Abstract
Individuals with type 1 diabetes are more vulnerable than the general population to morbidity and mortality from infectious disease, including COVID-19. Over the last 20 years, the >100-year-old tuberculosis vaccine, known as Bacille Calmette-Guerin (BCG), has been observed in global populations to protect from viral, bacterial and parasitic infections, among others. Our laboratory conducted the first and only trials in type 1 diabetics (T1Ds) to determine whether infectious disease protection could be conferred by multi-dose BCG vaccine as an immunotherapy. In two back-to-back randomized, placebo-controlled, double-blinded trials covering the entire course of the COVID-19 pandemic, we found that up to six doses of BCG were safe and protected against developing COVID-19 and other infections. Like other BCG-related off-target effects, these benefits took a minimum of 3 years to begin to materialize, yet they potentially may last for decades. A total of 12 worldwide clinical trials have evaluated largely single-dose BCG vaccines for COVID-19 prevention in other high-risk populations, like the elderly and health care workers. Five found BCG to be efficacious, while seven did not. The BCG trials that failed to find benefit were often too short in duration to obtain protection. We show, in a US T1D population, that full infectious disease protection takes up to 5 years. Also, many negative trials testing BCG efficacy were actually BCG booster (re-vaccination) trials in which placebo groups had also received prior neonatal BCG vaccines, thereby obscuring the possibility of finding a large benefit in the treatment group. Further, not all clinical trials utilized the most potent BCG strains. On the basis of our successful trials of multi-dose BCG in a vulnerable US population of T1D subjects, we conclude that this population stands to benefit from multi-dose BCG immunotherapy for protection from COVID-19 and other infectious diseases.
Keywords
BCG, COVID-19, Type 1 diabetes, High-risk population, Infectious disease, Platform vaccines
Introduction
Type 1 diabetics (T1Ds) are more vulnerable to infectious disease than the general population [1]. T1Ds are also at higher risk of morbidity and mortality from COVID-19, according to two nationwide studies, one from the US and the other from Greece. These studies find that T1Ds have up to 74% higher rate of hospitalization, up to 4 times higher rate of ICU admission, and up to 6 times higher mortality from COVID-19 than a reference population of type 2 diabetics (T2D) or non-diabetics [2,3]. The reasons why T1Ds are at such high-risk from COVID-19 and other infections are not known, but one study finds that immune T cells in autoimmune disease may have lower T cell receptor density and signal strength [4]. The T cell receptor is a driver of the immune response and helps B cells make life-saving immunoglobulins to prevent infectious diseases.
The Bacille Calmette-Guerin Vaccine
The Bacille Calmette-Guerin (BCG) vaccine has beguilingly broad protective properties against infectious disease. Originally introduced >100 years ago for tuberculosis prevention, BCG consists of a live attenuated version of the bacterium that causes tuberculosis in cattle (Mycobacterium bovis). It provides protection against Mycobacterium tuberculosis, the strain that causes human tuberculosis, in about 50% of recipients for a period of 10-20 years [5]. As early as 1931, one the founders of the vaccine, Albert Calmette, observed that the BCG vaccine had infectious disease benefits beyond tuberculosis: it curtailed childhood mortality unrelated to tuberculosis by four-fold [6]. Since then, research has established that the BCG vaccine protects against upper respiratory tract infections, leprosy, malaria, viral, and bacterial infections [7–21]. In our studies in the US, subjects do not frequently get parasites, malaria or yellow fever but frequently, especially with diabetes, have high incidences of bladder infection, skin infections related to glucose and insulin infusion devices, dental infections and more susceptible to cold and flu episodes, all which decrease after long term exposures to BCG [22,23].
BCG has been in continuous use since its introduction and is currently administered to neonates in more than 150 countries [24]. BCG is one of the safest vaccines ever developed. Around 3–4 billion people have been vaccinated thus far with minimal side effects.
