Abstract
Oxidative phosphorylation dysregulation (OXPHOS) has been demonstrated to be essential for the development of cancer. Therefore, it may be argued that chaperone and deacetylase activities modulate OXPHOS activity. For instance, a complicated network of interactions connects a cell’s bioenergetic features and neoplastic potential through the imbalance of sirtuin 3 (SIRT3) and succinate dehydrogenase (SDH) enzymatic activity in mitochondria. The studies outlined in this review indicate that targeting SDH regulators is a promising novel therapeutic strategy for this extremely resistant disease. Additionally, a viable therapeutic strategy may involve triggering the cell death mechanism in cancer cells by blocking mitochondrial metabolism with a natural substance. A naturally occurring flavonoid called naringenin (NAR) has been extensively investigated for its pharmacological properties, which include anti-tumor actions. However, due to its low bioavailability in this situation, nanoencapsulation is designed to improve NAR anticancer efficacy. NAR can be encapsulated by chitosan nanoparticles-TPP conjugates, thereby improving NAR cellular absorption and cytotoxicity against cancer cells. Consequently, we proposed naringenin nanoparticles as a novel therapeutic target for SDH regulators in cancer.
Keywords
Succinate dehydrogenase, Cancer, Chitosan, Naringenin, Oxidative phosphorylation
Background
Evidence indicating that OXPHOS plays a substantial role in the development of many cancer cells has led to the growing recognition of cancer as a metabolic disease. OXPHOS controls the environment for tumor growth, invasion, and metastasis in addition to providing enough energy for tumor tissue survival. In this review, we talk about new research on changes to SDH activity, such as SDH dysregulation or endogenous SDH inhibitors that cause succinate buildup. We also present a thorough analysis of the role of succinate in the initiation of metabolic and epigenetic changes that contribute to the development of cancer, as well as its effects on angiogenesis, cell invasion, and migration.
Succinate Dehydrogenase (SDH)
SDH is a mitochondrial enzyme (complex II) that participates in both the TCA cycle and the mitochondrial electron transport chain. SDH transports electrons to the ubiquinone (coenzyme Q) pool in the respiratory chain and catalyzes the oxidation of succinate to fumarate in the TCA cycle. SDH subunit A (SDHA) and subunit B (SDHB) comprise the catalytic subunits in the hydrophilic head that protrudes into the mitochondrial matrix. The catalytic subunits in the hydrophilic head that extends into the mitochondrial matrix are SDH subunits A (SDHA) and B (SDHB). The ubiquinone-binding and membrane-anchoring SDH subunits, SDHC and SDHD, respectively, are responsible for these functions. The flavination of SDHA, which is necessary for the formation of the SDH complex, depends on SDH assembly factor (SDHAF). The SDHA gene has 16 exons and is found on chromosome 5p15.33 [1]. SDHA is the primary catalytic component that changes succinate into fumarate. It includes the succinate binding site. While there are 8 exons in the SDHB gene, which is found on chromosome 1p35-36.1 [2]. It contains three Fe-S centers and mediates electron transfer to the ubiquinone pool. The gene encoding SDHC is located at 1q21 and has 6 exons [3], and the SDHD gene is found on chromosome 11q23 and has 4 exons [4]. By binding ubiquinone, SDHC and SDHD produce protons that eventually turn into ATP [5].
Regulation of SDH
Tumor necrosis factor receptor-associated protein 1 (TRAP1): TRAP1, a crucial member of the mitochondrial heat shock protein 90 family, is active in a variety of biological functions in various tumor types. TRAP1, also known as Hsp75, is the mitochondrial paralog of the molecular chaperone Hsp 90, with which it shares a high degree of amino acid sequence homology and domain organization [6]. Hsp90 family chaperones are molecular machines that regulate the degradation of proteins after aggregation, unfolding, or misfolding. They do this by allowing the proteins to undergo conformational changes, to reach specific subcellular localizations, or to form multimeric complexes [7]. The biochemical outputs of these functions consist in the integration of signaling and metabolic circuits, ultimately leading to a global proteostasis that can be seen as a dynamic process of quality control and efficiency maintenance of the proteome under changing environmental conditions [6]. In the instance of TRAP1, its mitochondrial location makes it an appropriate option for the tuning of metabolic activities that take place in these organelles as well as for reacting to toxic situations that influence mitochondrial physiology. In fact, a number of observations show that pathological situations increase TRAP1 expression or activity [8].
Additionally, TRAP1 mutations have been found in a case of acute auto-inflammation that was also accompanied with cellular redox disequilibrium [9], in a child with the mitochondrial disease Leigh syndrome [10] that connected to gastrointestinal dysmotility, exhaustion, and persistent pain [11] and in a small subset of patients with complex developmental syndromes marked by malformations in the kidney and other regions [12]. Therefore, it was suggested that TRAP1 protects mitochondria from harm in renal fibrosis models [13], and a proband with thyroid and kidney malignancies was found to have a TRAP1 mutation [14]. The primary source of information on a putative harmful role for the chaperone is cancer model studies because TRAP1 is highly expressed in patient cohorts with hepatocellular carcinoma (HCC) [15]. Small cell lung cancer high-grade glioma, breast cancer [16], ovarian, kidney, prostate, esophageal, and colorectal cancer are among the diseases where it is linked to advanced stage, metastasis, and a bad prognosis [17]. TRAP1 induction predates neoplastic alterations and is only present in lesions that develop to carcinoma in colorectal cancer linked with ulcerative colitis and in an animal model of HCC progression. While high TRAP1 expression was linked to a poor prognosis, robust TRAP1 expression was linked to an increased likelihood of lymph node metastasis. However, is yet unknown its pathogenic significance in cancer [7]. These findings point to a significant role for TRAP1 in the metabolic adjustments that support the growth of neoplasms and have stimulated studies aiming at mechanistically understanding its biochemical functions in various cancer contexts. Information on its structure, mode of action, interactors, and functional consequences have become clearer in recent years, along with the discovery of selective inhibitors. This information is beginning to paint a picture of TRAP1 acting as a master switch in tumor bioenergetics.
