Abstract
SIRPIDs (Stimulus Induced rhythmic, periodic, or ictal discharges) represent a relatively newer addition to the terminology of electroencephalography (EEG) monitoring. They are meant to represent findings that may have epileptogenic potential and are repeatedly produced in response to various stimuli. SIRPIDs have variable association with various pathologies and the concurrent occurrence of seizures, which adds to the difficulty in quantifying their epileptogenic potential and offering consistent treatment guidelines. We describe a rare case of a patient that presented with a left hemispheric subdural hematoma and developed left hemispheric onset seizures and right hemispheric onset SIRPIDs in response to physical stimulation. Our case aims to highlight the diverse nature of SIRPIDs and showcase unusual presentations of SIRPIDs that make recognizing this unusual condition more challenging and contribute to a better understanding of these weird EEG findings.
Keywords
Seizures, SIRPIDs, Status epilepticus, Video EEG monitoring, ICU EEG monitoring
Abbreviations
CT: Computed Tomography; MRI: Magnetic Resonance Imaging; EEG: Electroencephalography; ICU: Intensive Care Unit; VEEG: Video EEG Monitoring; ICU EEG: ICU EEG Monitoring; ASM: Anti-Seizure Medications; PT-INR: Prothrombin Time with International Normalized Ratios; SIRPIDs: Stimulus Induced Rhythmic, Periodic or Ictal Discharges; PET: Positron Emission Tomography; SPECT: Single Photon Emission Computed Tomography; MEG: Magnetoencephalography; NCSE: Nonconvulsive Status Epilepticus
Introduction
Video EEG (VEEG) represents one of the mainstays of diagnostics for the management of seizures and encephalopathy in inpatient settings and intensive care units (ICUs). VEEG studies show high prevalence of seizures and greatly correlate with rapid diagnostics and improved outcomes, when performed promptly and are used to guide management of care in these patients. Seizures are often readily captured on VEEG and can be caused by a variety of etiologies, such as strokes, tumors, intracranial hemorrhage, trauma, etc. The management of seizures on VEEG is relatively straightforward and follows existing and established guidelines. However, VEEG often captures findings of poorly defined etiology or unclear epileptogenic potential. Management of these findings is less well defined and often performed based on individual opinion of the interpreting EEG reader or ICU physician and requires expert opinion and consensus discussion amongst treatment teams to enable the optimal plan of action in such cases.
The American Clinical Neurophysiological Society (ACNS) has defined some of these unusual findings or terminologies and sought to standardize nomenclature and assist with developing consensus in treatment guidelines. Epileptiform discharges (spikes or sharp waves), lateralized rhythmic delta activity (LRDA) and lateralized periodic discharges (LPDs) are among the well-defined and commonly seen findings with high epileptogenic potential and established treatment guidelines. SIRPIDs represent a relatively newer phenomenon that remain incompletely understood and encompass EEG findings with diverse etiologies and patterns on EEG. Their epileptogenic potential varies depending on the cause and morphology on VEEG. This results in varying degrees of aggressiveness of treatment offered to patients with these findings. Some of these EEG terminologies are described in Table 1 for the convenience of the reader.
|
Terminology |
Location |
Modifiers / Types |
Clinical Significance |
|
Epileptiform discharges |
Lobar or multifocal or generalized or bilateral independent |
Spikes (<200 ms), sharp waves (>200 ms), spike-waves, polyspikes |
Possess significant epileptogenic potential and are accepted as having high risk for associated seizures |
|
Periodic discharges (PDs) |
Lobar or multifocal (L) or generalized (G) or bilateral independent |
Fast activity (+F) or rhythmic slowing (+R) or sharply contoured (+S) or triphasic morphology (T) |
Lateralized periodic discharges have high risk of associated seizures. Generalized periodic discharges usually reflect metabolic dysfunction – unless sharp or fast modifiers are seen which increase seizure risk. Fast activity or rhythmic slowing or both occur with these and were sometimes called afterdischarges—these have very high epileptogenic potential and associated risk for seizures. Triphasic morphology is often associated with metabolic dysfunction and low seizure risk unless sharp contour present. Presence of sharp contours often correlates with higher epileptogenic potential/seizure risk. |
|
Rhythmic delta activity/slowing (RDA) |
Generalized (G) or lateralized (L) |
Fast activity (+F) or sharply contoured (+S) |
Generalized rhythmic delta is usually suggestive of metabolic dysfunction with low seizure risk—presence of fast or sharply contoured activity suggests relatively higher seizure risk. Lateralized rhythmic delta has high risk of associated seizures—presence of fast or sharply contoured activity raises seizure risk even more. |
|
Stimulus induced rhythmic slowing or periodic (potentially ictal) discharges (SIRPIDs) |
Generalized (G) (common), Lateralized (L) (rare) |
Periodic discharges (PDs) or rhythmic slowing (RDA) |
These can occur from a variety of stimuli such as touch, sound, light exposure or other stimuli. Generalized SIRPIDs (periodic discharges or slowing) are more likely to reflect metabolic dysfunction and have undefined, but probably lower risk of associated seizures. Lateralized SIRPIDs (periodic discharges or slowing) are more likely to have higher risk of associated seizures. |
|
|
|
|
|
|
Spike (<200 ms duration) |
Sharp Wave (>200 ms duration) |
Sharp Wave (>200 ms duration) |
|
|
|
|
|
|
|
Polyspike |
Periodic discharges (PDs) |
Triphasic Waves (GPDs-T) |
|
|
|
|
|
|
|
Periodic discharges with following fast activity (afterdischarges) (PDs+F) |
Rhythmic slowing (RDA) |
Rhythmic slowing with intermixed fast activity (RDA+F) |
|
|
|
|
|
|
Our case report describes a patient who developed seizures along with contralateral SIRPIDs from a subdural hematoma and received aggressive treatment. We seek to share this data to contribute to the field of ICU EEG to enable better understanding and consensus in treatment guidelines for these uncommon and unusual findings.
Case Presentation
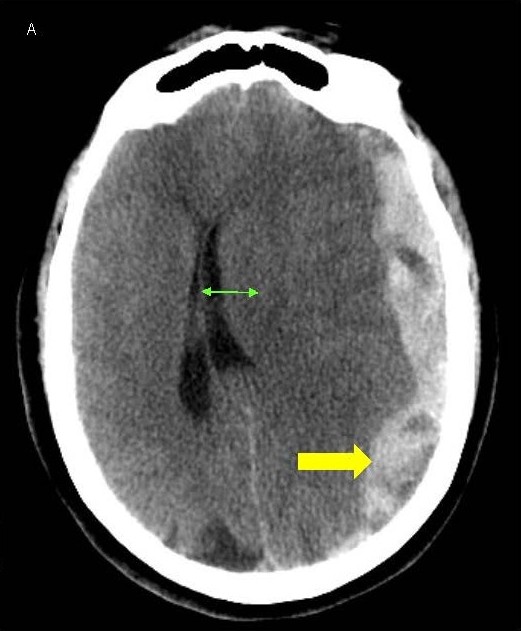
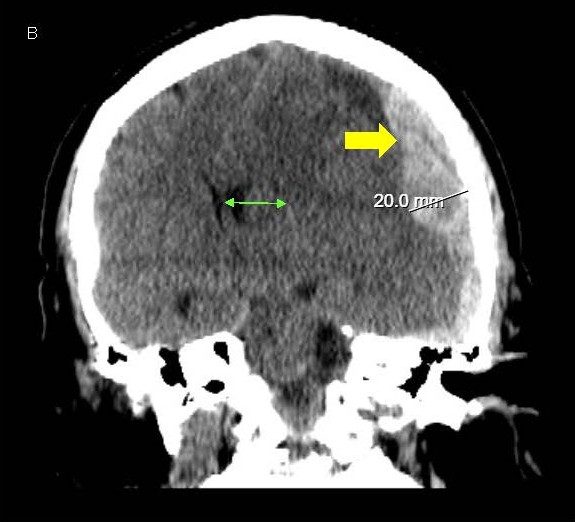
A 64-year-old woman presented to our emergency room with sudden onset severe headache and word finding difficulty that progressed to include lethargy and confusion. She had a history of pulmonary embolism treated with warfarin therapy, along with ischemic heart disease and heart failure, being treated with clopidogrel and controlled hypertension as well. She was disoriented to time and place and complained of a headache and word finding difficulty but had an otherwise normal exam. Lab tests were unremarkable other than an elevated PT-INR of 2.6 from warfarin. CT scans revealed a moderately large subdural hematoma over the left hemisphere, 2 cm thickness, with significant midline shift of 1.7 cm to the right and early left uncal herniation as well (Figures 1A and 1B). Due to the high risks of morbidity or mortality from the large hematoma and significant midline shift and herniation, aggressive treatment was instituted. She was given prothrombotic agents for reversal of warfarin and underwent emergent decompressive craniectomy.
Figure 1. A, B) CT scans of the head showing LEFT sided subdural hematoma (yellow arrow) with midline shift (green arrow).
