration of ZnO-NPs from 3 mM to 12 mM improved experiment efficiency and showed that P. expansum is more susceptible to ZnO-NPs treatment than B. cinerea [75]. F. graminearum, a wheat pathogen, is toxic to ZnO-NPs [76]. ZnO-NPs had antifungal action against three different phytopathogenic fungi: R. solani, A. alternative, and B. cinerea [77]. ZnO-NPs are highly effective against S. cerevisiae and have potential antibacterial action and fungicides capable of enhancing or obscuring their effectiveness against plant pathogens. [78]. ZnO-NPs have antifungal activity against the fungus Erythricium salmonicolor, a causative agent of pink disease (wilting and yellowing of the leaves, fruits, and stems). The ZnO-NPs with diameters ranging from 20–45 nm have a substantial effect on the growth rate of the fungus E. salmonicolor [79]. Hui et al. [80] made flower-shaped ZnO-NPs that killed harmful fungi and showed that the structure and shape of nanomaterials can affect antifungal activity in many ways.
Anti-inflammatory activity: In reaction to pathogens or chemicals, ZnO-NPs have anti-inflammatory effect [81]. ZnO-NPs are effective at reducing inflammation by (i) inhibiting the production of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-18 in activated mast cells and macrophages by inhibiting NF-κB and caspase 1; (ii) inhibiting mast cell proliferation by increasing p53 and decreasing thymic stromal lymphopoietin production related to IL-13, a TH2 cytokine [81]. Titanium dioxide (TiO2) nanotubes implanted in ZnO-NP shows antibacterial and anti-inflammatory properties [82]. Wiegand et al. [83] developed a ZnO-functionalized textile (Benevit Zink+, Benevit Van Clewe, Dingden, Germany) composed of 74% Lyocell fiber, 19% SmartCell sensitive fiber, and 7% spandex. This ZnO-functionalized textile improved antioxidative capacity and inhibited bacterial development on atopic dermatitis patients' skin. Ibuprofen, an anti-inflammatory drug, was kept inside and between the pores of ZnO-NPs in a magnesium/epoxy resin-ZnO/polycaprolactone microstructure with multiple functions [84].
Impact of ZnO-NPs in agricultural field: The introduction of nanotechnology has significantly contributed in different sectors to the improvement of different applications [85]. Agricultural land can be considered as the source of major nanoparticles based on various minerals. Nanotechnology has significant potential in the enhancement of nutrient delivery, quality, food safety, product traceability, as well as in the improvement of agricultural and food processing [86]. Agriculture is considered the backbone of third world economies, but now it is facing several problems, including urbanization, sustainable uses of resources, climate change, global warming, etc. Due to the increasing human population, food demand is increasing rapidly day by day. Therefore, we need to adopt efficient skills for the sustainable development of agriculture [87]. In this case, nanotechnology has an emphatic position in the alteration of agriculture and food products [88]. ZnO-NPs have the potential to enhance the growth and yield of food crops. The treatment of peanut seeds with ZnO-NPs has been shown to increase the rate of growth and development of peanut roots and stems [89]. Improved growth rates of cucumbers were observed when treated with a mixture of soil and ZnO-NPs [90]. While studying the growth rate of mung bean and chickpea with ZnO-NPs at low concentrations, Mahajan et al. [91] observed an improvement in root and shoot for mung bean and chickpea at concentrations of 20 mgL-1 and 1 mgL-1, respectively. Other than optimal concentration, there is no significant growth in roots and shoots observed [91]. Improved root elongation of germinated radish and rapeseed was observed when treated with ZnO-NPs at a concentration of 2 mgL-1. Similarly, improved growth of ryegrass was observed when treated with ZnO-NPs at the same concentration [92]. Starch, total protein, dry mass, and oil levels are related to effective photosynthesis in ZnO-NPs-mediated wheat and maize [93]. ZnO can be used as a fertiliser for the deficiency of Zn in soils [94]. Hence, the colloidal solution of ZnO-NPs can be used as fertilizer. Such nanoparticles play an emphatic role in agriculture. The nanoparticle-based fertilizer is a plant micronutrient [88]. Cucumber growth can be accelerated by ZnO-mediated nanosize particles [95]. ZnO-NPs treated tomato plants' roots at a concentration of 8 mgL-1 show a great enhancement of photosynthetic efficiency [96]. Despite the intense enhancement of nanotechnology, there is no sustainable competition in the agricultural sector. Regarding the current situation, the agriculture sector must be enhanced to minimize food demand. In this case, the nanoparticles can explore or help the way by providing suitable nutrition for the plants to increase the production rate of food for the global population [97].