High amounts of succinate are produced when TRAP1 blocks the activity of respiratory complex II, which in turn reduces SDH activity. TRAP1 inhibits PTP opening in mitochondria via regulating the activities of OXPHOS complexes II and IV (SDH and cytochrome c oxidase, respectively) [18,19]. The consequences of the ability of chaperon to switch between a dimer and a tetramer conformation are unknown. Src tyrosine kinase and Ras/MEK/ERK signaling are downstream effects of phosphorylation which enhance TRAP1 activity. After TRAP1-dependent SDH inhibition, succinate buildup results in epigenetic alterations, migration, and angiogenesis as shown in Figure 1. Also promote cellular invasion and migration by STAT3/MMP2 pathway [20].
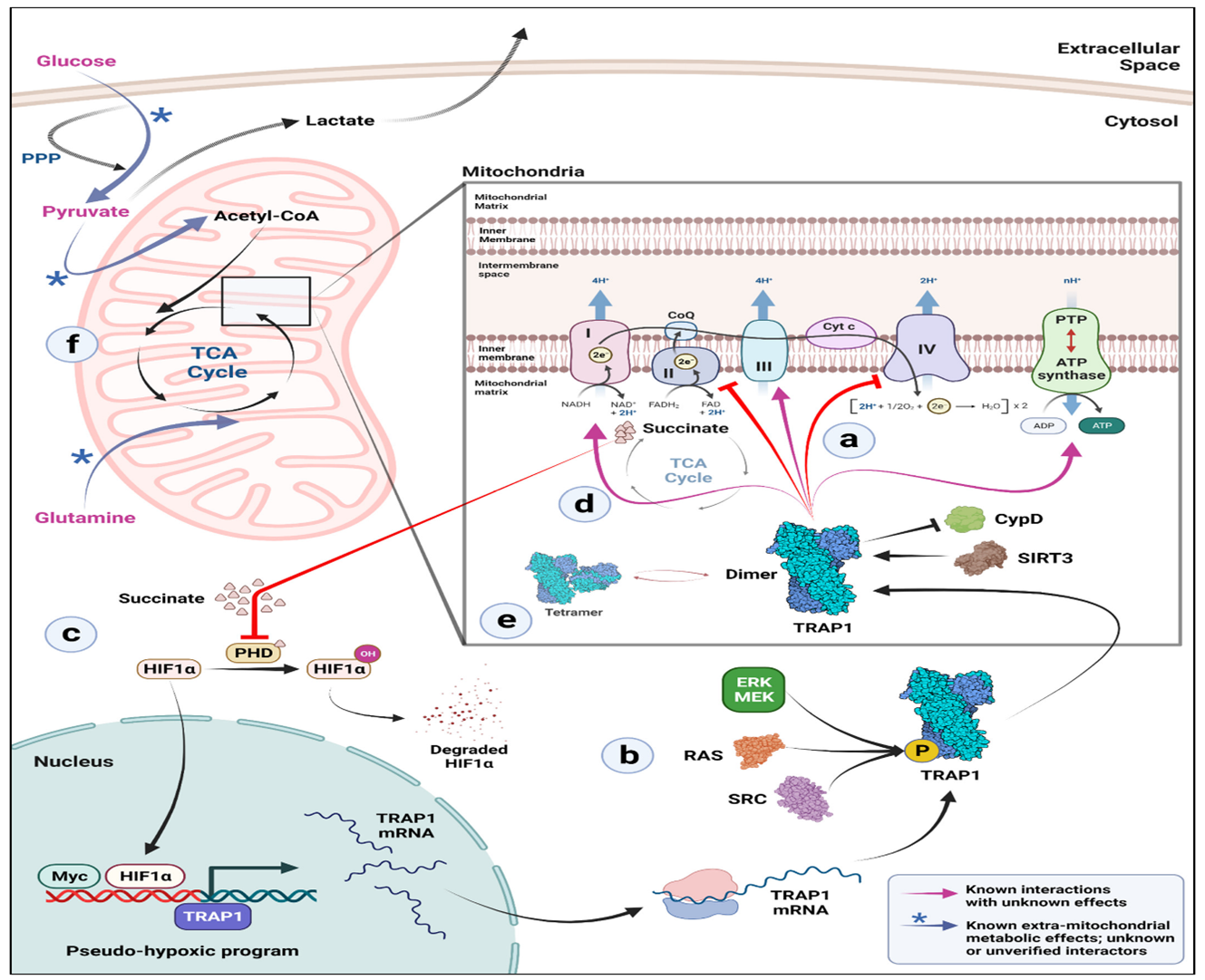
Figure 1. The biochemical mechanism of TRAP1 on SDH (a) In the mitochondria, TRAP1 binds to and prevents the activity of electron transport chain (ETC) complexes II and IV. As a result of its interaction with the protein deacetylase SIRT3, TRAP1 is known to suppress cyclophilin D (CypD), which prevents the opening of the permeability transition pore (PTP) and the subsequent induction of apoptosis by the release of cytochrome c. (b) In addition, phosphorylation of TRAP1 via several mechanisms increases its activity. Be aware that it is still unknown whether this occurs prior to or following the mitochondrial import of TRAP1. (c) Succinate builds up as a result of TRAP1's suppression of the ETC complex II, which in turn prevents prolyl hydroxylases from functioning in the cytosol to stabilize HIF1. A pseudo-hypoxic program is triggered by stabilized HIF1 and Myc, and this further upregulates the expression of the TRAP1 gene. (d) TRAP1 interacts with ETC complexes I, III, and V (ATP synthase) inside mitochondria as well, but the implications of this interaction are unknown. (e) Although TRAP1 tetramers and dimers coexist in the mitochondrial matrix, factors affecting the ratio of dimer to tetramer and their potential functional importance are still unknown. (f) The levels of TRAP1 have an impact on mitochondrial carbon preference. Glucose and pyruvate-derived carbon entry into the TCA cycle is downregulated in TRAP1 KO cells. A sizeable part of glucose is redirected to the pentose phosphate pathway (PPP), where it is used to synthesize NADPH reducing equivalents and make ribose sugars. This process may be done to combat the elevated reactive oxygen species (ROS) that are present in TRAP1 KO cells. Pyruvate generally enters the TCA cycle after decarboxylation and helps to create acetyl-CoA, a crucial TCA cycle intermediate [20,60].