BCG Immunotherapy for Type 1 Diabetes
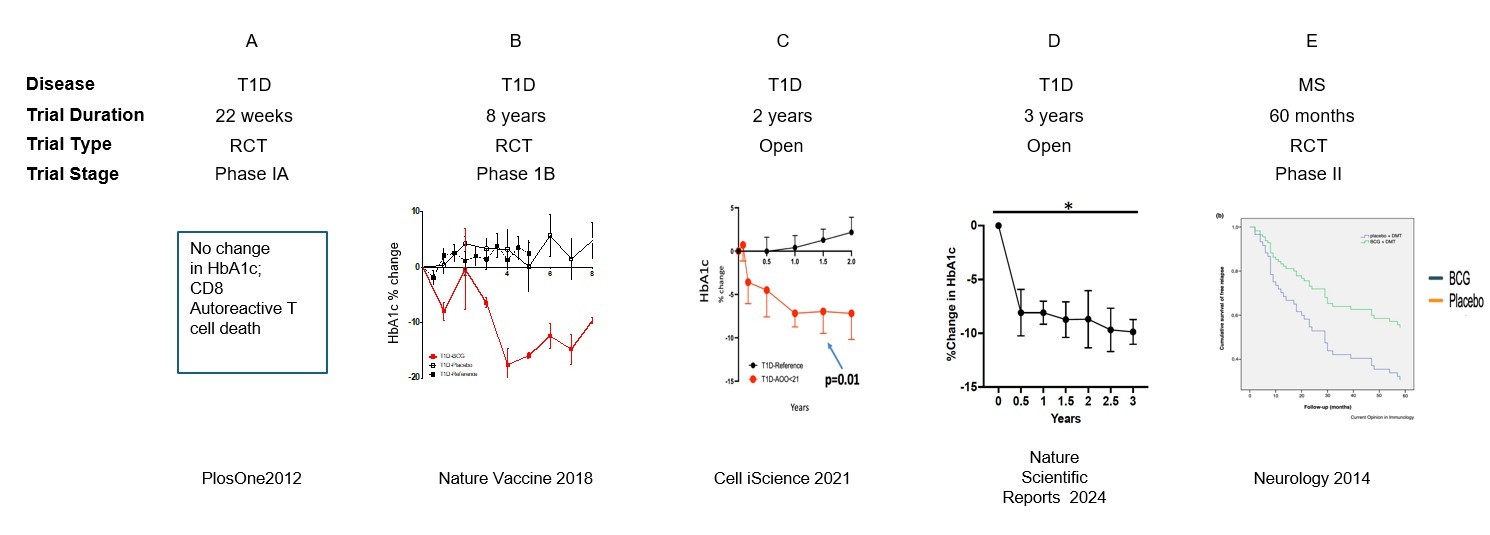
Our laboratory’s interest in BCG traces back more than a decade when we hypothesized that the BCG vaccine used not for prevention but as an immunotherapy would hold promise for treating type 1 diabetes based on its immunomodulatory effects. Our first randomized clinical trial found that multi-dose BCG produced favorable biomarker changes and transiently restored small amounts of pancreatic insulin—but did not lower hemoglobin A1c (HbA1c) by the conclusion of the 20-week trial [25]. We did observe the acute death of insulin autoreactive T cells from the known action of BCG to induce innate immunity and the release of TNF, a cytokine that at low dose kills only autoreactive T cells. Upon learning that BCG held therapeutic benefit for multiple sclerosis, another autoimmune disease, after a post-exposure latency period of years rather than months [26], we extended the period of observation of our trial long beyond 20 weeks. This made all the difference: we were able to show that BCG successfully reduced HbA1c levels to near normal in T1Ds after an interval of 3 years, and the benefit persisted for at least another 5 years [27]. Additional clinical trials, all conducted in the US, confirmed two more times that BCG successfully reduces HbA1c levels to near normal levels in T1Ds after a minimum interval of 2-3 years [28,29]. This exciting result was achieved in people with longstanding diabetes averaging 15–20 years duration (Figure 1).
Figure 1. Past adult BCG human clinical trials with autoimmune outcomes. The data shows, for both randomized double-blind trials (RCT) as well as for open label clinical trials, clinically meaningful outcomes in both autoimmune diabetes and multiple sclerosis. It is important to note for all clinical trials, at least in adults, a lag time of 2-3 years from the start of multi-dosing of BCG to the appearance of clinically meaningful outcomes.