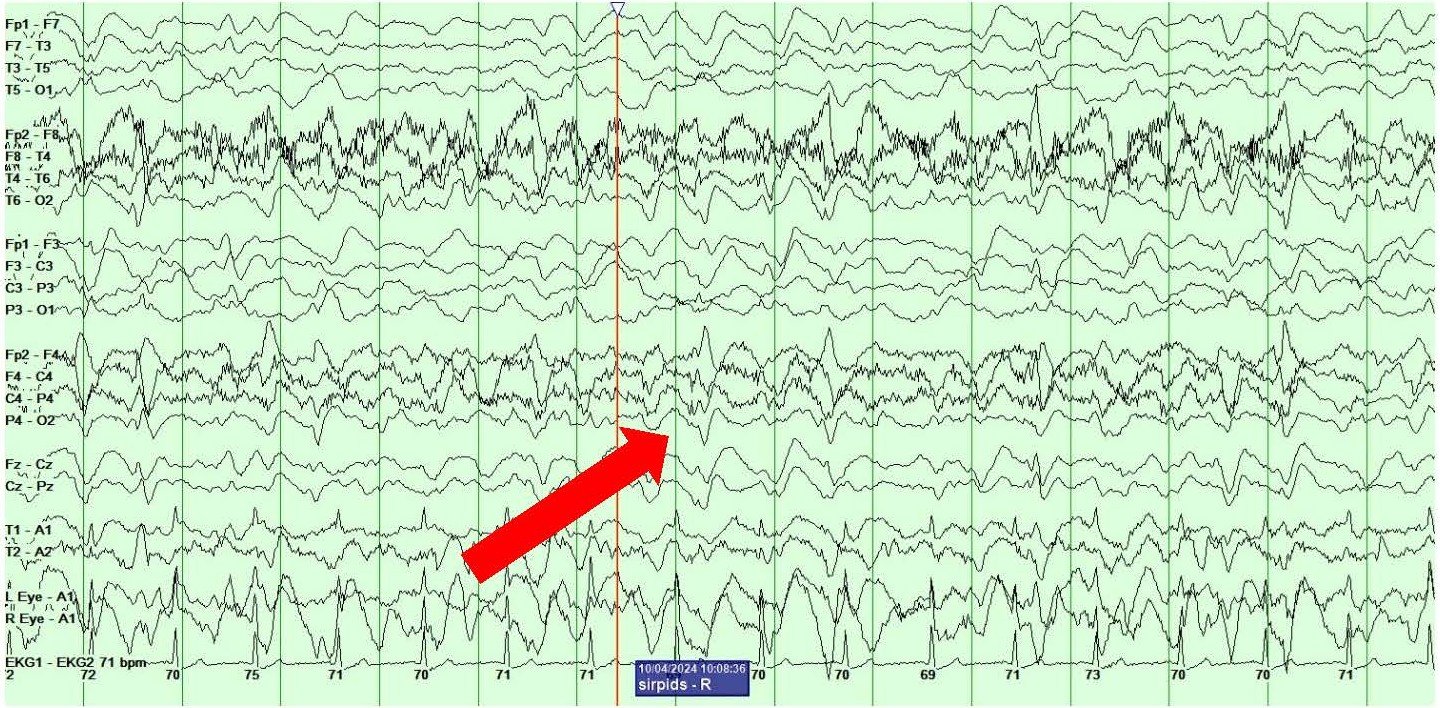
She remained awake but obtunded, after the surgery. Routine EEG done for evaluation of her obtundation, 2 days after the surgery showed diffuse polymorphic slowing along with left hemispheric polymorphic slowing and breach artifact along with occasional generalized periodic discharges/GPDs with triphasic morphology suggestive of postoperative and metabolic cerebral dysfunction (Figure 2). She continued to received supportive postoperative care in the ICU with minimal clinical improvement.
Figure 2. Routine EEG showing diffuse polymorphic slowing along with LEFT hemispheric polymorphic slowing and breach artifact and occasional triphasic morphology generalized periodic discharges (GPDs marked by red arrow).
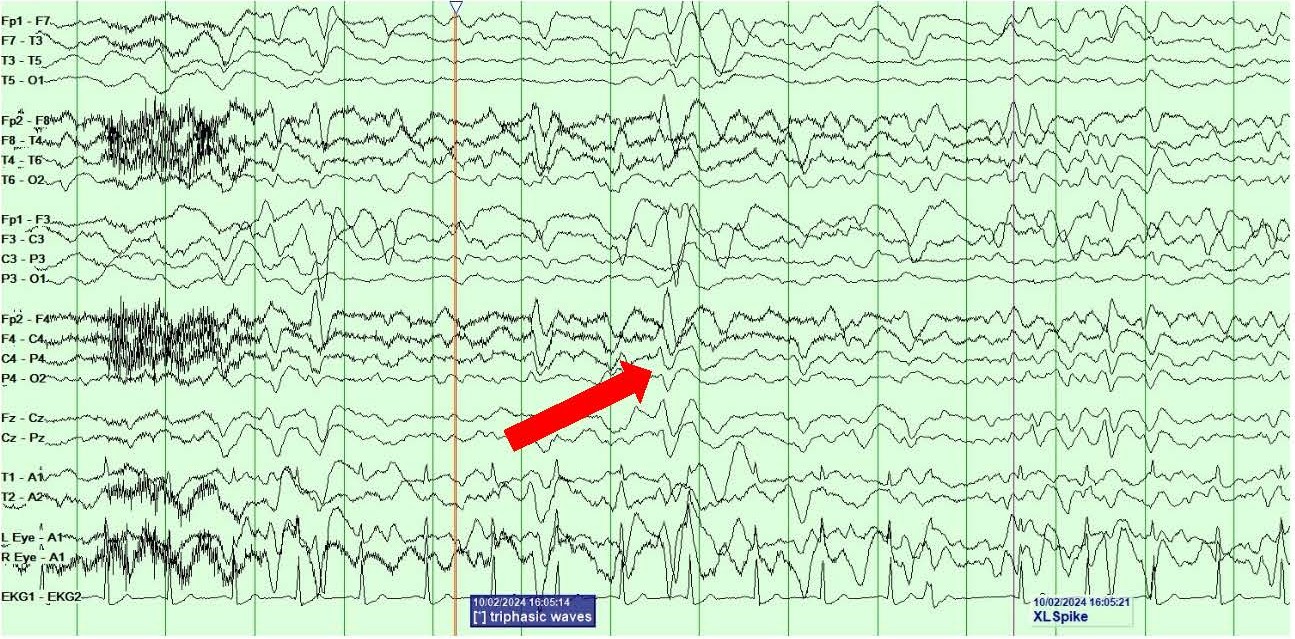
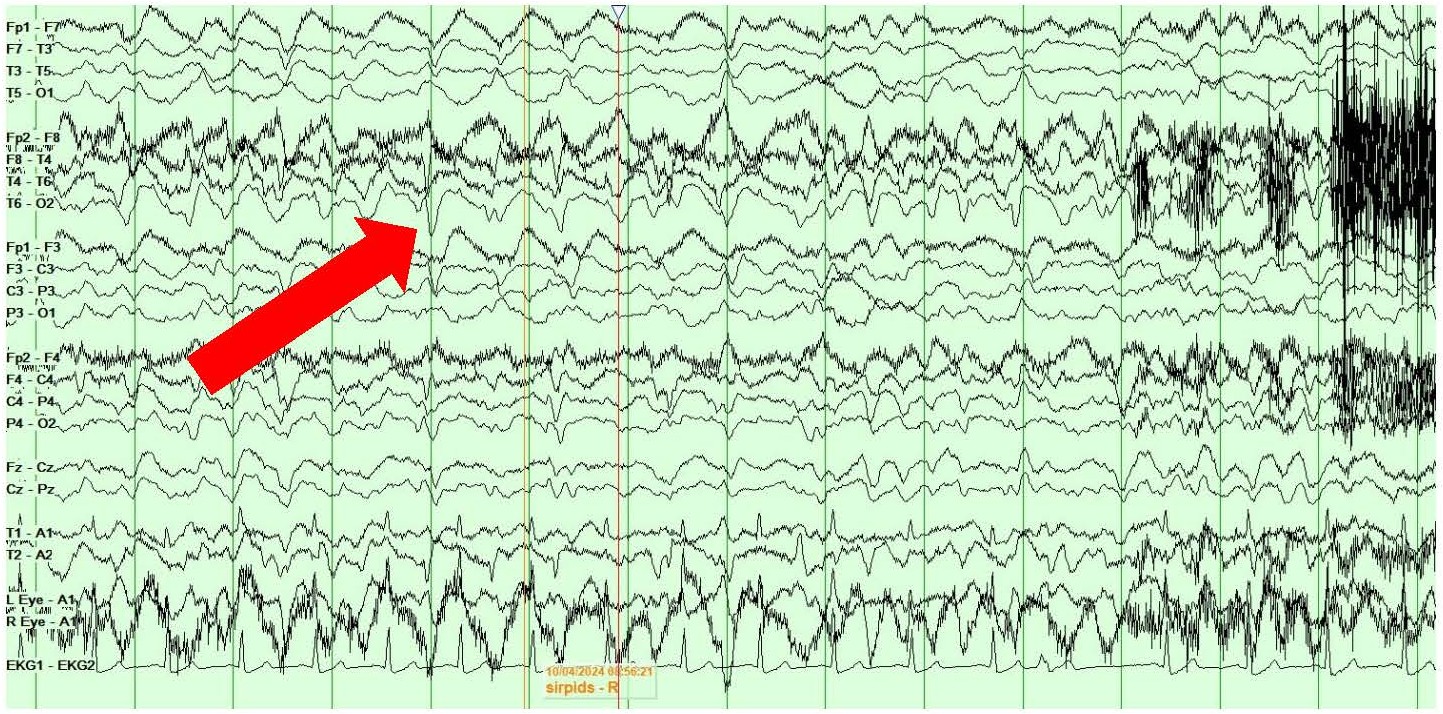
On day 4 of postoperative care, she developed right face and arm twitching along with rightward gaze deviation consistent with clinical focal onset motor seizures. VEEG showed the presence of left central onset epileptiform discharges, LPDs and multiple seizures with secondary generalization lasting 1-2 minutes each (Figures 3A and 3B). Clinically, the seizures presented with rightward gaze deviation, right jaw/mouth twitching and biting on the breathing tube and right arm twitching.