Delivery carrier
Due to their safety for human consumption [98], ZnO-NPs have also been investigated as drug delivery carriers, treatments, and diagnostics for human biomedical uses. The mesoporous ZnO-graphene oxide (GO) nanoparticles coupled with TiO2 nanoparticles might be employed for colon medication delivery. This is referred to as TiO2@ZnO-GO NPs. The TiO2@ZnO-GO NPs released drugs in a pH-dependent manner. The rate of drug release was faster at a neutral pH than at an acidic pH. Daunorubicin, doxorubicin, and plasmid DNA were covalently linked to ZnO-NPs to enable the delivery of cancer therapies to particular areas of the body [99]. Additionally, ZnO-NPs have been employed in the treatment of bacterial infections, diabetes, wounds, and inflammation [100]. ZnO nanostructures have been utilized to fabricate biosensors such as nanowires capable of detecting glucose and core-shell nanorods capable of detecting ultraviolet radiation and hydrogen [101]. These ZnO nanotherapeutics, which can be regulated in terms of particle size and porosity, are beneficial for biomedical applications such as drug delivery and therapeutic activity.
Biosensors
Biomedical diagnostic sensors based on ZnO-NPs have been developed for the detection of gases and biochemicals [102]. Internal surface adsorption is dependent on pore characteristics in gas sensors because they enable adsorbates to reach internal surfaces. Using ZnO-NPs gas sensors, they were able to detect ethanol and acetone swiftly and accurately [103]. Even in high relative humidity settings (>85%), Mn-doped ZnO-NPs can detect acetone as low as two parts per million (ppm). For the health of mice and diabetic canines, zinc oxide nanorod field-effect transistors (FETs) were used to assess glucose, cholesterol, and urea levels in the animals' blood [104]. For optical and impedimetric experiments, gold (Au)-ZnO hybrid NP films were used [105]. Zinc oxide electrodes on flexible porous polyimide substrates were made [106] so that cardiac troponin could be found.
Imaging Agents
Quantum dots (QDs) are semiconductor nanoparticles with unique optical and electrical characteristics and fluorescence for bioimaging applications (1–10 nm) [107]. Photoluminescent quantum yields are increased and protected against photobleaching in core-shell arrangements [107]. Nanospheres of SiO2 (150-200 nm) were coated with ZnO QDs (photoluminescent ZnO QDs, 3–4 nm) for bioimaging applications. QDs can be employed for imaging and medication delivery in pharmaceutical and biological applications [107-109]. For near-infrared excitation, nanocrystals with folic acid-modified ZnO nanocrystals have been used [110].
A recent study demonstrated that co-exposure of ZnO-NPs with GNs decreased the accumulation of Zn. The accumulation and removal of Zn from ZnO-NPs in blackfish (Capoeta fusca) is dependent on tissue, exposure concentration, and time, as well as graphene nanosheets (GN) [111]. Furthermore, chemically synthetized ZnO-NPs have been recognized as the strongest photocatalysts [112]. Recent studies have revealed that nanomaterials containing zinc, in particular zinc oxide nanoparticles (ZnO-NPs), are becoming increasingly desirable as innovative agents for medical applications, even though the significance of zinc as a trace element in the human body has been overlooked for a long time. Zinc oxide is biocompatible, which means that its antibacterial, antifungal, antiviral, and anticancer properties can be used in medicine [113].
Conclusion
Due to their diverse properties, functions, and applications, ZnO-NPs are one of the most versatile substances. ZnO-NPs can be produced via conventional (physical, chemical, and biological) and unconventional (microfluidic reactor-based) methods. Green synthesis of ZnO NPs is far safer and more ecologically friendly than chemical synthesis since it does not produce hazardous byproduct molecules. As colloidal solutions of ZnO NPs are utilized in nanofertilizers. For possible biological applications, the antioxidant, antibacterial, antifungal, anticancer, and antidiabetic properties of ZnO-NPs have all been explored. ZnO-NPs' superior anticancer mechanism is not fully known. Hence, in vitro and in vivo approaches may be needed for the treatment of a number of diseases, including cancer treatments and other biological applications. The mechanism of ZnO-NPs' toxicity, which has a significant inhibiting effect on malignant cells and microorganisms, remains unexplained. Because biomolecules are particularly sensitive to variations in temperature and pH, biological applications of ZnO-NPs require high-quality nanoparticles in aqueous solution at physiological pH and temperature. Conceptually, there is an urgent need for a better understanding of the relationship between the size, shape, and structure of ZnO-NPs as well as how to control their ability to interact electronically and chemically with biological molecules and their sensing (biological and chemical) capabilities.
Acknowledgement
The authors are thankful to the Director, Tea Research Association, the Principal, Darrang College and the Principal Eastern Karbi Anglong College for their encouragement and support.
References
2. Handy RD, Von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M. The Ecotoxicology and Chemistry of Manufactured Nanoparticles. Ecotoxicology. 2008 May;17(4):287-314.