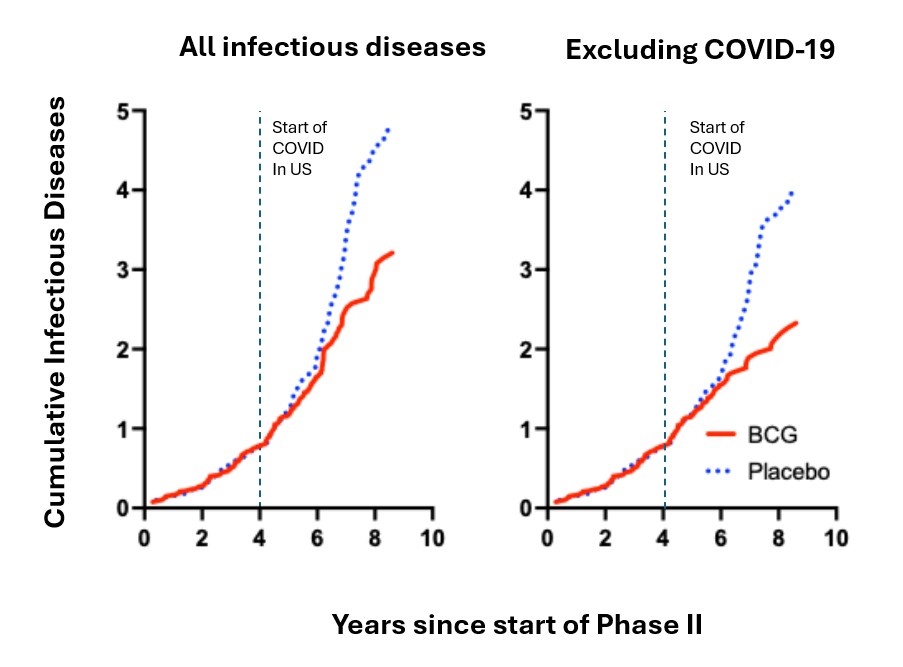
Later, in 2020, at the origin of the COVID-19 pandemic, we had underway a Phase 2 double-blind, placebo-controlled trial of multi-dose BCG for glycemic control in T1D. Patients already had received three doses of BCG or placebo two years earlier as part of a 5-year-long study. We adapted this trial into a parallel study to see if BCG could protect against primary infection with the original strain of SARS-CoV-2. The parallel study followed patients from January, 2020 until April, 2021, a 15-month period before the introduction of COVID-19 vaccines [22]. Our participants had received neither COVID-19 vaccine nor prior neonatal BCG vaccine, and they also were tuberculosis-negative. We found that multi-dose BCG was not only safe but was 92% effective compared to placebo in protecting type 1 diabetics from COVID-19 infection early in the pandemic [22] (Figure 2). Moreover, the BCG group (vs placebo group) had fewer infections of any type, fewer infectious symptoms, and lower infection severity (Figure 2). This kind of broad infectious disease protection suggested that BCG also might be advantageous against new SARS-CoV-2 variants. The trial’s strengths were multi-dose BCG, use of a potent strain of BCG (Tokyo-172), long follow-up period, and no systemic adverse events or patient drop-outs. The 92% efficacy was similar to that in both the Pfizer and Moderna mRNA clinical trials [30,31]. In sum, BCG was acting as a platform immunotherapy for infectious diseases and for all versions of the evolving COVID-19 virus.
Figure 2. All infectious diseases in Type 1 diabetics for 8 years: From Phase II RCT for diabetes outcomes, 8+ years. The data reveals in subjects receiving 6 BCG vaccines, two vaccines in year 01 followed by four yearly vaccines, that onset of infectious disease protection is a slow process whether for all infectious diseases or for COVID-19 protection. The dashed line is the start of the COVID-19 US pandemic. The data also shows the durability and growing stability of infectious disease protection against all infectious diseases with multi-dose BCG.