Figure 3. A, B) Screenshots from Video EEG showing LEFT hemispheric onset seizures (marked by red arrows).
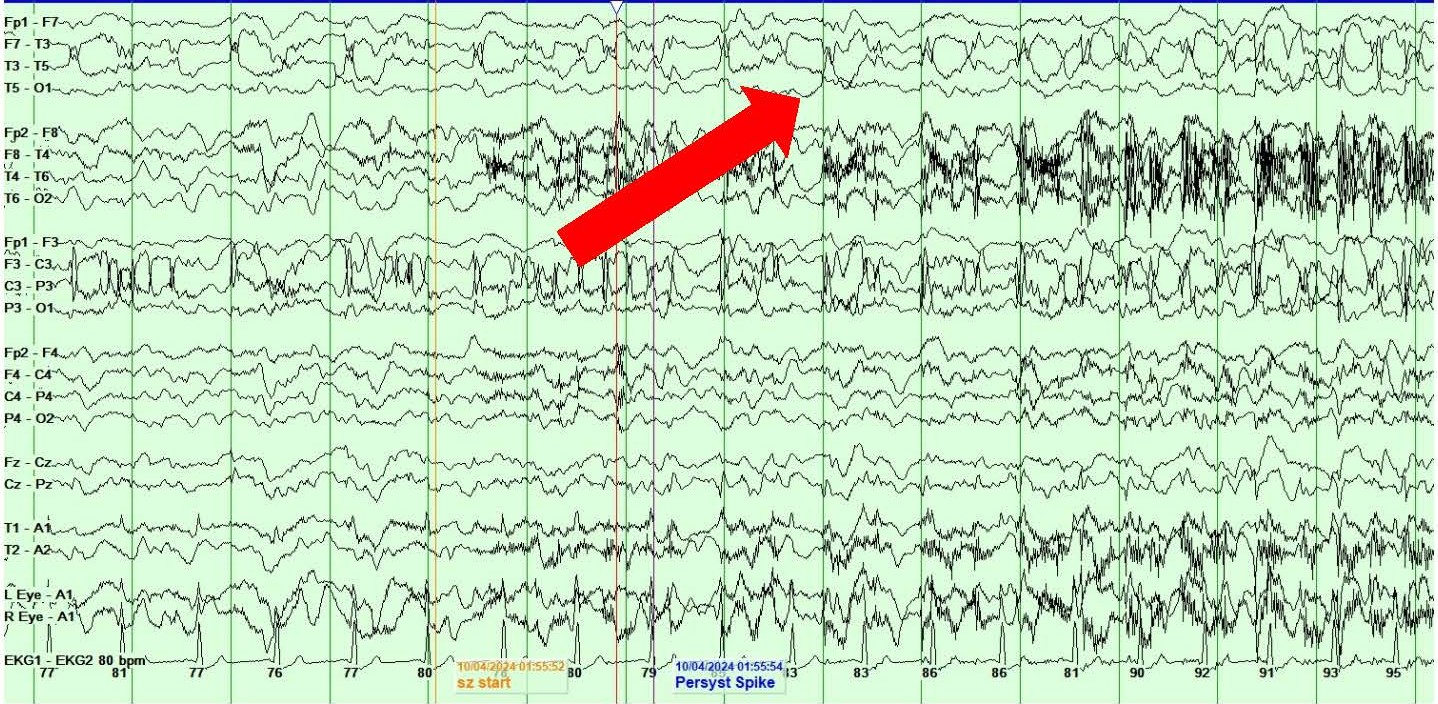
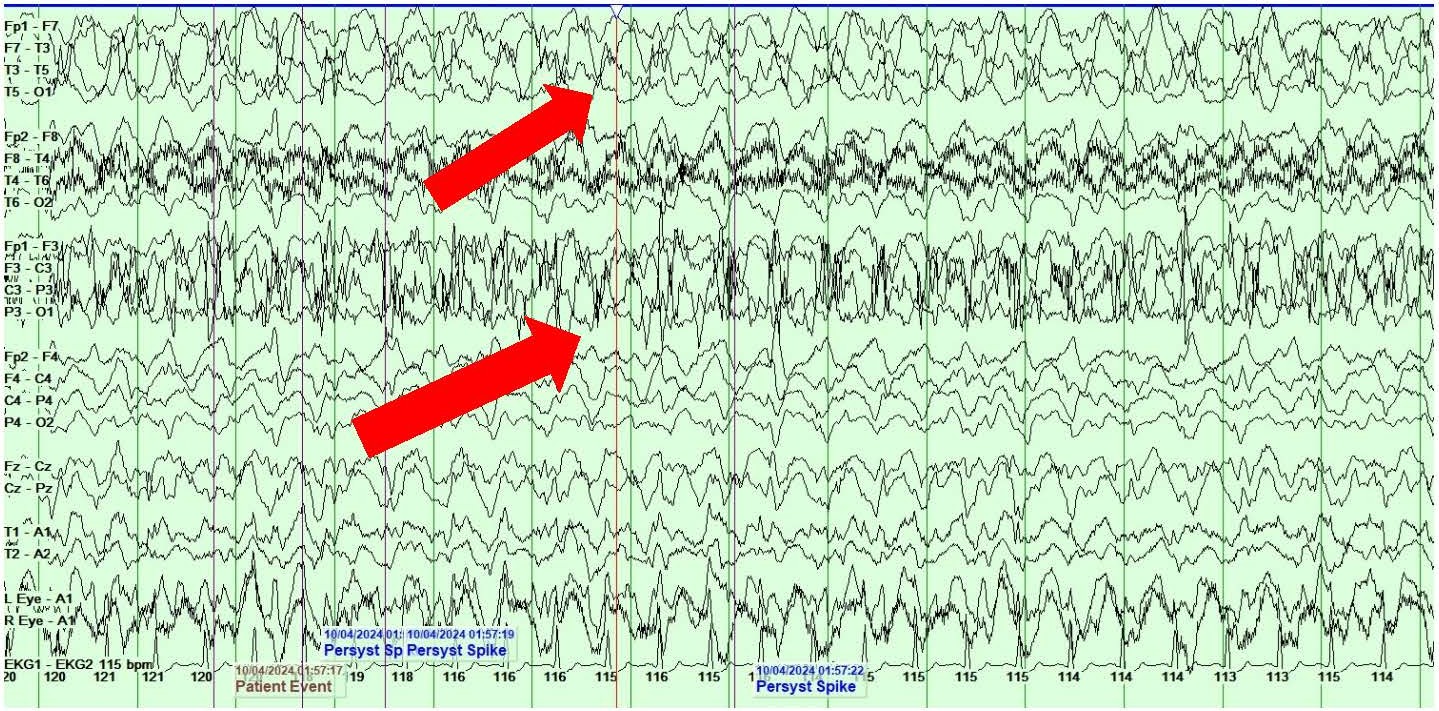
She also showed the presence of right hemispheric LPDs at 1-2 Hz and LRDA at 2-3 Hz that lasted for 1-10 minutes and occurred consistently with stimulation during physical manipulation or care by the medical providers and nursing staff (Figures 4A and 4B). These findings occurred independent of the previously described left hemispheric onset seizures mentioned above. They were felt to represent SIRPIDs (SI-LPDs and SI-LRDA).
Figures 4. A, B) Screenshots from Video EEG showing RIGHT hemispheric SIRPIDs provoked by stimulation (SI-LRDA and SI-LPDs) (marked by red arrows).
Due to these highly epileptogenic findings of seizures and SIRPIDs on a backdrop of subdural hematoma with herniation and cerebral edema, she was started on anti-seizure medications (ASMs) with the use of IV levetiracetam 2 gm IV followed by 1,500 mg IV twice daily maintenance, and lorazepam 2 mg IV every 6 hours as needed for convulsions or seizures, for treatment with continued ICU EEG for monitoring of her seizures and mental status. Her seizures slowed down over the first 24 hours of treatment without completely resolving, while her SIRPIDs resolved within the first 24 hours. Lacosamide at 200 mg IV twice daily was added to her treatment regimen on postoperative day 5 for better seizure control, and her seizures resolved in the next 24 hours. Levetiracetam and Lacosamide were used due to their proven efficacy in such situations and ease of use in critical care settings in patients with complex multisystemic pathologies, due to minimal pharmacokinetic and pharmacodynamic interactions. Lorazepam was used intermittently due to presence of multiple seizures and highly epileptogenic and lateralized appearance of her SIRPIDs.
After resolution of her seizures, she had persistent diffuse and left hemispheric polymorphic slowing with breach activity along with interictal right sided LPDs and LRDA, but no evidence of recurrent seizures or SIRPIDs. Video EEG was continued till day 7 postoperatively to ensure that her seizures or SIRPIDs did not recur. She underwent routine EEGs on days 8 and 9 postoperatively to confirm absence of seizures and evaluate her mental status and these EEGs showed findings of metabolic dysfunction similar to the routine EEG on day 3 postoperatively – similar to Figure 2.