3. Gowda R, Jones NR, Banerjee S, Robertson GP. Use Of Nanotechnology to Develop Multi-Drug Inhibitors for Cancer Therapy. Journal Of Nanomedicine & Nanotechnology. 2013 Dec 1;4(6).
4. Elshama SS, Abdallah ME, Abdel-Karim RI. Zinc Oxide Nanoparticles: Therapeutic Benefits and Toxicological Hazards. The Open Nanomedicine Journal. 2018 Jul 19;5(1).
5. Kolekar TV, Yadav HM, Bandgar SS, Deshmukh PY. Synthesis By Sol–Gel Method and Characterization of Zno Nanoparticles. Indian Streams Research Journal. 2011;1(1):1-4.
6. Shah MA, Al-Marzouki FM. Zinc Oxide Nanorods Prepared in Mixed Solvents. Materials Sciences and Applications. 2010 Jun 30;1(2):77-80.
7. McNeil SE. Nanoparticle Therapeutics: A Personal Perspective. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009 May;1(3):264-71.
8. Vizirianakis IS. Nanomedicine And Personalized Medicine Toward the Application of Pharmacotyping in Clinical Practice to Improve Drug-Delivery Outcomes. Nanomedicine: Nanotechnology, Biology and Medicine. 2011 Feb 1;7(1):11-7.
9. Agarwal H, Kumar SV, Rajeshkumar S. A Review on Green Synthesis of Zinc Oxide Nanoparticles–An Eco-Friendly Approach. Resource-Efficient Technologies. 2017 Dec 1;3(4):406-13.
10. Agarwal H, Menon S, Kumar SV, Rajeshkumar S. Mechanistic Study on Antibacterial Action of Zinc Oxide Nanoparticles Synthesized Using Green Route. Chemico-Biological Interactions. 2018 Apr 25; 286:60-70.
11. Raja A, Ashokkumar S, Marthandam RP, Jayachandiran J, Khatiwada CP, Kaviyarasu K, et al. Eco-Friendly Preparation of Zinc Oxide Nanoparticles Using Tabernaemontana Divaricata and Its Photocatalytic and Antimicrobial Activity. Journal Of Photochemistry and Photobiology B: Biology. 2018 Apr 1; 181:53-8.
12. Rajabairavi N, Raju CS, Karthikeyan C, Varutharaju K, Nethaji S, Hameed AS, et al. Biosynthesis of Novel Zinc Oxide Nanoparticles (Zno Nps) Using Endophytic Bacteria Sphingobacterium Thalpophilum. Inrecent Trends in Materials Science and Applications 2017 (pp. 245-254). Springer, Cham.
13. Rauf MA, Owais M, Rajpoot R, Ahmad F, Khan N, Zubair S. Biomimetically Synthesized Zno Nanoparticles Attain Potent Antibacterial Activity Against Less Susceptible S. Aureus Skin Infection in Experimental Animals. RSC Advances. 2017;7(58):36361-73.
14. Taran M, Rad M, Alavi M. Biosynthesis of Tio2 and Zno Nanoparticles by Halomonas Elongata IBRC-M 10214 In Different Conditions of Medium. Bioimpacts: BI. 2018;8(2):81.
15. Kalpana VN, Kataru BA, Sravani N, Vigneshwari T, Panneerselvam A, Rajeswari VD. Biosynthesis Of Zinc Oxide Nanoparticles Using Culture Filtrates of Aspergillus Niger: Antimicrobial Textiles and Dye Degradation Studies. OpenNano. 2018 Jan 1; 3:48-55.
16. Moghaddam AB, Moniri M, Azizi S, Rahim RA, Ariff AB, Saad WZ, et al. Biosynthesis of Zno Nanoparticles by A New Pichia Kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules. 2017 Jun;22(6):872.
17. Król A, Pomastowski P, Rafińska K, Railean-Plugaru V, Buszewski B. Zinc Oxide Nanoparticles: Synthesis, Antiseptic Activity and Toxicity Mechanism. Advances In Colloid and Interface Science. 2017 Nov 1; 249:37-52.
18. Ambika S, Sundrarajan M. Green Biosynthesis of Zno Nanoparticles Using Vitex Negundo L. Extract: Spectroscopic Investigation of Interaction Between Zno Nanoparticles and Human Serum Albumin. Journal Of Photochemistry and Photobiology B: Biology. 2015 Aug 1; 149:143-8.
19. Divya M, Vaseeharan B, Abinaya M, Vijayakumar S, Govindarajan M, Alharbi NS, et al. Biopolymer Gelatin-Coated Zinc Oxide Nanoparticles Showed High Antibacterial, Antibiofilm and Anti-Angiogenic Activity. Journal Of Photochemistry and Photobiology B: Biology. 2018 Jan 1; 178:211-8.