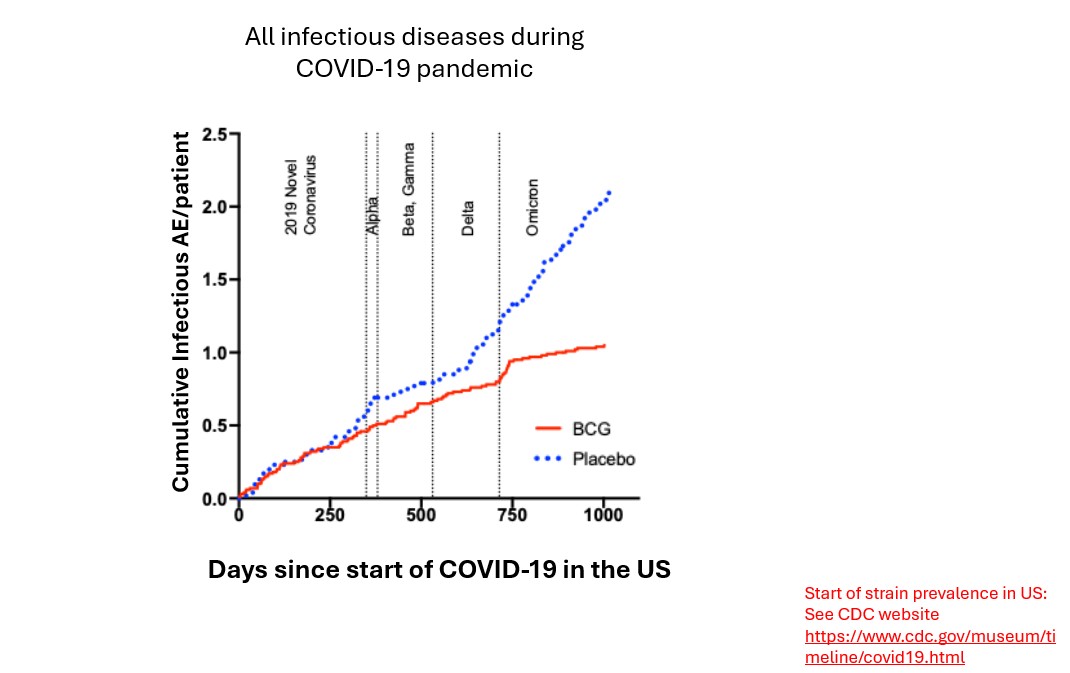
Our second trial, a Phase 3 trial, was a 19-month-long trial conducted late in the pandemic during the period of delta and omicron variant predominance, again in vulnerable T1D subjects. We found that BCG had an efficacy of 43.2% vs placebo against SARS-CoV-2 infection (p=0.023) [23]. When we combined results of the Phase 2 and 3 trials, we reported an efficacy of 54.3%. Also, BCG was acting like a platform vaccine and continued to work against evolving strains of the COVID-19 virus. In contrast, the COVID-19 mRNA vaccines not only had short duration of protection in the general US population, but also rapid obsolescence against viral drift during the pandemic. Our Phase 3 trial also demonstrated that mRNA vaccines had no efficacy in the type 1 diabetic population [23].
It is important to point out that like the multi-dose BCG clinical trials in autoimmunity, the clinically significant protection from all infectious disease or from COVID-19 required about 4 years for the placebo and BCG treatment groups to diverge (Figure 2). This re-set of the immune system was slow but stable and increased in magnitude with the passage of time.
Two other trials in high-risk populations supported our findings in high-risk T1D subjects. The first, known as ACTIVATE-2, was a multi-center, double-blind, placebo-controlled, phase III randomized trial of BCG for protection against COVID-19 among high-risk patients in Greece [32]. The high-risk patients were over 50 years old and nearly 20% had T2D. No T1Ds were included. The trial found a 68% reduced risk of symptom-based and/or laboratory-based COVID-19 infection compared to placebo. While it did not study protection against other pathogens, an earlier trial by the same investigators, known as ACTIVATE, reported that BCG (vs placebo) reduced the incidence of non-COVID upper respiratory tract infections in elderly individuals 65 years or older [12].