In spite of all this treatment, she remained encephalopathic and minimally responsive to painful stimuli. She continued to receive supportive care for her encephalopathy and medical needs in the ICU from the neurosurgical and critical care teams. Unfortunately, she did not show much improvement in her clinical status despite all the offered treatment, and her prognosis was felt to be poor due to her age, postoperative course from the subdural hematoma and her medical comorbidities including pulmonary embolism and ischemic heart disease. This was discussed with her family who opted to withdraw care due to her declining condition and overall poor prognosis and transition to terminal palliative care. She was extubated and died on day 10 of her postoperative course due to lack of significant improvement from treatment for her presenting hemorrhage and cerebral edema with superimposed extensive medical comorbidities.
Discussion
Prevalence
According to retrospective studies, 10% to 34% of patients receiving EEG monitoring experience SIRPIDs, making them a quite common occurrence in the critically ill [1–3]. Hirsch et al.'s [1] first retrospective analysis revealed that 33 out of 150 patients (22%) had SIRPIDs; the majority of these patients were in an ICU. SIRPIDs were found in 43/416 critically sick patients with EEG (10.3%) in a retrospective international multicenter research [2]. SIRPIDs were seen in 4 out of 33 (12.1%) patients who had VEEG monitoring in critical care units in a short retrospective single-center investigation [4]. SIRPIDs were eventually identified in 19.3% of patients in the prospective cohort of Alsherbini et al. [5], using a uniform stimulation technique (although at least one electroencephalographer read SIRPIDs in 38.5% of patients). In a multicenter study of 4,772 patients undergoing continuous EEG monitoring, Rodriguez Ruiz et al. [6] discovered that 19.9% of recorded sessions with periodic or rhythmic patterns had a stimulus-induced character. Variability of prevalence across different centers is felt to be related to differences in patient profiles and diagnoses, and definitions and criteria for monitoring and recognizing SIRPIDs, along with a reasonable degree of inter-rater variability in recognizing SIRPIDs.
Longer periods of periodic discharges (i.e., longer than 5 days) were significantly associated with SIRPIDs, P value 0.019, according to Ong et al. [3] 's study of patients undergoing prolonged EEG monitoring (duration of 10 days or more). This relatively high prevalence found with prolonged monitoring may indicate that SIRPIDs are more common than otherwise reported, but transient phenomenon occurring during a phase of a patient's recovery from brain dysfunction.
Etiology
The most frequent cause of SIRPIDs, according to Hirsch et al. [1], is subarachnoid hemorrhage, and cerebral hemorrhage, along with female sex and prior status epilepticus, is an independent predictor of SIRPIDs. In the prospective study by Alsherbini et al. [5], traumatic brain injury was the most common cause, occurring in 39% of patients. This was followed by encephalopathy (i.e., toxic-metabolic abnormalities, drug overdose, anoxia) in 15%, stroke in 13%, intracerebral or intraventricular hemorrhage in 12%, status epilepticus in 11%, aneurysmal subarachnoid hemorrhage in 10%, and meningitis or encephalitis in 8% of patients. Three of Van Straten et al.'s four SIRPID patients had a traumatic brain injury etiology, which was noticeably greater than that of the control group of SIRPID patients. According to multivariable analysis, SIRPIDs were substantially linked to anoxic brain injury in the retrospective multicenter investigation by Braksick et al., [2] with an odds ratio of 3.80 (1.73–8.33, P,0.001).
In patients who have anoxic damage following cardiac arrest, rhythmic, periodic, or ictal discharges triggered by stimuli are rather prevalent. Among 105 patients who survived cardiac arrest and received therapeutic hypothermia 13.3% had SIRPIDs, according to Alvarez et al. [7]. It's also worthwhile to mention more uncommon etiologies. Creutzfeldt-Jakob disease (CJD) patients may have PDs that respond to stimulation. A CJD case that began with nonconvulsive status epilepticus (NCSEs) and progressed through SIRPIDs and nonstimulus sensitive GPDs following treatment was reported by Rossetti et al. [8].
Pathophysiology
Periodic or rhythmic discharges produced by stimulation (SIRPIDs) have an unknown etiology. After a thorough analysis of individuals with a range of periodic discharges, it was shown that periodicity in general necessitates a disruption of both cortical and subcortical systems [9–13]. Periodic discharges "represent an abnormal response of the cortex, thalamocortical neurons, or both to rhythmic burst firing generated by the reticular thalamic nucleus," according to this idea, which was primarily put up by Gloor and most recently summed up by Gross et al. [14]. It also seems that metabolic disorders are a significant factor [11,15,16]. It is debatable how to interpret periodic discharges on EEG. Periodic patterns like periodic lateralized epileptiform discharges (PLEDs) are sometimes ictal [17,18], but most experts think they are typically interictal [13,19,20], a terminal phase of status epilepticus, or on an unstable interictal–ictal continuum [21]. The presence of multifocal periodic discharges or modifiers like fast activity or rhythmic slowing can also affect the probability of epileptogenicity by raising the risk of associated seizures.
Why would such EEG patterns result from alerting stimuli? A complicated circuit comprising reciprocal connections between the cortex and thalamus mediates arousal, which starts in the upper brainstem, particularly the midbrain reticular formation [22,23]. Cortical projections have been shown in experiments to have significant impacts on intrinsic thalamic oscillations [24]. Therefore, it should come as no surprise that rhythmic or periodic discharges can be observed when the arousal circuit is activated. The natural occurrence of hypnagogic hypersynchrony (high-voltage rhythmic delta activity during slumber in infants [25]) and the same but aberrant frontal rhythmic delta observed on waking in encephalopathic adults are likely related to a similar process. The latter pattern is known as "paradoxical arousal" [26,27]. Other EEG examples include the well-known (albeit underreported in the literature) rise in prominence of the triphasic waves [28,29] that some patients experience following alerting stimuli and the periodic discharges of CJD [30–32].
The last two instances could serve as useful models for researching SIRPIDs. According to certain data, brain oscillations—especially those involving the thalamus—determine behavioral state, and certain seizures are also linked to the thalamocortical projections in this arousal circuit [23].
The almost universal occurrence of infantile spasms immediately upon arousal [33,34] and startle-induced seizures, which typically originate from the supplementary sensorimotor area in the frontal lobe (which has close ties to the thalamus) [35], are clinical examples of the epileptogenic effect of alerting or arousal. Patients with epilepsia partialis continua have been observed by us and others [36] to exhibit a sharp rise in focal clonic activity upon waking. According to Niedermeyer [37], the main abnormality, or "prime mover," in individuals with primary generalized epilepsy is disordered arousal. This is shown clinically by seizures upon awakening and electrochemically by the appearance of widespread epileptiform discharges on K-complexes (also known as "dyshormia"). Niedermeyer also highlights and examines the evidence that demonstrates a wide degree of overlap in the neuroanatomical pathways related to arousal, spindles, and generalized epileptiform discharges [38]. The phenomenon of stimulation-induced massive myoclonus with bursts of epileptiform activity observed in patients with severe postanoxic injury may be more pertinent to our population of critically ill patients [39]. These phenomena all demonstrate the significant impact that arousal can have on epileptiform activity manifestation.
Cefepime induced neurotoxicity has become an increasingly common diagnosis seen in ICUs and is often recognized first in encephalopathic patterns treated with the drug due to typical EEG patterns. It was originally seen in patients with impaired renal function and presumed to be from retention of the drug, but has since been seen in patients with normal renal function, reflecting inherent neurotoxic effects of the drug at normal concentrations [44]. VEEG correlates include generalized rhythmic delta activity (GRDA), GPDs, triphasic GPDs, SIRPIDs with RDA or PD morphology, and in rare cases, NCSEs with myoclonic jerks affecting various muscle groups [45]. SIRPIDs in these patients have usually had generalized patterns rather than focal, unless they had underlying cerebral lesions as well [44]. The SIRPIDs seen in such patients have often responded to treatment with benzodiazepines or conventional ASMs, although cessation of cefepime use has been the most effective maneuver to result in cessation of these patterns on EEG and produce clinical improvement [44]. The cefepime neurotoxicity model is felt to be a classic example of cerebral electrical dysfunction in ICU settings due to VEEG findings and underlying mechanisms of neurotoxicity of the drug on subcortical circuits and is a frequently studied phenomenon to help improve understanding of SIRPIDs.
Various radiological and neurophysiological modalities have been used to understand and explain the phenomenon of SIRPIDs. While most of the work has involved patients with periodic discharges rather than rhythmic slowing, the principles underlying these have substantially improved our understanding of ICU patterns and expanded our definitions of SIRPIDs and the newly coined ictal-interictal continuum [46]. SIRPIDs can result in transient focal diffusion restriction on diffusion-weighted imaging (DWI) images with corresponding apparent diffusion coefficient (ADC) signal reduction. These findings likely result from increased metabolism, hyperperfusion, and cytotoxic edema. Many patients had restricted diffusion in the thalamic regions supporting the hypotheses of thalamocortical dysregulation producing SIRPIDs [47]. These findings were seen in patients with both focal and generalized patterns of SIRPIDs. Restricted diffusion in MRI has also been seen with SIRPIDs in patients with posterior reversible encephalopathy syndrome (PRES), with or without the presence of concomitant seizures—another finding that supports the genesis of SIRPIDs by subcortical networks [48].