20. Hao N, Zhang M, Zhang JX. Microfluidics For Zno Micro-/Nanomaterials Development: Rational Design, Controllable Synthesis, And On-Chip Bioapplications. Biomaterials Science. 2020;8(7):1783-801.
21. Kang HW, Leem J, Yoon SY, Sung HJ. Continuous Synthesis of Zinc Oxide Nanoparticles in A Microfluidic System for Photovoltaic Application. Nanoscale. 2014;6(5):2840-6.
22. Janasek D, Franzke J, Manz A. Scaling and The Design of Miniaturized Chemical-Analysis Systems. Nature. 2006 Jul;442(7101):374-80.
23. Xing Y, Dittrich PS. One-Dimensional Nanostructures: Microfluidic-Based Synthesis, Alignment and Integration Towards Functional Sensing Devices. Sensors. 2018 Jan;18(1):134.
24. Li LL, Li X, Wang H. Microfluidic Synthesis of Nanomaterials for Biomedical Applications. Small Methods. 2017 Aug;1(8):1700140.
25. Elvira KS, Wootton RC, deMello AJ. The Past, Present and Potential for Microfluidic Reactor Technology in Chemical Synthesis. Nature Chemistry. 2013 Nov;5(11):905-15.
26. Azzouz I, Habba YG, Capochichi-Gnambodoe M, Marty F, Vial J, Leprince-Wang Y, et al. Zinc Oxide Nano-Enabled Microfluidic Reactor for Water Purification and Its Applicability to Volatile Organic Compounds. Microsystems & Nanoengineering. 2018 Feb 26;4(1):1-7.
27. Joo J, Chow BY, Prakash M, Boyden ES, Jacobson JM. Face-Selective Electrostatic Control of Hydrothermal Zinc Oxide Nanowire Synthesis. Nature Materials. 2011 Aug;10(8):596-601.
28. Kraus I, Li S, Knauer A, Schmutz M, Faerber J, Serra CA, et al. Continuous-Microflow Synthesis and Morphological Characterization of Multiscale Composite Materials Based on Polymer Microparticles and Inorganic Nanoparticles. Journal Of Flow Chemistry. 2014 Jul 1;4(2):72-8.
29. Shen C, James SA, de Jonge MD, Turney TW, Wright PF, Feltis BN. Relating Cytotoxicity, Zinc Ions, And Reactive Oxygen in Zno Nanoparticle–Exposed Human Immune Cells. Toxicological Sciences. 2013 Nov 1;136(1):120-30.
30. Jiang J, Pi J, Cai J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorganic Chemistry and Applications. 2018 Oct;2018.
31. Soren S, Kumar S, Mishra S, Jena PK, Verma SK, Parhi P. Evaluation of Antibacterial and Antioxidant Potential of The Zinc Oxide Nanoparticles Synthesized by Aqueous and Polyol Method. Microbial Pathogenesis. 2018 Jun 1; 119:145-51.
32. Zago MP, Oteiza PI. The Antioxidant Properties of Zinc: Interactions with Iron and Antioxidants. Free Radical Biology and Medicine. 2001 Jul 15;31(2):266-74.
33. Badkoobeh P, Parivar K, Kalantar SM, Hosseini SD, Salabat A. Effect of Nano-Zinc Oxide on Doxorubicin-Induced Oxidative Stress and Sperm Disorders in Adult Male Wistar Rats. Iranian Journal of Reproductive Medicine. 2013 May;11(5):355.
34. Dawei AI, Zhisheng W, Anguo Z. Protective Effects of Nano-Zno on The Primary Culture Mice Intestinal Epithelial Cells In In Vitro Against Oxidative Injury.
35. Das D, Nath BC, Phukon P, Dolui SK. Synthesis of Zno Nanoparticles and Evaluation of Antioxidant and Cytotoxic Activity. Colloids And Surfaces B: Biointerfaces. 2013 Nov 1; 111:556-60.
36. Sharma V, Anderson D, Dhawan A. Zinc Oxide Nanoparticles Induce Oxidative DNA Damage And ROS-Triggered Mitochondria Mediated Apoptosis in Human Liver Cells (HepG2). Apoptosis. 2012 Aug;17(8):852-70.
37. Atef HA, Mansour MK, Ibrahim EM, Sayed El-Ahl RM, Al-Kalamawey NM, El Kattan YA, et al. Efficacy of Zinc Oxide Nanoparticles and Curcumin in Amelioration the Toxic Effects in Aflatoxicated Rabbits. Int. J. Curr. Microbiol. Appl. Sci. 2016 Dec;5(12):795-818.