The second confirmatory trial, known as BRIC, was a phase 3, quadruple-blind, placebo-controlled, multi-center trial in high-risk adults (n=495; ages 18-60 years) in 3 hospitals in India [33]. Participation was excluded if patients had been vaccinated against COVID-19. Fifty percent of patients had diabetes, but the authors did not specify which type(s). A history of tuberculosis was detected in 6% of the BCG group and 4% of the placebo group. Participants were given single-dose BCG (the strain was unreported) or placebo and followed for 9 months. The primary outcome, new COVID-19 infection, was assessed by two methods: definite COVID-19 by cartridge-based nucleic acid amplification, or probable COVID-19 by symptom profile. The trial found that BCG did not prevent definite COVID-19, but it did prevent probable COVID-19. The BCG group displayed a 62% decrease in probable COVID-19 (vs placebo), based on an odds ratio of 0.38 (95% confidence interval, 0.20-0.72). The BCG group had fewer cases of severe COVID-19, fewer hospitalizations, and fewer cases requiring oxygen. All three of these secondary outcomes showed 0 cases in the BCG group and 6 cases (2.4%) in the placebo group, each with a P value of 03. The trial found no vaccine-related serious adverse events (grade 3 or grade 4). The US population is particularly well-suited for BCG human clinical trials because in the US the BCG vaccine has never been used routinely and has never been administered at birth like in most third-world countries.
Studies of Off-target Effects Need Long Term Follow-up Plus Other Design Features
When looking at the body of evidence, there have been 12 randomized clinical trials of BCG for COVID-19 prevention. Five of them, including our two US based clinical trials, show BCG efficacy against primary COVID-19 infection, while seven did not. Post-hoc analysis of 8 of these trials found that BCG was superior to placebo in preventing death from COVID-19 [34].
Design features of the negative trials may have precluded observing a BCG effect on primary prevention. First, the trial may not have excluded people with prior BCG or tuberculosis exposure, both of which confer long-term protection against infectious diseases [35]. Inclusion of BCG-vaccinated or tuberculosis-exposed subjects would have obscured finding a BCG benefit with respect to SARS-CoV-2 infection. Second, the trial may not have used the most potent strains of BCG. The strain we used, Tokyo 174, is the most potent [36]. Third, the trial may have used only a single dose instead of a multi-dose regimen of up to 6 doses. Multiple BCG doses work best to achieve the metabolic and T cell changes that occur in the adaptive and innate immune system, according to our mechanistic studies [4,28,29,37]. Fourth, the trial may have been in health care workers who as a group are resistant to infections and lack immune compromise. Fifth, and arguably the most important feature, the trial may not have been long enough in duration. BCG benefit takes at least 3–5 years to be realized against primary COVID-19 infection, but once obtained may last years to decades [27,35] instead of the months-long protection afforded by mRNA vaccines against primary infection [38,39]. Figure 1 summarizes the years-long interval needed to observe benefit from BCG in autoimmune patients never previously immunized with BCG. It captures our experience with BCG for improving glycemic control in T1D, as well as for preventing infectious diseases. The figure also summarizes the experience of the team studying BCG for the treatment of multiple sclerosis (MS) [26].
A key question is why BCG immunotherapy in US-based adults takes 2-3 years to show autoimmunity benefits and even 5 years for full infectious disease protection. In the case of T1D, BCG triggers a shift in host energy metabolism from oxidative phosphorylation to aerobic glycolysis, a transformation that occurs gradually over a 2-3 year interval [27]. We also have recently reported, on the basis of human imaging with positron emission tomography, that BCG’s glucose-lowering effect in T1Ds unfolds gradually in the spleen, where BCG takes up residence over a similar years-long time course [40].