Fluorodeoxyglucose positron emission tomography (FDG-PET) measures cerebral metabolism by evaluating glucose uptake. It frequently displays hypermetabolism during seizures and has been used to study epileptogenic potential of SIRPIDs to guide treatment. In most studies, lateralized patterns have been associated with hypermetabolism, whereas generalized patterns have been associated with both hypometabolism and hypermetabolism. FDG-PET hypermetabolism is believed to serve as a common biomarker of epileptogenic activity or potential. In some studies, 80% of patients had hypometabolism on PET during interictal states, while ictal states displayed hypermetabolism and increased cerebral blood flow. Lateralized patterns correlated with increased regional blood flow and local cerebral metabolic rate in all patients that showed them [49]. These findings have often resolved with treatment and coincided with clinical improvement supporting the clinical utility of PET in patient care.
ICU care for neurological patients often includes advanced multimodal brain monitoring, such as intracranial pressure, brain tissue PO2 (PbtO2), and cerebral microdialysis (CMD). These modalities are intended to diminish secondary cerebral injury by measuring and optimizing cerebral perfusion pressure, oxygen therapy, red blood cell transfusion, and metabolic control and represent some of the cutting-edge monitoring systems being used for the management of severely compromised neurological patients. Pathological spreading cortical depressions result in significant metabolic disturbance evidenced by alterations in CMD biomarkers, such as reduced extracellular glucose and elevated lactate-pyruvate ratios and glutamate levels, thus producing a metabolic crisis. Many SIRPIDs have similar metabolic crisis profile compared with seizures. These findings have prompted clinicians to treat many SIRPIDs and seizures similarly [50].
Single positron emitted computed tomography (SPECT) is used to study regional cerebral blood flow, a marker of functional impairment. Increased localized isotope uptake occurs during ictal events (defined as hyperperfusion), whereas a decreased isotope uptake could be seen interictally and postictally (hypoperfusion). SPECT reflects increased neuronal activity that could most probably be secondary to an ictal event. SPECT is a good tool to detect functional impairment, especially when the brain MRI is unremarkable or lags in findings. This is due to commonly believed hypothesis that functional impairment in cerebral disease can precede structural abnormalities. Hyperperfusion (positivity) on SPECT has been used as a marker to determine the need for treatment of SIRPIDs with ASMs and has correlated with outcomes and clinical benefit in many cases and reflects the strong utility of SPECT in determining need for aggressive vs milder treatment in SPECT positive vs SPECT negative patients with SIRPIDs [42]. Similar principles have also been used to study abnormal EEG findings in CJD patients, suggesting a similar pathophysiology between SIRPIDs and startle myoclonus, and other electroclinical manifestations of CJD [51].
Functional near-infrared spectroscopy (f-NIRS) is a noninvasive optical imaging modality that permits continuous monitoring of cerebral tissue oxygenation and can be performed at the bedside simultaneously with scalp EEG recordings. This is a newer modality that is being investigated for its utility in studying SIRPIDs and other ICU EEG patterns. Magnetoencephalography (MEG) measures magnetic fields associated with intracellular current flow within the neurons and has great utility in studying interictal epileptiform discharges in epilepsy patients for evaluation for epilepsy surgery. MEG has a promising future role in identifying neuronal networks involved in the generation of discharges, particularly LPDs. MEG has identified generation of discharges in lesional patients to the junction between lesional and non-lesional tissue; while generation of discharges in non-lesional patients is often localized to electrical networks in the temporal lobes that are electrically connected to the epileptogenic networks, irrespective of their location. For these electrophysiological reasons, MEG is felt to have poor utility in distinguishing epileptic vs metabolic SIRPIDs or other discharges and is unlikely to be used for further evaluation of such patients [52]. Moreover, practical utility of MEG in ICU patients is difficult, along with its limited availability as well.
We speculate that in a substantially aberrant brain with a hyperexcitable cortex, the etiology of SIRPIDs most likely entails dysregulation of subcortico-cortical projections, especially thalamocortical. Different forms of stimulation can activate these aberrant circuits by supplying an afferent input into the thalamus through sensory or arousal pathways.
Types of SIRPIDs
When Hirsch et al. initially defined SIRPIDs, they included ictal-appearing patterns along with periodic and rhythmic patterns that could be lateralized, generalized, or bilateral in the case of periodic discharges. According to Hirsch et al., ictal-appearing discharges were the most prevalent kind of SIRPIDs, occurring in 18 out of 33 patients (63.6%). The next most prevalent type of rhythmic delta activity was frontal-predominant, which was seen in 14 out of 33 individuals (42.4%). Thirty-three percent of patients experienced simulation-induced generalized periodic discharges (SI-GPDs), and twenty-seven percent experienced SI-LPDs (patients may exhibit multiple patterns during the recording) [1]. On the other hand, generalized rhythmic delta activity (SI-GRDA), which is typically frontally predominant and occurs in 47.5% of stimulated intervals, was the most prevalent type of SIRPIDs in the study. SI-GPDs with triphasic morphology were less common (26.3%) than SI-GPDs without triphasic morphology (10.5%) and lateralized rhythmic delta (SI-LRDA), whereas just 1 patient (5.3%) had lateralized periodic discharges (SI-LPDs). Additionally, Braksick et al. discovered a significant correlation between periodic discharges (PDs) or occasional sharp waves and SIRPIDs (OR 2.59, 1.13–5.92, P ¼ 0.02), as well as between SIRPIDs and GPDs with triphasic shape (OR 3.66, 1.67–8.02, P value 0.001). The majority of SIRPIDs were categorized as ictal (9.3%), rhythmic (37.2%), and periodic (53.5%) [2,4]. After cardiac arrest, the majority of stimulus-induced rhythmic, periodic, or ictal discharges have been classified as periodic (57.2%) [7]. SIRPIDs have been shown in certain investigations to occur in the more damaged side of the brain [40] or to originate from the same site as focal seizures [1]. The presence or absence of SIRPIDs has not been linked in other investigations to the location of brain injury (focal, bilateral, or multifocal/diffuse) [5]. SIRPIDs may also be located in the opposite hemisphere from the focal seizures [1,4] or on the opposite side of more severe injury when focal seizures and focal SIRPIDs coincide [41,42].
Association between SIRPIDs and seizures
In 51.5% of individuals with SIRPIDs, Hirsch et al. [1] discovered concurrent spontaneous electrographic seizures at some point during EEG monitoring (often prior to SIRPIDs being observed). However, none of the stimulus-induced patterns in the prospective investigation by Alsherbini et al. [5] were found to be ictal seeming, and no patients with SIRPIDs experienced electrographic seizures. According to Braksick et al. [2], SIRPIDs were substantially linked to electrographic seizures, and 10 out of 43 (23.3%) patients with SIRPIDs experienced a clinical seizure during their hospital stay. At some time during their hospital stays, all four of the SIRPID patients described by Van Straten et al. [4] also experienced nonstimulus-induced seizure activity. In a large multicenter research of 4,772 patients receiving continuous EEG monitoring, Rodriguez Ruiz et al. [6] discovered a substantial correlation between seizures and LPDs, LRDAs, and GPDs, particularly at higher frequencies [43] 2 Hz, in the cases of GPDs and LRDAs), but that the connection with seizures was unaffected by whether the underlying pattern was stimulus-induced or not (P. 0.10). They do, however, warn that their findings may be limited because there were only a small percentage of sessions with SIRPIDs overall (9.8% of all patient sessions reported). When they occur, focal seizure onset sites frequently do not correlate with focal SIRPID locations. The connection between seizures and SIRPIDs is yet unclear. We found no correlation between SIRPIDs of any kind and clinical seizures other than status epilepticus when comparing patients with and without SIRPIDs. Only SIRPIDs that were focal or ictal in appearance showed a link with clinical status epilepticus. Additionally, it seems that intracerebral hemorrhage was an independent predictor of SIRPIDs, especially focused SIRPIDs. SIRPIDs have been most commonly noted after resolution of NCSE, but have also preceded it or occurred concurrently with it in many cases. They are associated with therapy-refractory course of status epilepticus and high mortality, although the high mortality may reflect the effects of underlying pathology, rather than the damaging effects of SIRPIDs themselves. These findings corroborate the probable hypothesis that SIRPIDs might represent a state with increased epileptogenic potential, commonly co-occurring with NCSE [53].