38. Richard JL. Some Major Mycotoxins and Their Mycotoxicoses—An Overview. International Journal of Food Microbiology. 2007 Oct 20;119(1-2):3-10.
39. Sharma H, Kumar K, Choudhary C, Mishra PK, Vaidya B. Development and Characterization of Metal Oxide Nanoparticles for The Delivery of Anticancer Drug. Artificial Cells, Nanomedicine, And Biotechnology. 2016 Feb 17;44(2):672-9.
40. Zhang Y, R Nayak T, Hong H, Cai W. Biomedical Applications of Zinc Oxide Nanomaterials. Current Molecular Medicine. 2013 Dec 1;13(10):1633-45.
41. Martínez-Carmona M, Gun’Ko Y, Vallet-Regí M. Zno Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials. 2018 Apr;8(4):268.
42. Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C. Selective Cytotoxic Effect of Zno Nanoparticles on Glioma Cells. Nano Research. 2009 Nov;2(11):882-90.
43. Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, Alrokayan SA. Zinc Oxide Nanoparticles Selectively Induce Apoptosis in Human Cancer Cells Through Reactive Oxygen Species. International Journal of Nanomedicine. 2012; 7:845.
44. Rao MD, Gautam P. Synthesis and Characterization of Zno Nanoflowers Using C Hlamydomonas Reinhardtii: A Green Approach. Environmental Progress & Sustainable Energy. 2016 Jul;35(4):1020-6.
45. Chakraborti S, Chakraborty S, Saha S, Manna A, Banerjee S, Adhikary A, et al. PEG-Functionalized Zinc Oxide Nanoparticles Induce Apoptosis in Breast Cancer Cells Through Reactive Oxygen Species-Dependent Impairment of DNA Damage Repair Enzyme NEIL2. Free Radical Biology and Medicine. 2017 Feb 1; 103:35-47.
46. Tanino R, Amano Y, Tong X, Sun R, Tsubata Y, Harada M, et al. Anticancer Activity of ZnO Nanoparticles Against Human Small-Cell Lung Cancer in An Orthotopic Mouse Model. Molecular Cancer Therapeutics. 2020 Feb 1;19(2):502-12.
47. Bai DP, Zhang XF, Zhang GL, Huang YF, Gurunathan S. Zinc Oxide Nanoparticles Induce Apoptosis and Autophagy in Human Ovarian Cancer Cells. International Journal of Nanomedicine. 2017; 12:6521.
48. Sharma V, Singh P, Pandey AK, Dhawan A. Induction of Oxidative Stress, DNA Damage and Apoptosis in Mouse Liver After Sub-Acute Oral Exposure to Zinc Oxide Nanoparticles. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2012 Jun 14;745(1-2):84-91.
49. Arakha M, Roy J, Nayak PS, Mallick B, Jha S. Zinc Oxide Nanoparticle Energy Band Gap Reduction Triggers the Oxidative Stress Resulting into Autophagy-Mediated Apoptotic Cell Death. Free Radical Biology and Medicine. 2017 Sep 1; 110:42-53.
50. Zhang J, Qin X, Wang B, Xu G, Qin Z, Wang J, et al. Zinc Oxide Nanoparticles Harness Autophagy to Induce Cell Death in Lung Epithelial Cells. Cell Death & Disease. 2017 Jul;8(7): e2954-.
51. Zimmet P, Alberti KG, Shaw J. Global and Societal Implications of The Diabetes Epidemic. Nature. 2001 Dec;414(6865):782-7.
52. Arthur BC, Chausmer S. Zinc, Insulin and Diabetes. J Am Coll Nutri. 1998;17(2):109-15.
53. Malizia R, Scorsone A, D'Angelo P, Pitrolo L, Giordano C. Zinc Deficiency and Cell-Mediated and Humoral Autoimmunity of Insulin-Dependent Diabetes in Thalassemic Subjects. Journal Of Pediatric Endocrinology & Metabolism: JPEM. 1998 Jan 1; 11:981-4.
54. Nazarizadeh A, Asri-Rezaie S. Comparative Study of Antidiabetic Activity and Oxidative Stress Induced by Zinc Oxide Nanoparticles and Zinc Sulfate in Diabetic Rats. AAPS PharmSciTech. 2016 Aug;17(4):834-43.
55. Hussein J, El-Banna M, Razik TA, El-Naggar ME. Biocompatible Zinc Oxide Nanocrystals Stabilized Via Hydroxyethyl Cellulose for Mitigation of Diabetic Complications. International Journal of Biological Macromolecules. 2018 Feb 1; 107:748-54.
56. Bayrami A, Parvinroo S, Habibi-Yangjeh A, Rahim Pouran S. Bio-Extract-Mediated Zno Nanoparticles: Microwave-Assisted Synthesis, Characterization and Antidiabetic Activity Evaluation. Artificial Cells, Nanomedicine, And Biotechnology. 2018 May 19;46(4):730-9.