Current Covid Vaccines Do Not Protect Type 1 Diabetics from SARS-CoV-2 Infection
In clinical trials of COVID-19 mRNA vaccines, people with diabetes were successfully protected against SARS-CoV-2 infection. We know this from subgroup analyses of the Pfizer and Moderna trials, as reported in FDA briefing documents. The documents revealed that the mRNA vaccine efficacy in diabetics ranged from 94 to 100%, a range similar to that seen in the general population. The problem is that the COVID-19 clinical trial data did not specify the type of diabetes. Since T1Ds account for only 6% of all diabetics [41], we assert that these trial results do not necessarily apply to T1Ds. In fact, there is evidence to the contrary. T1Ds are at least twice as likely to die from COVID-19 compared to T2D [3]. At least two real-world studies report that diabetics are more susceptible to breakthrough infection after COVID vaccination than nondiabetics [42,43]. In addition, when we separately analyzed the no-BCG placebo recipients in our trials, we found that the COVID-19 vaccine-treated subset developed COVID-19 at a rate no different from that in placebo subjects who never received COVID-19 vaccinations [23]. To reiterate, none of these placebo T1D subjects received prior BCG vaccine. Others have also found that the administration of the COVID-19 mRNA vaccines in T1D populations have low efficacy [44]. Others report, even more disconcertingly, that either COVID-19 mRNA vaccines or COVID-19 infection also can trigger new-onset diabetic disease [45,46]. Published research has also indicated that diabetics as a group are at greater risk of breakthrough infection after receipt of mRNA vaccine [42,43], which further suggests that the existing mRNA vaccines are less effective for diabetics when compared with non-diabetics. If diabetics, as a group, are at risk of breakthrough infection, then T1Ds, in particular, are likely to have an even poorer outcome. This is a reasonable inference based on findings wherein T1Ds, when compared to T2Ds, are at least twice more prone to dying from COVID-19 [3]. Taken together, this suggests an unmet need in T1Ds for an effective vaccine against all infections and/or COVID-19. We conclude that this group of highly infectious disease-susceptible adults, who also have poor responses to mRNA COVID-19 vaccines, may uniquely stand to gain from multi-dose BCG immunotherapy.
References
2. Barrett CE, Park J, Kompaniyets L, Baggs J, Cheng YJ, Zhang P, et al. Intensive Care Unit Admission, Mechanical Ventilation, and Mortality Among Patients With Type 1 Diabetes Hospitalized for COVID-19 in the U.S. Diabetes Care. 2021 Aug;44(8):1788–96.
3. Demirci I, Haymana C, Tasci I, Satman I, Atmaca A, Sahin M, et al. Higher rate of COVID-19 mortality in patients with type 1 than type 2 diabetes: a nationwide study. Endokrynol Pol. 2022;73(1):87–95.
4. Takahashi H, Kühtreiber WM, Keefe RC, Lee AH, Aristarkhova A, Dias HF, et al. BCG vaccinations drive epigenetic changes to the human T cell receptor: Restored expression in type 1 diabetes. Sci Adv. 2022 Nov 18;8(46):eabq7240.
5. Nieuwenhuizen NE, Kaufmann SHE. Next-Generation Vaccines Based on Bacille Calmette-Guérin. Front Immunol. 2018 Feb 5;9:121.
6. Calmette A. Preventive Vaccination Against Tuberculosis with BCG. Proc R Soc Med. 1931 Sep;24(11):1481–90.
7. Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011 Jul 15;204(2):245–52.
8. Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin Infect Dis. 2017 Oct 1;65(7):1183–90.
9. Biering-Sørensen S, Aaby P, Napirna BM, Roth A, Ravn H, Rodrigues A, et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr Infect Dis J. 2012 Mar;31(3):306–8.
10. de Castro MJ, Pardo-Seco J, Martinón-Torres F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis. 2015 Jun 1;60(11):1611–9.
11. Garly ML, Martins CL, Balé C, Baldé MA, Hedegaard KL, Gustafson P, et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003 Jun 20;21(21-22):2782–90.
12. Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020 Oct 15;183(2):315–23.e9.
13. Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008 Nov 13;337:a2052.
14. Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ. 2000 Dec 9;321(7274):1435–8.
15. Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med. 2018 Jul 12;379(2):138–49.
16. Pönnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992 Mar 14;339(8794):636–9.
17. Roth A, Gustafson P, Nhaga A, Djana Q, Poulsen A, Garly ML, et al. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol. 2005 Jun;34(3):540–7.
18. Shann F. The non-specific effects of vaccines. Arch Dis Child. 2010 Sep;95(9):662–7.
19. Stensballe LG, Nante E, Jensen IP, Kofoed PE, Poulsen A, Jensen H, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005 Jan 26;23(10):1251–7.
20. Walk J, de Bree LCJ, Graumans W, Stoter R, van Gemert GJ, van de Vegte-Bolmer M, et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat Commun. 2019 Feb 20;10(1):874.
21. Wardhana, Datau EA, Sultana A, Mandang VV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011 Jul;43(3):185–190.
22. Faustman DL, Lee A, Hostetter ER, Aristarkhova A, Ng NC, Shpilsky GF, et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep Med. 2022 Sep 20;3(9):100728.