General principles for treatment of various types of SIRPIDs
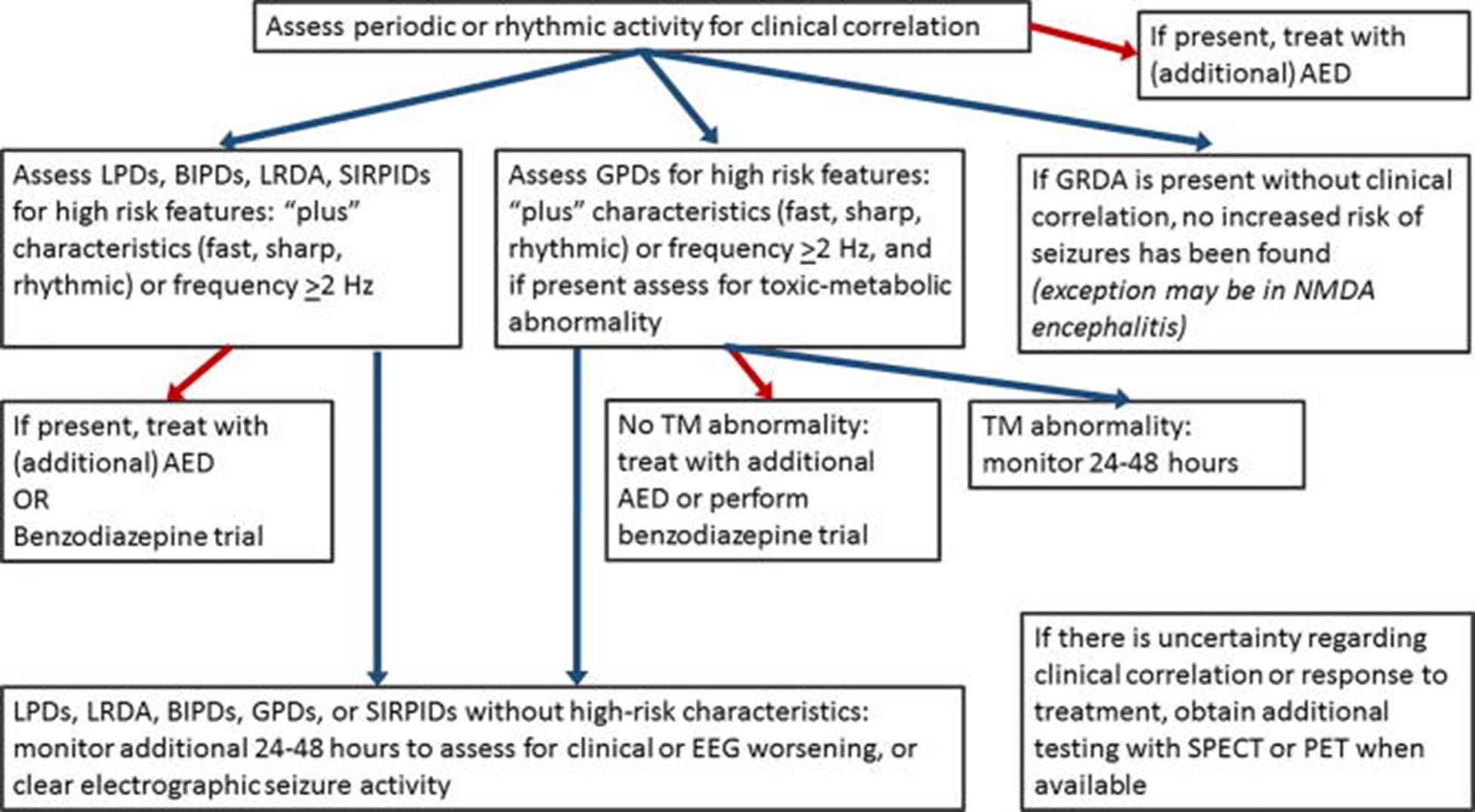
The treatment principles for SIRPIDs follow paradigms shown below in Figure 5. Decisions to treat and choice of treatment are often based on the presence of findings with greater or lesser epileptogenic potential, as stated below [54]. The presence of clinical changes, concurrent presence of seizures, improvement or worsening of underlying conditions like hemorrhage, stroke, tumor, encephalitis, would all influence treatment decisions.
Presence of clinical correlate to the SIRPIDs pattern would meet criteria for being an electroclinical seizure and necessitate the use or addition of an ASM. Presence of high-risk characteristics like SI-LPDs, SI-LRDA, sharp, fast or rhythmic modifiers, or frequency >2 Hz require the use of a benzodiazepine challenge or an ASM. SI-GPDs without high-risk characteristics or <2 Hz only need monitoring. For SI-GPDs with high-risk characteristics (>2 Hz, or additional rhythmic or fast activity): assess for underlying toxic-metabolic abnormality and if found, continue monitoring for 24–48 h; if no toxic-metabolic etiology is present, treat with an ASM, or perform benzodiazepine trial to assess for clinical improvement. For SI-GRDA, no increased risk of seizures has been found, and in the absence of clinical correlation, usually no treatment is advised – with rare exceptions for conditions like NMDA encephalitis, which have been shown to have GRDA + Fast modifiers in addition to GRDA [55].
If there is uncertainty regarding clinical correlation or response to treatment, further testing with functional imaging like SPECT or PET is recommended, as hyperperfusion with the captured SIRPIDs pattern supports treatment. Other modalities like neuromonitoring (CMD and similar technologies) may be used anytime during the process to complement diagnostic accuracy and guide treatment.
The choice of ASMs is not usually determined by the pattern of SIRPIDs. Patient comorbidities and physician preference often accounts for choice of ASM. Levetiracetam, lacosamide, brivaracetam are among the most preferred options due to efficacy, safety and favorable pharmacokinetic profiles. Valproic acid is often used as second- or third-line therapy after these agents, but higher probability of hepatic disease in these populations limits its frequency of use. These account for first and second lines of therapy. Older generation ASMs like phenytoin are avoided due to unfavorable pharmacokinetic profiles, adverse reaction profile and high probability of interactions with other medications used to treat comorbidities. Anesthetic agents may be used as needed, but are often chosen for treating comorbidities or concurrent seizures. The presence of concurrent seizures with SIRPIDs may influence choice of ASMs and anesthetics, and need for escalation based on the clinical need to control seizures and reduce their severity, rather than presence or type of SIRPIDs [56].
Benzodiazepines comprise a common treatment for the management of status epilepticus and have also been used to treat some of the ICU EEG patterns that fall into the ictal-interictal continuum. SIRPIDs are often treated with a benzodiazepine challenge to study electroclinical response to such therapy. A successful challenge consists of electrical cessation of the offending pattern with concurrent clinical improvement, often in mentation. The definition of a successful challenge is often varied due to difficulties in achieving improvements in mentation and encephalopathy in obtunded patients in the ICU, that may have multiple medical comorbidities contributing to their level of wakefulness. Most EEG readers agree that a benzodiazepine challenge is highly recommended for the presence of most SIRPIDs in the ICU, but cannot completely agree on the definition of a successful challenge. The EEG improvement in triphasic GPDs with a benzodiazepine challenge does not always correlate with improved mentation and may not often contribute to useful insight into the epileptogenic potential of such SI-GPDs with triphasic morphology. Benzodiazepine challenges are more likely to be offered with the presence of periodic discharges on EEG, rather than GRDA, which is felt to be more likely to be metabolic in nature. The presence of concurrent seizures with SIRPIDs makes a compelling case for the use of benzodiazepines, but also transfers any observed benefit of benzodiazepine use to seizure treatment, rather than the treatment of SIRPIDs [57]. Most guidelines pertaining to the use of benzodiazepines in the ICU are geared towards the treatment of status epilepticus, rather than SIRPIDs. Most clinicians agree that benzodiazepine use for SIRPIDs should be less aggressive than seizure protocols, but this can vary depending on the degree of epileptogenicity of the SIRPIDs. Various benzodiazepines are used in the ICU, with lorazepam, midazolam, diazepam being the most commonly used options. These 3 agents can be used as IV options, which is of great value in the ICU setting and enable fast and reasonably prolonged duration of action against SIRPIDs and seizures. Alprazolam and clonazepam also have some utility against SIRPIDs and seizures, but are usually administered orally and are more likely to be used in awake and obtunded patients off ventilatory support. The risk of respiratory compromise and risks of subsequent intubation and mechanical ventilation often play into calculations for the need of a benzodiazepine challenge in obtunded patients with SIRPIDs. These are lesser concerns for patients that are already intubated. Risks of hypotension from benzodiazepines are also a concern in the ICU and may also play a role in the decision to perform or avoid a benzodiazepine challenge. Benzodiazepine challenges for SIRPIDs may also be affected by internalization and maladaptive changes in the GABA receptor—a fairly common scenario in patients with prior or ongoing status epilepticus and/or prolonged use of benzodiazepine infusions like midazolam in the ICU for sedation [58]. In spite of all these caveats, benzodiazepines continue to play a prominent role in the ICU EEG setting for the management of SIRPIDs, with or without the presence of concurrent seizures [57].
The treatment of SIRPIDs follows the principles stated above. However, the management of the patient often varies depending on the underlying medical etiology of their presentation and the presence of systemic comorbidities. Despite the associations between SIRPIDs and various etiologies producing them, patient outcomes are often the result of concomitant seizures and medical comorbidities, rather than the underlying patterns seen in the SIRPIDs themselves. These facts need to be kept in mind to assist with choice of treatment for SIRPIDs in ICU patients.
Relevance of this case report
Our case report highlights a patient situation that is complex and unusual and yet offers sufficient food for thought, with regards to SIRPIDs and their pathophysiology. Our patient presented with an intracranial hemorrhage and associated midline shift with cerebral edema—a common etiology for SIRPIDs and seizures. It is a matter of interest that our patient developed seizures arising in the left hemisphere that was affected by the hemorrhage, but developed her SIRPIDs from the right hemisphere. We postulate that she suffered greater dysfunction in the left hemisphere but had substantial, yet lesser, dysfunction in the right hemisphere; which permitted greater excitability and arousal in the right hemispheric electrical circuits and supported the production of SIRPIDs. SIRPIDs and seizures have occurred concurrently, but their concurrent presence in opposite hemispheres is of great interest, and probably represents different bilateral rates and effects of cerebral dysfunction due to the hemorrhage and its sequelae.