57. Amiri A, Dehkordi RA, Heidarnejad MS, Dehkordi MJ. Effect Of the Zinc Oxide Nanoparticles and Thiamine for The Management of Diabetes in Alloxan-Induced Mice: A Stereological and Biochemical Study. Biological Trace Element Research. 2018 Feb;181(2):258-64.
58. Wahba NS, Shaban SF, Kattaia AA, Kandeel SA. Efficacy of Zinc Oxide Nanoparticles in Attenuating Pancreatic Damage in A Rat Model of Streptozotocin-Induced Diabetes. Ultrastructural Pathology. 2016 Nov 1;40(6):358-73.
59. Umrani RD, Paknikar KM. Zinc Oxide Nanoparticles Show Antidiabetic Activity in Streptozotocin-Induced Type 1 And 2 Diabetic Rats. Nanomedicine. 2014 Jan;9(1):89-104.
60. Gawade VV, Gavade NL, Shinde HM, Babar SB, Kadam AN, Garadkar KM. Green Synthesis of Zno Nanoparticles by Using Calotropis Procera Leaves for The Photodegradation of Methyl Orange. Journal Of Materials Science: Materials in Electronics. 2017 Sep;28(18):14033-9.
61. Wahab R, Kim YS, Mishra A, Yun SI, Shin HS. Formation Of Zno Micro-Flowers Prepared Via Solution Process and Their Antibacterial Activity. Nanoscale Research Letters. 2010 Oct;5(10):1675-81.
62. Sirelkhatim A, Mahmud S, Seeni A, Kaus NH, Ann LC, Bakhori SK, et al. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Letters. 2015 Jul;7(3):219-42.
63. Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective Toxicity of Zinc Oxide Nanoparticles to Prokaryotic and Eukaryotic Systems. Applied Physics Letters. 2007 May 21;90(21):213902.
64. Dutta RK, Sharma PK, Bhargava R, Kumar N, Pandey AC. Differential Susceptibility of Escherichia Coli Cells Toward Transition Metal-Doped and Matrix-Embedded Zno Nanoparticles. The Journal of Physical Chemistry B. 2010 Apr 29;114(16):5594-9.
65. Shi LE, Li ZH, Zheng W, Zhao YF, Jin YF, Tang ZX. Synthesis, Antibacterial Activity, Antibacterial Mechanism and Food Applications of Zno Nanoparticles: A Review. Food Additives & Contaminants: Part A. 2014 Feb 1;31(2):173-86.
66. Zhang ZY, Xiong HM. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials. 2015 Jun;8(6):3101-27.
67. Jiang J, Pi J, Cai J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorganic Chemistry and Applications. 2018 Oct;2018.
68. Sarwar S, Chakraborti S, Bera S, Sheikh IA, Hoque KM, Chakrabarti P. The Antimicrobial Activity of ZnO Nanoparticles Against Vibrio Cholerae: Variation in Response Depends on Biotype. Nanomedicine: Nanotechnology, Biology and Medicine. 2016 Aug 1;12(6):1499-509.
69. Spadaro D, Garibaldi A, Gullino ML. Control of Penicillium Expansum and Botrytis Cinerea on Apple Combining a Biocontrol Agent with Hot Water Dipping and Acibenzolar-S-Methyl, Baking Soda, Or Ethanol Application. Postharvest Biology and Technology. 2004 Aug 1;33(2):141-51.
70. Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir. 2002 Aug 20;18(17):6679-86.
71. Xu J, Zhao X, Han X, Du Y. Antifungal Activity of Oligochitosan Against Phytophthora Capsici and Other Plant Pathogenic Fungi In Vitro. Pesticide Biochemistry and Physiology. 2007 Mar 1;87(3):220-8.
72. Ouda SM. Antifungal Activity of Silver and Copper Nanoparticles on Two Plant Pathogens, Alternaria Alternata and Botrytis Cinerea. Research Journal of Microbiology. 2014 Jan 1;9(1):34.
73. Chatterjee AK, Sarkar RK, Chattopadhyay AP, Aich P, Chakraborty R, Basu T. A Simple Robust Method for Synthesis of Metallic Copper Nanoparticles of High Antibacterial Potency Against E. Coli. Nanotechnology. 2012 Feb 1;23(8):085103.
74. Padmavathy N, Vijayaraghavan R. Enhanced Bioactivity of ZnO Nanoparticles—An Antimicrobial Study. Science And Technology of Advanced Materials. 2008 Sep 1.
75. He L, Liu Y, Mustapha A, Lin M. Antifungal Activity of Zinc Oxide Nanoparticles Against Botrytis Cinerea and Penicillium Expansum. Microbiological Research. 2011 Mar 20;166(3):207-15.