Post-translational regulation:
Phosphorylation: Through many mechanisms, particularly phosphorylation, the activity of SDH is also controlled at the post-translational level. For example, c-Src kinase must phosphorylate the Y215 of the SDHA in order for Complex II electron transfer to be effective [21,22]. Other studies have demonstrated that under some circumstances, such as oxidative stress, SDHA phosphorylation is increased, enhancing Complex II-dependent respiration [23]. Additionally, it has been demonstrated that the dephosphorylation process mediated by PTEN-like mitochondrial phosphatase-1 (PTPMT1) is associated to a glucose level adaptation and is involved in the deregulation of SDH activity [24].
Acetylation by a NAD-dependent deacetylase (SIRT3): Thirteen of the lysines in SDHA have been shown to be acetylated, and it has been shown that SIRT3, a NAD-dependent deacetylase, is a key regulator of SDH function because its absence reduces the activity of the enzyme [25]. However, further studies are needed to shed light on the crosstalk between SDH and SIRT3 and the relative adaptation of the cell to metabolic alterations. SDH loss, reduction or dysregulation induce an altered metabolic phenotype by action of succinate accumulation [5,26]. It was revealed for Naringenin, activates AMPK-SIRT3 signaling to prevent mitochondrial oxidative stress damage and maintain mitochondrial function during ischemia-reperfusion injury. A promising candidate for the therapy of mitochondrial dysfunction caused by a variety of disorders may be naringenin [27].
Dysregulated induces an altered metabolic phenotype
Otto Warburg effect: It was first postulated in the early 1920s that tumor cells may alter their glucose metabolism and, consequently, their energy metabolism, producing energy mostly through glycolysis and giving rise to a process known as "aerobic glycolysis" [28]. Although this hypothesis initially seems illogical, it has now been proven to be true [29]. The biological characteristic of cellular energy adaptation to support high neoplastic proliferation most effectively was reprogramming of the TCA cycle, which later supported the etiology of cancer during its multistep evolution [29,30].
Epigenetic alteration: According to recent investigations, succinate is a new "epigenetic hacker" that inhibits DNA and histone demethylases [31]. Additionally, succinylation is a post-translational alteration in which an amide bond holds a succinyl moiety to a lysine residue. A major conformational change is most likely the effect of this alteration, which conceals the positive charge on lysine. Multiple subcellular compartments, particularly mitochondria, experience global lysine hyper-succinylation as a result of succinate buildup brought on by SDH depletion leading to an epigenetic change in carcinomas [32]. Studies have validated the role of succinate in cellular transformation and cancer at both the biochemical and genomic levels [33,34].
ROS production: Oxyradicals generated from mitochondria called reactive oxygen species (ROS) are involved in oxygen metabolism. Superoxide anion (O2-), a kind of ROS, is one example. It is becoming more and more known that O2- causes oxidative damage to a variety of physiological and pathological processes, including signal transduction [35], cell apoptosis [36] and gene mutagenesis [37]. Reduced complex II enzyme activity, ROS generation, and increased DNA mutation can narrowly be avoided in transgenic mouse cells transfected with SDHC loss-of-function [38]. Later investigations revealed that complex II enzymatic activity in mitochondria will be disrupted by any abnormalities in SDHB, SDHC, or SDHD but not SDHA. The inactivation of the TCA cycle enzyme causes mitochondria to malfunction, which directly and indirectly causes an excess generation of ROS. When ROS levels rise in the cytosol, they can oxidize amino acid residues in fatty acids and proteins result in genomic instability and irreversible DNA damage, which promote carcinogenesis and tumorigenesis [39,40].