The appearance of SIRPIDs and seizures about 4 days after craniectomy is consistent with most published literature and likely reflects the duration of developing cerebral dysfunction in such cases. The morphology of her SIRPIDs included PDs and LRDA which greatly supported their epileptogenic potential and is consistent with the high-risk features of SIRPIDs that support more aggressive treatment of such patterns. These patterns, coupled with the concurrence of seizures, prompted our team to treat her aggressively with ASMs. We do note that her SIRPIDs resolved within 24 hours on her first ASM with mild use of benzodiazepine, reflecting their relatively lower epileptogenic potential. Her seizures needed another 24 hours to resolve, along with the use of a second ASM. It is also of interest that she had GPDs with triphasic morphology for 48 hours before the appearance of her SIRPIDs, which points to an underlying metabolic dysfunction produced by her presentation and may have served as a substrate for the development of her SIRPIDs. Another point of interest remains that she had occasional, but persistent interictal LRDA and LPDs over the right hemisphere for 48 hours after her SIRPIDs resolved with treatment—a finding that supports underlying epileptogenic potential with likely lower excitability due to the use of ASMs. These interictal findings resolved after 48 hours as well to be replaced by triphasic GPDs similar to those seen over the first 3 days of her course.
Our patient sustained significant morbidity and eventual mortality despite receiving aggressive treatment and monitoring meeting all standards of care. We believe that her overall poor health, significant medical comorbidity and poor recovery from intracranial hemorrhage and craniectomy for it contributed to her death. Her SIRPIDs were diagnosed quickly and treated promptly with rapid cessation within 24 hours. Her seizures were treated quickly and aggressively and were terminated within 48 hours. Mortality from concurrent SIRPIDs and seizures is higher and mortality from seizures and intracranial hemorrhage is known to occur commonly. We postulate that her hemorrhage was the biggest contributor to her mortality, rather than SIRPIDs or seizures—an association with mortality from intracranial hemorrhage in such cases, that has also been reported in prior case series.
Overall, our case report represents an interesting phenomenon of opposite lateralization of concurrent SIRPIDs and seizures, that adds to the literature on SIRPIDs and offers strong supporting evidence for their prompt and aggressive treatment.
Limitations of our case report
Our case report does add a very interesting presentation of SIRPIDs to the literature. There are a few limitations we would like to discuss. Many patients presenting with encephalopathy are often monitored with VEEG immediately, but our patient only received routine EEGs for the first 3 days and was monitored only from day 4 till day 7 post operatively. She also received routine EEGs from day 8 till her death on day 10. While many prior case series include VEEG monitoring for extended periods of time for 10-21 days or even longer, we did perform regular EEGs on her, which compares somewhat to other studies that included daily monitoring of patients.
Our patient received prompt neurosurgical care but did not have advanced neuromonitoring, a technique that is often used at many centers in such cases. Our center’s protocols did not support the need for such monitoring as she was relatively stable post operatively. While we feel that this would not have changed her outcome, we would still have had some more data to evaluate her progress, had monitoring been performed. She did not receive functional imaging like SPECT or PET at our center either, due to lack of availability. Many studies have shown utility in performing such testing in SIRPIDs to determine need for further aggressive treatment. We state that we were justified in avoiding such evaluation as we initiated aggressive treatment that resolved her SIRPIDs in 24 hours and seizures in 48 hours.
Our patient received care for 10 days postoperatively before she passed away from withdrawal of care. Many other studies highlight longer durations of care, up to 4 weeks, if need be, to achieve good outcomes. Our patient’s poor baseline for health, coupled with her family’s wishes to withdraw care, prompted the decision to let her pass away peacefully by day 10. We postulate that she would likely have had poor outcomes from her bad health at baseline, worsened by seizures, SIRPIDs and her extensive intracranial hemorrhage with related complications. The mortality data from studies on similar patients supports our assertion in this regard.
Our patient did not undergo an autopsy, which probably deprived us of some data to finalize the cause of death and rule out other findings that may have been of insight to us. We however believe that the amount of data available to us was adequate to make reasonably accurate decision making and determine cause of morbidity and mortality.
While we detail some limitations in our case report, we believe that these are minor and do not significantly reduce the significance of the findings noted in our report. We feel that the observations learnt from this report are a worthy and useful addition to the medical literature.
Conclusion
Continuous EEG monitoring frequently reveals stimulus-induced activity, or SIRPIDs, in patients suffering from toxic metabolic disorders, anoxic damage, traumatic brain injury, or hemorrhage. Recent research has not demonstrated an elevated risk of seizures in individuals with stimulus-induced patterns as opposed to spontaneous, periodic, or rhythmic patterns, despite some studies indicating a greater prevalence of seizures among patients with SIRPIDs. The seemingly incongruous results about SIRPIDs might be a reflection of how our knowledge of them is developing. The negative SPECT imaging seen in patients with SIRPIDs suggests that stimulus-induced rhythmic, periodic, or ictal discharges in the critically ill may be more similar to stimulus-induced phenomena, such as periodic discharges in CKD, than to stimulus-induced seizures. Instead of using harsh treatment, as with seizures, SIRPIDs should be treated according to procedures for the underlying pattern. Though more research is needed, it is thought that the pathophysiology of SIRPIDs is caused by thalamo-cortical projections acting on an aberrant or hyperexcitable brain. This case report further stresses the importance on delving deeper into the topic of SIRPIDS and to keep an open mind while analyzing EEGs related to SIRPIDS instead of dismissing them as random potentially epileptic activity.
Acknowledgements
The authors are grateful to the EEG lab at SSM Health for their assistance in acquiring these EEG images.
Financial and Scientific Disclosures
The authors have no relevant financial disclosures to make and have no relevant conflicts of interest with the published research. There was no funding involved in this publication. The patient’s family consented to the publication of data in this paper. All data published in this paper is consistent with ethical and scientific standards and guidelines in clinical care, research and publication. The study followed institutional guidelines for research at BMC and SSM Health and was approved for publication with IRB approval exemptions from BMC and SSM in keeping with guidelines for case reports. All relevant data was added to the paper with continued access available, should the need for further review arise. AR was involved in writing the manuscript, performing data analysis and pertinent literature review. AS was involved in writing the manuscript, performing data analysis, pertinent literature review, caring for the patient, and creating the hand drawn illustrations. Both authors reviewed the manuscript and approved the final version after necessary changes were made.
References
2. Braksick SA, Burkholder DB, Tsetsou S, Martineau L, Mandrekar J, Rossetti AO, et al. Associated Factors and Prognostic Implications of Stimulus-Induced Rhythmic, Periodic, or Ictal Discharges. JAMA Neurol. 2016 May 1;73(5):585-90.
3. Ong C, Gilmore E, Claassen J, Foreman B, Mayer SA. Impact of prolonged periodic epileptiform discharges on coma prognosis. Neurocrit Care. 2012 Aug;17(1):39-44.
4. Van Straten AF, Fesler JR, Hakimi R, Sheng T, Thompson DM, Hakimi AS. SIRPIDs: prevalence and outcome in critically ill patients. J Clin Neurophysiol. 2014 Oct;31(5):418-21.
5. Alsherbini KA, Plancher JM, Ficker DM, Foreman BP, Adeoye OM, Ying J, et al. Stimulus-Induced Rhythmic, Periodic, or Ictal Discharges in Coma-Incidence and Interrater Reliability of Continuous EEG After a Standard Stimulation Protocol: A Prospective Study. J Clin Neurophysiol. 2017 Jul;34(4):375-80.
6. Rodriguez Ruiz A, Vlachy J, Lee JW, Gilmore EJ, Ayer T, Haider HA, et al. Association of Periodic and Rhythmic Electroencephalographic Patterns With Seizures in Critically Ill Patients. JAMA Neurol. 2017 Feb 1;74(2):181-88.
7. Alvarez V, Oddo M, Rossetti AO. Stimulus-induced rhythmic, periodic or ictal discharges (SIRPIDs) in comatose survivors of cardiac arrest: characteristics and prognostic value. Clin Neurophysiol. 2013 Jan;124(1):204-8.
8. Rossetti AO, Dunand M. Creutzfeldt-Jakob disease: evolution from nonconvulsive status epilepticus, through SIRPIDs, to generalized periodic discharges. Clin Neurophysiol. 2007 Nov;118(11):2533-6.
9. Kuroiwa Y, Celesia GG. Clinical significance of periodic EEG patterns. Arch Neurol. 1980 Jan;37(1):15-20.
10. Gloor P. Generalized cortico-reticular epilepsies. Some considerations on the pathophysiology of generalized bilaterally synchronous spike and wave discharge. Epilepsia. 1968 Sep;9(3):249-63.
11. Chatrian GE, Shaw CM, Leffman H. The significance of periodic lateralized epileptiform discharges in EEG: an electrographic, clinical and pathological study. Electroencephalogr Clin Neurophysiol. 1964 Aug; 17:177-93.
12. Karnaze DS, Bickford RG. Triphasic waves: a reassessment of their significance. Electroencephalogr Clin Neurophysiol. 1984 Mar;57(3):193-8.
13. Fisch BJ. Electrographic seizure patterns, pseudoperiodic patterns, and pseudoepileptiform patterns. Fisch and Spehlmann's EEG primer: basic principles of digital and analog EEG. 1999:307-48.