76. Dimkpa CO, McLean JE, Britt DW, Anderson AJ. Antifungal Activity of ZnO Nanoparticles and their Interactive Effect with a Biocontrol Bacterium on Growth Antagonism of the Plant Pathogen Fusarium Graminearum. Biometals. 2013 Dec;26(6):913-24.
77. Al-Dhabaan FA, Shoala T, Ali AA, Alaa M, Abd-Elsalam K, Abd-Elsalam K. Chemically-Produced Copper, Zinc Nanoparticles and Chitosan-Bimetallic Nanocomposites and Their Antifungal Activity Against Three Phytopathogenic Fungi. Int. J. Agric. Technol. 2017;13(5):753-69.
78. Jamdagni P, Rana JS, Khatri P, Nehra K. Comparative Account of Antifungal Activity of Green and Chemically Synthesized Zinc Oxide Nanoparticles in Combination with Agricultural Fungicides. International Journal of Nano Dimension. 2018 Apr 1;9(2):198-208.
79. Arciniegas-Grijalba PA, Patiño-Portela MC, Mosquera-Sánchez LP, Guerrero-Vargas JA, Rodríguez-Páez JE. ZnO Nanoparticles (ZnO-NPs) And Their Antifungal Activity Against Coffee Fungus Erythricium Salmonicolor. Applied Nanoscience. 2017 Jun;7(5):225-41.
80. Hui A, Liu J, Ma J. Synthesis and Morphology-Dependent Antimicrobial Activity of Cerium Doped Flower-Shaped ZnO Crystallites Under Visible Light Irradiation. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2016 Oct 5; 506:519-25.
81. Agarwal H, Nakara A, Shanmugam VK. Anti-Inflammatory Mechanism of Various Metal and Metal Oxide Nanoparticles Synthesized Using Plant Extracts: A Review. Biomedicine & Pharmacotherapy. 2019 Jan 1; 109:2561-72.
82. Yao S, Feng X, Lu J, Zheng Y, Wang X, Volinsky AA, et al. Antibacterial Activity and Inflammation Inhibition of ZnO Nanoparticles Embedded TiO2 Nanotubes. Nanotechnology. 2018 Apr 16;29(24):244003.
83. Wiegand C, Hipler UC, Boldt S, Strehle J, Wollina U. Skin-Protective Effects of a Zinc Oxide-Functionalized Textile and Its Relevance for Atopic Dermatitis. Clinical, Cosmetic and Investigational Dermatology. 2013; 6:115.
84. Dong H, Li Q, Tan C, Bai N, Cai P. Bi-Directional Controlled Release of Ibuprofen and Mg2+ From Magnesium Alloys Coated by Multifunctional Composite. Materials Science and Engineering: C. 2016 Nov 1; 68:512-8.
85. Nikalje AP. Nanotechnology and Its Applications in Medicine. Med Chem. 2015;5(2):081-9.
86. Dasgupta N, Ranjan S, Ramalingam C. Applications of Nanotechnology in Agriculture and Water Quality Management. Environmental Chemistry Letters. 2017 Dec;15(4):591-605.
87. Chen H, Yada R. Nanotechnologies in Agriculture: New Tools for Sustainable Development. Trends In Food Science & Technology. 2011 Nov 1;22(11):585-94.
88. Sabir S, Arshad M, Chaudhari SK. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. The Scientific World Journal. 2014 Oct;2014.
89. Prasad TN, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, et al. Effect of Nanoscale Zinc Oxide Particles on the Germination, Growth and Yield of Peanut. Journal of Plant Nutrition. 2012 Apr 1;35(6):905-27.
90. Zhao L, Sun Y, Hernandez-Viezcas JA, Servin AD, Hong J, Niu G, et al. Influence of CeO2 and ZnO Nanoparticles on Cucumber Physiological Markers and Bioaccumulation of Ce and Zn: A Life Cycle Study. Journal Of Agricultural and Food Chemistry. 2013 Dec 11;61(49):11945-51.
91. Mahajan P, Dhoke SK, Khanna AS. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna Radiata) and Gram (Cicer Arietinum) Seedlings Using Plant Agar Method. Journal Of Nanotechnology. 2011 Oct;2011.
92. Lin D, Xing B. Phytotoxicity of Nanoparticles: Inhibition of Seed Germination and Root Growth. Environmental Pollution. 2007 Nov 1;150(2):243-50.
93. Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, et al. Zinc and Iron Oxide Nanoparticles Improved the Plant Growth and Reduced the Oxidative Stress and Cadmium Concentration in Wheat. Chemosphere. 2019 Jan 1; 214:269-77.
94. Mortvedt JJ. Crop Response to Level of Water-Soluble Zinc in Granular Zinc Fertilizers. Fertilizer Research. 1992 Oct;33(3):249-55.