Migration & angiogenesis: Additionally, it has been demonstrated that succinate causes cell migration via activating the SUCNR1 receptor. The succinate receptor, also known as GPR91, belongs to the P2Y purinoreceptor family of G protein-coupled receptors [5]. Several tissues, including blood cells, adipose tissue, the liver, retina, and kidney, express it. This receptor, along with its ligand succinate, has just come to be recognized in these tissues as novel mediators of local stress conditions such ischemia, hypoxia, toxicity, and hyperglycemia. By activating PKCs phosphorylation and p38 MAPK subsequent activation SUCNR1 caused migration as in Figure 2 [41]. Additionally, succinate induces neovascularization through the activation of extracellular regulated kinase (ERK) 1/2 and signal transducer and activator of transcription 3 (STAT3) via the particular succinate receptor GPR91, which demonstrates that this occurs in an HIF-independent manner [41,42]. There is a growing body of research that connects the ERK1/2 signaling pathway to angiogenesis [43,44]. Moreover, according to Loriot et al. [45], hypermethylation caused by succinate buildup is sufficient to trigger Epithelial mesenchymal transition (EMT). Additionally, Wang et al. demonstrated that SDHB knockdown activates the TGFß signaling pathway in colorectal cancer cells by increasing the activity of the tight-junction transcriptional repression complex SNAIL1-SMAD3/SMAD4, which produces EMT and facilitates cell migration and invasion [46].
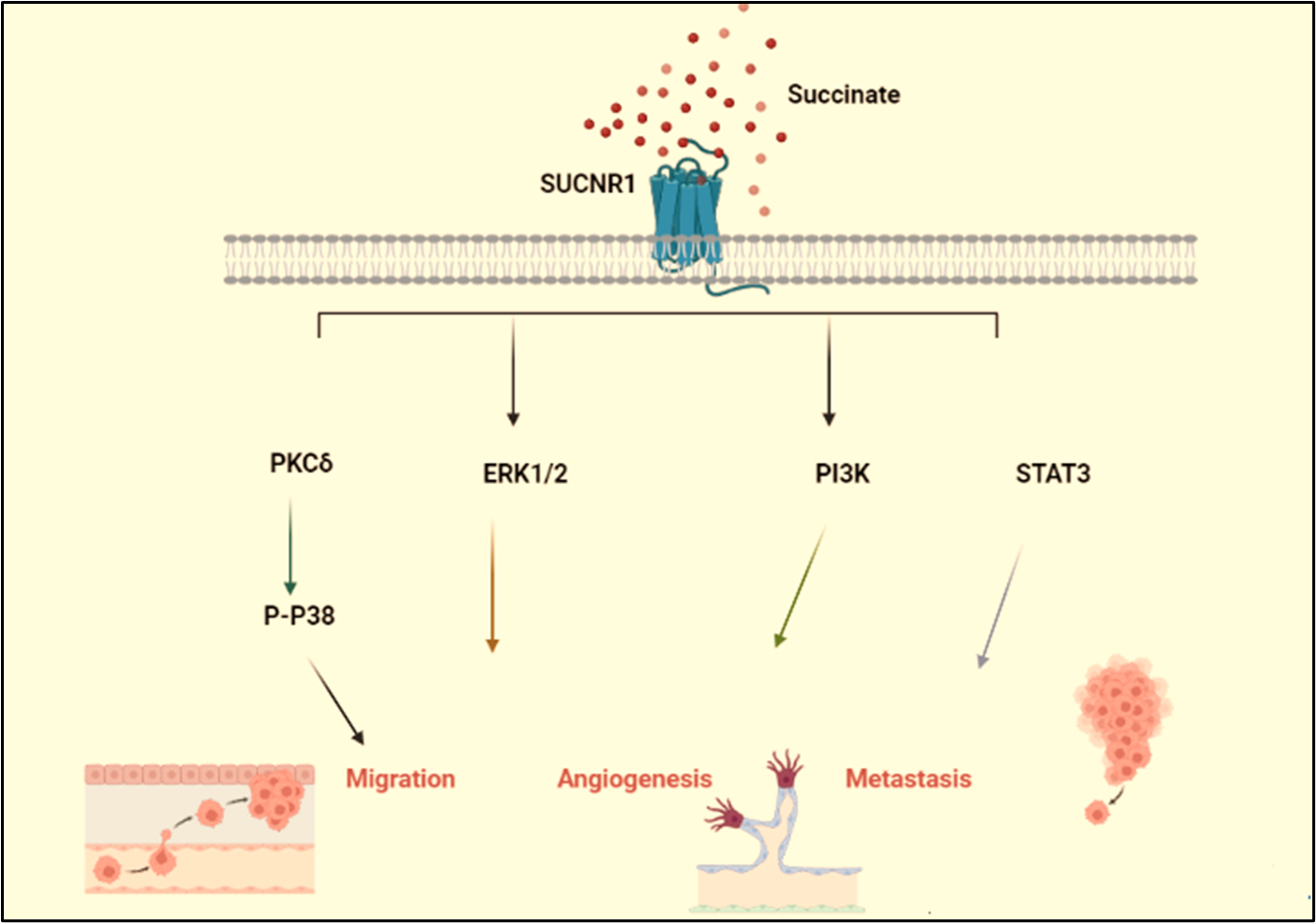
Figure 2. Schematic illustration of the signaling pathway via which succinate drive cancer. The signaling pathway via which succinate phosphorylate Drp-1 and induces mitochondrial fission. ROS generated drive cancer cell migration. Extracellular regulated kinases (ERK1/2), phosphatidyl kinases (PI3K)/ STAT3 induce metastasis & angiogenesis.