23. Kühtreiber WM, Hostetter ER, Wolfe GE, Vaishnaw MS, Goldstein R, Bulczynski ER, et al. Late in the US pandemic, multi-dose BCG vaccines protect against COVID-19 and infectious diseases. iScience. 2024 May 22;27(6):109881.
24. BCG vaccines: WHO position paper – February 2018. Wkly Epidemiol Rec. 2018 Feb 23;93(8):73-96.
25. Faustman DL, Wang L, Okubo Y, Burger D, Ban L, Man G, et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS One. 2012;7(8):e41756.
26. Ristori G, Romano S, Cannoni S, Visconti A, Tinelli E, Mendozzi L, et al. Effects of Bacille Calmette–Guerin after the first demyelinating event in the CNS. Neurology. 2014 Jan 7;82(1):41–8.
27. Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018 Jun 21;3:23.
28. Kühtreiber WM, Takahashi H, Keefe RC, Song Y, Tran L, Luck TG, et al. BCG Vaccinations Upregulate Myc, a Central Switch for Improved Glucose Metabolism in Diabetes. iScience. 2020 May 22;23(5):101085.
29. Dias HF, Kühtreiber WM, Nelson KJ, Ng NC, Zheng H, Faustman DL. Epigenetic changes related to glucose metabolism in type 1 diabetes after BCG vaccinations: A vital role for KDM2B. Vaccine. 2022 Mar 8;40(11):1540–54.
30. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403–16.
31. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–15.
32. Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front Immunol. 2022 Jul 5;13:873067.
33. Sinha S, Ajayababu A, Thukral H, Gupta S, Guha SK, Basu A, et al. Efficacy of Bacillus Calmette-Guérin (BCG) Vaccination in Reducing the Incidence and Severity of COVID-19 in High-Risk Population (BRIC): a Phase III, Multi-centre, Quadruple-Blind Randomised Control Trial. Infect Dis Ther. 2022 Dec;11(6):2205–17.
34. Benn CS, Netea MG, Aaby P. BCG to Protect against Covid-19 in Health Care Workers. N Engl J Med. 2023 Jul 13;389(2):191–2.
35. Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA. 2004 May 5;291(17):2086–91.
36. Angelidou A, Conti MG, Diray-Arce J, Benn CS, Shann F, Netea MG, et al. Licensed Bacille Calmette-Guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine. 2020 Feb 24;38(9):2229–40.
37. Keefe RC, Takahashi H, Tran L, Nelson K, Ng N, Kühtreiber WM, et al. BCG therapy is associated with long-term, durable induction of Treg signature genes by epigenetic modulation. Sci Rep. 2021 Jul 22;11(1):14933.
38. Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022 Jun 2;13(1):3082.
39. Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022 Mar 9;376:e069761.
40. Dias HF, Fu JF, Luck TG, Wolfe GE, Hostetter ER, Ng NC, et al. The spleen assumes a major role in blood glucose regulation in type 1 diabetes patients treated with BCG. Sci Rep. 2024 Jul 30;14(1):17611.
41. Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, et al. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018 Mar 30;67(12):359–361.
42. Basso P, Negro C, Cegolon L, Larese Filon F. Risk of Vaccine Breakthrough SARS-CoV-2 Infection and Associated Factors in Healthcare Workers of Trieste Teaching Hospitals (North-Eastern Italy). Viruses. 2022 Feb 7;14(2):336.
43. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021 Nov;27(11):1652–7.
44. D'Addio F, Sabiu G, Usuelli V, Assi E, Abdelsalam A, Maestroni A, et al. Immunogenicity and Safety of SARS-CoV-2 mRNA Vaccines in a Cohort of Patients With Type 1 Diabetes. Diabetes. 2022 Aug 1;71(8):1800–6.
45. Karavanaki K, Rodolaki K, Soldatou A, Karanasios S, Kakleas K. Covid-19 infection in children and adolescents and its association with type 1 diabetes mellitus (T1d) presentation and management. Endocrine. 2023 May;80(2):237–52.
46. Yano M, Morioka T, Natsuki Y, Sasaki K, Kakutani Y, Ochi A, et al. New-onset Type 1 Diabetes after COVID-19 mRNA Vaccination. Intern Med. 2022 Apr 15;61(8):1197–200.