95. Moghaddasi S, Fotovat A, Karimzadeh F, Khazaei HR, Khorassani R, Lakzian A. Effects of Coated and Non-Coated Zno Nano Particles on Cucumber Seedlings Grown in Gel Chamber. Archives of Agronomy and Soil Science. 2017 Jul 3;63(8):1108-20.
96. Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, et al. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiology and Biochemistry. 2021 Apr 1; 161:122-30.
97. Mba C, Guimaraes EP, Ghosh K. Re-Orienting Crop Improvement for The Changing Climatic Conditions of the 21st Century. Agriculture & Food Security. 2012 Dec;1(1):1-7.
98. Huang X, Zheng X, Xu Z, Yi C. ZnO-Based Nanocarriers for Drug Delivery Application: From Passive to Smart Strategies. International Journal of Pharmaceutics. 2017 Dec 20;534(1-2):190-4.
99. Liu J, Huang H, Zhao H, Yan X, Wu S, Li Y, et al. Enhanced Gas Sensitivity and Selectivity on Aperture-Controllable 3D Interconnected Macro–Mesoporous ZnO Nanostructures. ACS Applied Materials & Interfaces. 2016 Apr 6;8(13):8583-90.
100. Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc Oxide Nanoparticles: A Promising Nanomaterial for Biomedical Applications. Drug Discovery Today. 2017 Dec 1;22(12):1825-34.
101. Li X, Zhao C, Liu X. A Paper-Based Microfluidic Biosensor Integrating Zinc Oxide Nanowires for Electrochemical Glucose Detection. Microsystems & Nanoengineering. 2015 Aug 3;1(1):1-7.
102. Nasiri N, Clarke C. Nanostructured Gas Sensors for Medical and Health Applications: Low to High Dimensional Materials. Biosensors. 2019 Mar;9(1):43.
103. Zhou X, Wang J, Wang Z, Bian Y, Wang Y, Han N, et al. Transilient Response to Acetone Gas Using the Interlocking P+ N Field-Effect Transistor Circuit. Sensors. 2018 Jun;18(6):1914.
104. Ahmad R, Tripathy N, Park JH, Hahn YB. A Comprehensive Biosensor Integrated with A ZnO Nanorod FET Array for Selective Detection of Glucose, Cholesterol and Urea. Chemical Communications. 2015 Jul 14;51(60):11968-71.
105. Perumal V, Hashim U, Gopinath SC, Haarindraprasad R, Liu WW, Poopalan P, et al. Thickness Dependent Nanostructural, Morphological, Optical and Impedometric Analyses of Zinc Oxide-Gold Hybrids: Nanoparticle to Thin Film. PLoS One. 2015 Dec 22;10(12): e0144964.
106. Shanmugam NR, Muthukumar S, Prasad S. Ultrasensitive and Low-Volume Point-Of-Care Diagnostics on Flexible Strips–A Study with Cardiac Troponin Biomarkers. Scientific Reports. 2016 Sep 16;6(1):1-0.
107. Matea CT, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia Cet al. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. International Journal of Nanomedicine. 2017; 12:5421.
108. Deka B, Nisha SN, Baruah C, Babu A, Sarkar S, Phukan H, et al. Journal of Applied Nanotechnology Agricultural Pest Management with Plant-Derived Nanopesticides: Prospects and Challenges.
109. Deka B, Babu A, Baruah C, Barthakur M. Nanopesticides: A Systematic Review of Their Prospects with Special Reference to Tea Pest Management. Frontiers In Nutrition. 2021;8.
110. Liu TM, Conde J, Lipiński T, Bednarkiewicz A, Huang CC. Revisiting the Classification of NIR-Absorbing/Emitting Nanomaterials for in Vivo Bioapplications. NPG Asia Materials. 2016 Aug;8(8): e295.
111. Sayadi MH, Pavlaki MD, Martins R, Mansouri B, Tyler CR, Kharkan J, et al. Bioaccumulation and Toxicokinetics of Zinc Oxide Nanoparticles (Zno Nps) Co-Exposed with Graphene Nanosheets (Gns) in the Blackfish (Capoeta Fusca). Chemosphere. 2021 Apr 1; 269:128689.
112. Król-Górniak A, Rafińska K, Monedeiro F, Pomastowski P, Buszewski B. Comparison Study of Cytotoxicity of Bare and Functionalized Zinc Oxide Nanoparticles. International Journal of Molecular Sciences. 2021 Jan;22(17):9529.
113. Wiesmann N, Tremel W, Brieger J. Zinc Oxide Nanoparticles for Therapeutic Purposes in Cancer Medicine. Journal Of Materials Chemistry B. 2020;8(23):4973-89.