SDH Target for Anticancer Therapy
Recently, natural substances have attention due to block tumor-specific changes in the mitochondrial metabolism and could be an attractive therapeutic strategy for activating the cell death machinery in cancer cells [47-49]. Flavanone group member naringenin (NAR) [2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) was discovered by Power and Tutin in 1907 [50]. The hydrophobic molecule NAR (C15 H12 O5) has a molecular weight of 272.25 g/mol. It is primarily found in the form of aglycones and is a derivative of naringin or narirutin hydrolysis. Oranges, tomatoes, grapefruits, and lemons are the main sources of NAR [50,51]. Numerous investigations have shown that NAR and its derivatives possess significant pharmacological capabilities. These properties include estrogen-like activity or anti-cancer effects through inactivating carcinogens and cell cycle arrest. Additionally, p53 and members of the mitogen-activated protein kinase (MAPK) family, which are overexpressed and play a critical role in mediating pro-apoptotic effects in many cancers, induce apoptosis as shown in Figure 3 [52,53]. Flavonoids were shown to interfere with the UbQ-binding site between the SDHC and SDHD subunits in recent research, which demonstrated that they greatly inhibited SDH activity in cancer cells [48,54,55].
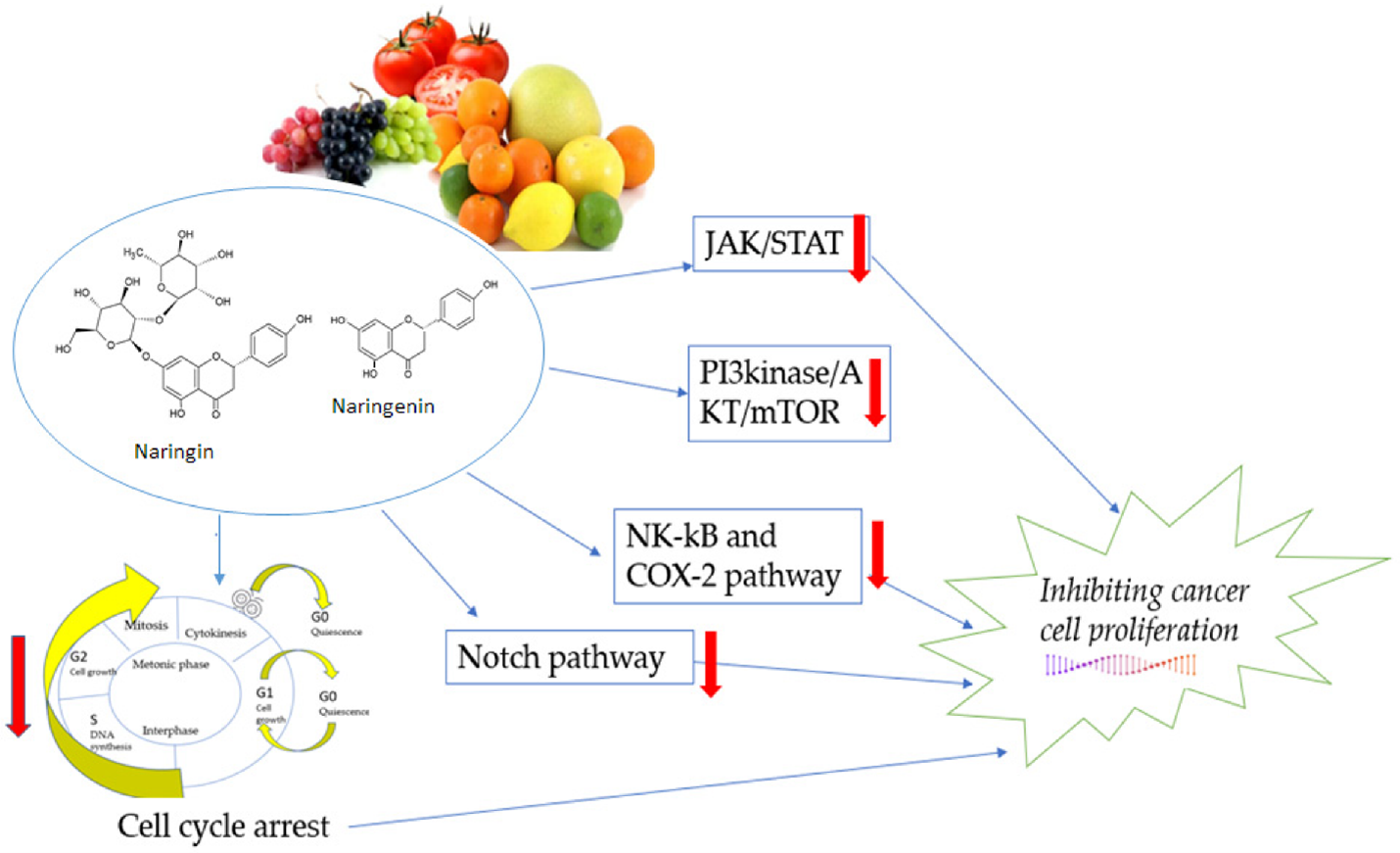
Figure 3. The mechanisms of naringenin in the proliferation pathway. JAK: Janus kinase, a group of intracellular, non-receptor tyrosine kinases that use the JAK-STAT pathway to translate cytokine-mediated signals: transcription factors STAT, PI3K, AKT, protein kinase B, an important cell survival mediator, and mTOR, the mammalian target of rapamycin: NF-B, also known as nuclear factor-kappa-light-chain-enhancer of activated B cells, COX-2, or cyclooxygenase 2, and Notch, a highly conserved cell signaling pathway seen in the majority of animals [61].