14. Gross DW, Quesney LF, Sadikot AF. Chronic periodic lateralized epileptiform discharges during sleep in a patient with caudate nucleus atrophy: insights into the anatomical circuitry of PLEDs. Electroencephalogr Clin Neurophysiol. 1998 Dec;107(6):434-8.
15. Neufeld MY, Vishnevskaya S, Treves TA, Reider I, Karepov V, et al. Periodic lateralized epileptiform discharges (PLEDs) following stroke are associated with metabolic abnormalities. Electroencephalogr Clin Neurophysiol. 1997 Apr;102(4):295-8.
16. Raroque HG Jr, Gonzales PC, Jhaveri HS, Leroy RF, Allen EC. Defining the role of structural lesions and metabolic abnormalities in periodic lateralized epileptiform discharges. Epilepsia. 1993 Mar-Apr;34(2):279-83.
17. Terzano MG, Parrino L, Mazzucchi A, Moretti G. Confusional states with periodic lateralized epileptiform discharges (PLEDs): a peculiar epileptic syndrome in the elderly. Epilepsia. 1986 Jul-Aug;27(4):446-57.
18. Handforth A, Cheng JT, Mandelkern MA, Treiman DM. Markedly increased mesiotemporal lobe metabolism in a case with PLEDs: further evidence that PLEDs are a manifestation of partial status epilepticus. Epilepsia. 1994 Jul-Aug;35(4):876-81.
19. Brenner RP, Schaul N. Periodic EEG patterns: classification, clinical correlation, and pathophysiology. J Clin Neurophysiol. 1990 Apr;7(2):249-67.
20. Snodgrass SM, Tsuburaya K, Ajmone-Marsan C. Clinical significance of periodic lateralized epileptiform discharges: relationship with status epilepticus. J Clin Neurophysiol. 1989 Apr;6(2):159-72.
21. Pohlmann-Eden B, Hoch DB, Cochius JI, Chiappa KH. Periodic lateralized epileptiform discharges--a critical review. J Clin Neurophysiol. 1996 Nov;13(6):519-30.
22. Steriade M. Arousal: revisiting the reticular activating system. Science. 1996 Apr 12;272(5259):225-6.
23. Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101(2):243-76.
24. Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex. 1997 Sep;7(6):583-604.
25. Kellaway P. Orderly Approach to Visual Analysis: Elements of the Normal EEG and Their Characteristics in Children and Adults. In: Ebersole JS, Pedley TA, Editors. Current Practice of Clinical Electroencephalography, 3rd Edition. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 100-59.
26. Vas GA. Diffuse encephalopathies. In: Daly DD, Pedley TA. Current practice of clinical electroencephalography. New York: Raven Press; 1990. P. 371-400.
27. Niedermeyer E. Cerebrovascular disorders and EEG. In: Niedermeyer E, Lopes da Silva FH, Editors. Electroencephalography: basic principles, clinical applications, and related fields. 3rd ed. Baltimore: Williams & Wilkins;1993. P. 305-28.
28. Niedermeyer E. Metabolic central nervous system disorders. In: Niedermeyer E, Lopes da Silva FH, Editors. Electroencephalography: basic principles, clinical applications, and related fields. 3rd ed. Baltimore: Williams & Wilkins;1993. P. 405-18.
29. Fisch BJ, Klass DW. The diagnostic specificity of triphasic wave patterns. Electroencephalogr Clin Neurophysiol. 1988 Jul;70(1):1-8.
30. Olkinuora M. Organic brain syndromes and dementia. In: Daly DD, Pedley TA. Current practice of clinical electroencephalography. New York: Raven Press; 1990. P. 401- 24.
31. Westmoreland BF. The EEG in cerebral inflammatory processes. In: Niedermeyer E, Lopes da Silva FH, Editors. Electroencephalography: basic principles, clinical applications, and related fields. 3rd ed. Baltimore: Williams & Wilkins;1993. pp. 291-304.
32. Cobb WA. Electroencephalographic changes in viral encephalitis. In: Illis LS, Editor. Viral diseases of the central nervous system. Baltimore: Williams & Wilkins; 1975. P. 76-89.
33. Kellaway P, Hrachovy RA, Frost JD Jr, Zion T. Precise characterization and quantification of infantile spasms. Ann Neurol. 1979 Sep;6(3):214-8.
34. Daly DD, Pedley TA. Current practice of clinical electroencephalography. New York: Raven Press; 1990.
35. Zifkin BG. Epilepsy with reflex seizures. In: Wyllie E, Editor. The treatment of epilepsy: principles and practice. 2nd ed. Baltimore: Williams & Wilkins; 1997. p. 573-83.
36. Fröscher W. Sleep and prolonged epileptic activity (status epilepticus). Epilepsy Res Suppl. 1991;2:165–76.
37. Niedermeyer E. Epileptic seizure disorders. In: Niedermeyer E, Lopes da Silva FH, Editors. Electroencephalography: basic principles, clinical applications, and related fields. 3rd ed. Baltimore: Williams & Wilkins;1993. p. 461-564.
38. Niedermeyer E. Primary (idiopathic) generalized epilepsy and underlying mechanisms. Clin Electroencephalogr. 1996 Jan;27(1):1-21.
39. Van Cott AC, Blatt I, Brenner RP. Stimulus-sensitive seizures in postanoxic coma. Epilepsia. 1996 Sep;37(9):868-74.
40. Johnson E, Hannawi Y, Martinez NC, Ritzl EK. Cefepime-Associated SIRPIDs in a Patient With Normal Renal Function. Neurohospitalist. 2016 Oct;6(4):167-9.
41. Dixit S, Kurle P, Buyan-Dent L, Sheth RD. Status epilepticus associated with cefepime. Neurology. 2000 Jun 13;54(11):2153-5.
42. Herlopian A, Struck AF, Rosenthal E, Westover BM. Neuroimaging Correlates of Periodic Discharges. J Clin Neurophysiol. 2018 Jul;35(4):279-94.
43. Rennebaum F, Kassubek J, Pinkhardt E, Hübers A, Ludolph AC, Schocke M, et al. Status epilepticus: Clinical characteristics and EEG patterns associated with and without MRI diffusion restriction in 69 patients. Epilepsy Res. 2016 Feb;120:55–64.
44. Bhatt A, Farooq MU, Bhatt S, Majid A, Kassab MY. Periodic lateralized epileptiform discharges: an initial electrographic pattern in reversible posterior leukoencephalopathy syndrome. Neurol Neurochir Pol. 2008 Jan-Feb;42(1):55-9.
45. Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic Correlates of the Ictal-Interictal Continuum: FDG-PET During Continuous EEG. Neurocrit Care. 2016 Jun;24(3):324-31.
46. Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016 Apr;79(4):579-90.
47. Kan R, Takahashi Y, Sato K, Watabe M, Tago H, Yashima Y, et al. Serial changes of SPECT in periodic synchronous discharges in a case with Creutzfeldt-Jakob disease. Jpn J Psychiatry Neurol. 1992 Mar;46(1):175-9.
48. Shvarts V, Zoltay G, Bowyer SM, Zillgitt A, Moran JE, Mason K, et al. Periodic Discharges: Insight From Magnetoencephalography. J Clin Neurophysiol. 2017 May;34(3):196-206.
49. Zeiler SR, Turtzo LC, Kaplan PW. SPECT-negative SIRPIDs argues against treatment as seizures. J Clin Neurophysiol. 2011 Oct;28(5):493-6.
50. Johnson EL, Kaplan PW, Ritzl EK. Termination patterns of stimulus-induced rhythmic, periodic, or ictal patterns and spontaneous electrographic seizures. Clin Neurophysiol. 2017 Nov;128(11):2279-85.
51. Smith CC, Tatum WO, Gupta V, Pooley RA, Freeman WD. SPECT-negative SIRPIDs: less aggressive neurointensive care?. Journal of Clinical Neurophysiology. 2014 Jun 1;31(3):e6-10.
52. Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013 Feb;30(1):1-27.
53. Capecchi F, di Giacopo A, Keller E, Mothershill I, Imbach LL. Stimulus Induced Rhythmic, Periodic, or Ictal Discharges (SIRPIDs) and its Association with Non-convulsive Status Epilepticus in Critically Ill Patients. Clin EEG Neurosci. 2023 May;54(3):247-54.
54. Rodríguez V, Rodden MF, LaRoche SM. Ictal-interictal continuum: A proposed treatment algorithm. Clin Neurophysiol. 2016 Apr;127(4):2056–64.
55. Parwani J, Ortiz JF, Alli A, Lalwani A, Ruxmohan S, Tamton H, et al. Understanding Seizures and Prognosis of the Extreme Delta Brush Pattern in Anti-N-Methyl-D-Aspartate (NMDA) Receptor Encephalitis: A Systematic Review. Cureus. 2021 Sep 21;13(9):e18154.
56. Orinx C, Legros B, Gaspard N. Recent antiseizure medications in the Intensive Care Unit. Minerva Anestesiol. 2017 Aug;83(8):878–887.
57. Kienitz R, Kay L, Beuchat I, Gelhard S, von Brauchitsch S, Mann C, et al. Benzodiazepines in the Management of Seizures and Status Epilepticus: A Review of Routes of Delivery, Pharmacokinetics, Efficacy, and Tolerability. CNS Drugs. 2022 Sep;36(9):951–75.
58. Singh A, Stredny CM, Loddenkemper T. Pharmacotherapy for Pediatric Convulsive Status Epilepticus. CNS Drugs. 2020 Jan;34(1):47–63