However, water solubility, low oral bioavailability, and instability of NAR, which are mostly caused by its hydrophobic and crystalline structure. Which provide obstacles to its effective medicinal application. Nano-drug delivery methods have been employed for formulation to address these physicochemical problems [53,56]. These nanocarriers, mostly through surface modification, shield the encapsulated molecule from lysis and pH changes, enhance its solubility, and enable sustained medication release to target cells. These factors have led to the development of a variety of nanocarriers, such as polymeric nanoparticles based on chitosan [57]. In particular, the advancement of oncological therapy has drawn attention to the use of nanoparticles made of biodegradable polymer materials like chitosan (CS). D-glucosamine and N-acetyl-D-glucosamine, which were joined by a (1,4) glycosidic link, each make up two subunits of CS [52,58]. Because it provides a strong and reactive positive charge, the amine group on the glucosamine unit of the CS is a crucial component. The positive charge of CS can combine with an anion molecule to produce a complex. Additionally, CS can speed up the route of medication through cell membranes [54,59]. CS (positive) and tri-sodium polyphosphate (TPP) were ionically crosslinked to create chitosan nanoparticles that were naringenin-loaded. Usually, one of the most important factors influencing the short- and long-term stability of NPs is their surface charge. In our earlier research, we found that the particles' surface charge had increased as measured by zeta potential. Less neutralization of the NH3 groups by the TPP crosslinker may be the cause of the rise in surface charge. High positive charge particles are more stable and have cohesion and tissue permeability characteristics. Due to their positive zeta potential, chitosan nanoparticles are more stable and can carry drugs more effectively by more easily adhering to negatively charged cell membranes [54]. Due to its distinct sub-cellular size in comparison to the microscopic system, the formulation of CS with NAR is anticipated to produce significant intracellular absorption [58]. As a result, while using the initial molecule, in this example NAR, the potential for being anti-cancer can be improved.
Conclusion
The unbalance in SDH activity, regulation, or mitochondria chaperones leading to succinate accumulation. Succinate plays a significant role in the growth of cancer through the production of epigenetic and metabolic changes as well as through its impact on angiogenesis, invasion, and cell migration. Therefore, there must be approaches that keep succinate from building up. Recently, flavonoids played an important role in SDH activity regulation. One of these is NAR, a substance that is naturally contained in many fruits. In clinical trials, the probability of serious side effects manifesting seems to be minimal, and it is anticipated to have a safer profile when compared to other chemotherapeutic drugs. However, the bioavailability and release of new formulations for encapsulating NAR like chitosan nanoparticles must also be further studied in relation to clinical trials, as to date the results of in vitro and in vivo models, opening the way for the development of new cancer therapies.
Declarations
Acknowledgements
Thanks to the Biochemistry department, Faculty of Science Tanta University, Egypt.
Author contributions
RE: writing original draft preparation, designing all figures, and editing. GD: conceptualization. MT: supervision and investigation. KA: reviewing and editing. The authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that this publication does not encompass known disputes.
Availability of data and materials
Not applicable.
Funding
Not applicable.
References
2. Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. The American Journal of Human Genetics. 2001 Jul 1;69(1):49-54.
3. Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nature Genetics. 2000 Nov;26(3):268-70.
4. Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000 Feb 4;287(5454):848-51.
5. Dalla Pozza E, Dando I, Pacchiana R, Liboi E, Scupoli MT, Donadelli M, et al. Regulation of succinate dehydrogenase and role of succinate in cancer. Seminars in Cell & Developmental Biology. 2020 Feb 1;98:4-14.
6. Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011 Jul 21;475(7356):324-32.
7. Ou Y, Liu L, Xue L, Zhou W, Zhao Z, Xu B, et al. TRAP1 shows clinical significance and promotes cellular migration and invasion through STAT3/MMP2 pathway in human esophageal squamous cell cancer. Journal of Genetics and Genomics. 2014 Oct 20;41(10):529-37.
8. Xu L, Voloboueva LA, Ouyang Y, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. Journal of Cerebral Blood Flow & Metabolism. 2009 Feb;29(2):365-74.
9. Standing AS, Hong Y, Paisan-Ruiz C, Omoyinmi E, Medlar A, Stanescu H, et al. TRAP1 chaperone protein mutations and autoinflammation. Life Science Alliance. 2020 Feb 1;3(2).
10. Skinner SJ, Doonanco KR, Boles RG, Chan AK. Homozygous TRAP1 sequence variant in a child with Leigh syndrome and normal kidneys. Kidney International. 2014 Oct 1;86(4):860.
11. Boles RG, Hornung HA, Moody AE, Ortiz TB, Wong SA, Eggington JM, et al. Hurt, tired and queasy: specific variants in the ATPase domain of the TRAP1 mitochondrial chaperone are associated with common, chronic “functional” symptomatology including pain, fatigue and gastrointestinal dysmotility. Mitochondrion. 2015 Jul 1;23:64-70.
12. Saisawat P, Kohl S, Hilger AC, Hwang DY, Gee HY, Dworschak GC, et al. Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney International. 2014 Jun 1;85(6):1310-17.
13. Bhreathnach U, Griffin B, Brennan E, Ewart L, Higgins D, Murphy M. Profibrotic IHG-1 complexes with renal disease associated HSPA5 and TRAP1 in mitochondria. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2017 Apr 1;1863(4):896-906.
14. Nicolas E, Demidova EV, Iqbal W, Serebriiskii IG, Vlasenkova R, Ghatalia P, et al. Interaction of germline variants in a family with a history of early-onset clear cell renal cell carcinoma. Molecular Genetics & Genomic Medicine. 2019 Mar;7(3):e556.
15. Megger DA, Bracht T, Kohl M, Ahrens M, Naboulsi W, Weber F, et al. Proteomic differences between hepatocellular carcinoma and nontumorous liver tissue investigated by a combined gel-based and label-free quantitative proteomics study. Molecular & Cellular Proteomics. 2013 Jul 1;12(7):2006-20.
16. Hu T, Zhang D, Wang J. A meta-analysis of the trait resilience and mental health. Personality and Individual Differences. 2015 Apr 1;76:18-27.
17. Leav I, Plescia J, Goel HL, Li J, Jiang Z, Cohen RJ, et al. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. The American Journal of pathology. 2010 Jan 1;176(1):393-401.
18. Matassa DS, Agliarulo I, Avolio R, Landriscina M, Esposito F. TRAP1 regulation of cancer metabolism: dual role as oncogene or Tumor Suppressor. Genes. 2018 Apr 5;9(4):195.
19. Sarkar S, Halder B. The role of TRAP1, the mitochondrial Hsp90 in cancer progression and as a possible therapeutic target. Current Science (00113891). 2023 Mar 25;124(6).
20. Masgras I, Laquatra C, Cannino G, Serapian SA, Colombo G, Rasola A. The molecular chaperone TRAP1 in cancer: From the basics of biology to pharmacological targeting. Seminars in Cancer Biology. 2021 Nov 1;76:45-53.
21. Ogura M, Yamaki J, Homma MK, Homma Y. Mitochondrial c-Src regulates cell survival through phosphorylation of respiratory chain components. Biochemical Journal. 2012 Oct 15;447(2):281-9.
22. Salvi M, Morrice NA, Brunati AM, Toninello A. Identification of the flavoprotein of succinate dehydrogenase and aconitase as in vitro mitochondrial substrates of Fgr tyrosine kinase. FEBS Letters. 2007 Dec 11;581(29):5579-85.
23. Garaude J, Acín-Pérez R, Martínez-Cano S, Enamorado M, Ugolini M, Nistal-Villán E, et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nature Immunology. 2016 Sep;17(9):1037-45.
24. Perry JJ, Ballard GD, Albert AE, Dobrolecki LE, Malkas LH, Hoelz DJ. Human C6orf211 encodes Armt1, a protein carboxyl methyltransferase that targets PCNA and is linked to the DNA damage response. Cell Reports. 2015 Mar 3;10(8):1288-96.
25. Xiao X, Wu ZC, Chou KC. A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PloS One. 2011 Jun 17;6(6):e20592.
26. Neeli PK, Gollavilli PN, Mallappa S, Hari SG, Kotamraju S. A novel metadherinΔ7 splice variant enhances triple negative breast cancer aggressiveness by modulating mitochondrial function via NFĸB-SIRT3 axis. Oncogene. 2020 Mar 5;39(10):2088-102.
27. Yu LM, Dong X, Xue XD, Zhang J, Li Z, Wu HJ, et al. Naringenin improves mitochondrial function and reduces cardiac damage following ischemia-reperfusion injury: the role of the AMPK-SIRT3 signaling pathway. Food & Function. 2019;10(5):2752-65.
28. Ochoa-Ruiz E, Diaz-Ruiz R. Anaplerosis in Cancer: Another step beyond the warburg effect. American Journal of Molecular Biology. 2012;2:4.
29. Eijkelenkamp K, Osinga TE, Links TP, van der Horst-Schrivers ANA. Clinical implications of the oncometabolite succinate in SDHx-mutation carriers. Clin Genet. 2020 Jan;97(1):39-53.
30. Fliedner SM, Kaludercic N, Jiang XS, Hansikova H, Hajkova Z, Sladkova J, et al. Warburg effect's manifestation in aggressive pheochromocytomas and paragangliomas: Insights From a Mouse Cell Model Applied to Human Tumor Tissue. PLoS One. 2012;7(7):e40949.
31. Liu C, Liu Y, Chen L, Zhang M, Li W, Cheng H, et al. Quantitative proteome and lysine succinylome analyses provide insights into metabolic regulation in breast cancer. Breast Cancer. 2019 Jan 22;26:93-105.
32. Smestad J, Erber L, Chen Y, Maher LJ. Chromatin succinylation correlates with active gene expression and is perturbed by defective TCA cycle metabolism. Iscience. 2018 Apr 27;2:63-75.
33. Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. The Journal of Clinical Investigation. 2013 Sep 3;123(9):3652-8.
34. Vikramdeo KS, Sharma A, Anand S, Sudan SK, Singh S, Singh AP, et al. Mitochondrial Alterations in Prostate Cancer: Roles in Pathobiology and Racial Disparities. International Journal of Molecular Sciences. 2023 Feb 24;24(5):4482.
35. Else T, Marvin ML, Everett JN, Gruber SB, Arts HA, Stoffel EM, et al. The clinical phenotype of SDHC-associated hereditary paraganglioma syndrome (PGL3). The Journal of Clinical Endocrinology & Metabolism. 2014 Aug 1;99(8):E1482-6.
36. Bickmann JK, Sollfrank S, Schad A, Musholt TJ, Springer E, Miederer M , et al. Phenotypic variability and risk of malignancy in SDHC-linked paragangliomas: lessons from three unrelated cases with an identical germline mutation (p. Arg133*). The Journal of Clinical Endocrinology & Metabolism. 2014 Mar 1;99(3):E489-96.
37. Hannah-Shmouni F, Londo-Mendoza R. “A Unique Case of Metastatic, Functional, Hereditary Paraganglioma Associated With an SDHC Germline Mutation”. The Journal of Clinical Endocrinology & Metabolism. 2019 Apr;104(4):1158-9.
38. Bourdeau I, Grunenwald S, Burnichon N, Khalifa E, Dumas N, Binet MC, et al. A SDHC founder mutation causes paragangliomas (PGLs) in the French Canadians: new insights on the SDHC-related PGL. The Journal of Clinical Endocrinology & Metabolism. 2016 Dec 1;101(12):4710-8.
39. Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proceedings of the National Academy of Sciences. 2011 Jan 4;108(1):314-8.
40. Shaikh A, Neeli PK, Singuru G, Panangipalli S, Banerjee R, Maddi SR, et al. A functional and self-assembling octyl-phosphonium-tagged esculetin as an effective siRNA delivery agent. Chemical Communications. 2021;57(92):12329-32.
41. Park SY, Le CT, Sung KY, Choi DH, Cho EH. Succinate induces hepatic fibrogenesis by promoting activation, proliferation, and migration, and inhibiting apoptosis of hepatic stellate cells. Biochemical and Biophysical Research Communications. 2018 Feb 5;496(2):673-8.
42. Bobkov YV, Walker III WB, Cattaneo AM. Altered functional properties of the codling moth Orco mutagenized in the intracellular loop-3. Scientific Reports. 2021 Feb 16;11(1):3893.
43. Vickers NJ. Animal communication: when i'm calling you, will you answer too?. Current Biology. 2017 Jul 24;27(14):R713-5.
44. Rapizzi E, Ercolino T, Fucci R, Zampetti B, Felici R, Guasti D, et al. Succinate dehydrogenase subunit B mutations modify human neuroblastoma cell metabolism and proliferation. Hormones and Cancer. 2014 Jun;5:174-84.
45. Loriot C, Burnichon N, Gadessaud N, Vescovo L, Amar L, Libe R, et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. The Journal of Clinical Endocrinology & Metabolism. 2012 Jun 1;97(6):E954-62.
46. Neeli PK, Sahoo S, Karnewar S, Singuru G, Pulipaka S, Annamaneni S, et al. DOT1L regulates MTDH‐mediated angiogenesis in triple‐negative breast cancer: intermediacy of NF‐κB‐HIF1α axis. The FEBS Journal. 2023 Jan;290(2):502-20.
47. Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. International Journal of Molecular Sciences. 2010 May 19;11(5):2188-99.
48. Salimi A, Roudkenar MH, Seydi E, Sadeghi L, Mohseni A, Pirahmadi N, et al. Chrysin as an anti-cancer agent exerts selective toxicity by directly inhibiting mitochondrial complex II and V in CLL B-lymphocytes. Cancer Investigation. 2017 Mar 16;35(3):174-86.
49. Ragab EM, El Gamal DM, Mohamed TM, Khamis AA. Impairment of electron transport chain and induction of apoptosis by chrysin nanoparticles targeting succinate-ubiquinone oxidoreductase in pancreatic and lung cancer cells. Genes & Nutrition. 2023 Dec;18(1):1-5.
50. Bhia M, Motallebi M, Abadi B, Zarepour A, Pereira-Silva M, Saremnejad F, et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics. 2021 Feb 23;13(2):291.
51. Arafah A, Rehman MU, Mir TM, Wali AF, Ali R, Qamar W, et al. Multi-therapeutic potential of naringenin (4', 5, 7-trihydroxyflavonone): experimental evidence and mechanisms. Plants. 2020 Dec 16;9(12):1784.
52. Vieira IR, Conte-Junior CA. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Critical Reviews in Food Science and Nutrition. 2022 Jul 25:1-26.
53. Dadwal V, Gupta M. Recent developments in citrus bioflavonoid encapsulation to reinforce controlled antioxidant delivery and generate therapeutic uses. Critical Reviews in Food Science and Nutrition. 2023 Apr 3;63(9):1187-207.
54. Ragab EM, El Gamal DM, Mohamed TM, Khamis AA. Study of the inhibitory effects of chrysin and its nanoparticles on mitochondrial complex II subunit activities in normal mouse liver and human fibroblasts. Journal of Genetic Engineering and Biotechnology. 2022 Dec;20(1):1-5.
55. Seydi E, Rahimpour Z, Salimi A, Pourahmad J. Selective toxicity of chrysin on mitochondria isolated from liver of a HCC rat model. Bioorganic & Medicinal Chemistry. 2019 Dec 15;27(24):115163.
56. Rudrapal M, Mishra AK, Rani L, Sarwa KK, Zothantluanga JH, Khan J, et al. Nanodelivery of dietary polyphenols for therapeutic applications. Molecules. 2022 Dec 8;27(24):8706.
57. Sharma S, Hafeez A, Usmani SA. Nanoformulation approaches of naringenin-an updated review on leveraging pharmaceutical and preclinical attributes from the bioactive. Journal of Drug Delivery Science and Technology. 2022 Aug 28:103724.
58. Winarti L, Sari LO, Nugroho AE. Naringenin-Loaded Chitosan Nanoparticles Formulation, and its In Vitro Evaluation Against T47D Breast Cancer Cell Line. Indonesian J Pharm. 2015;26(3):147-57.
59. Fitriyani A, Winarti L, Muslichah S, Nuri N. Anti-inflammatory Activityy of Piper crocatum Ruiz & Pav. Leaves metanolic extract in rats. Majalah Obat Tradisional. 2011;16(1):34-42.
60. Joshi A, Ito T, Picard D, Neckers L. The mitochondrial HSP90 paralog TRAP1: structural dynamics, interactome, role in metabolic regulation, and inhibitors. Biomolecules. 2022 Jun 24;12(7):880.
61. Stabrauskiene J, Kopustinskiene DM, Lazauskas R, Bernatoniene J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines. 2022 Jul 13;10(7):1686.
