Abstract
Posterior quadrant epilepsy is relatively uncommon and refractory seizures from these regions are difficult to diagnose and manage. A 28-year-old woman presented for evaluation of her seizures. Scalp Electroencephalogram (EEG) showed seizures with independent onset over the right posterior and left anterior regions. Positron emission tomography (PET) scans revealed multiple regions of hypometabolism in the brain with maximum decrease in metabolism seen over the left and right precuneus and both occipital lobes. Magnetoencephalography (MEG) revealed epileptogenic dipoles over bilateral precuneus regions. Intracranial EEG revealed seizure onset from the left precuneus and left frontal regions with rapid generalization. She underwent Responsive Neurostimulation System (RNS) implantation targeting the left precuneus and left frontal regions for network modulation. She has had a 90 percent seizure reduction and remains on 2 medications as she continues to follow up in clinic. Posterior quadrant epilepsy remains a challenging condition to manage, and the use of neuromodulation may improve response rates and improve our understanding of these networks. Definite guidelines on location of electrode placement of RNS in such cases remain absent and improved understanding of such seizure networks is expected to improve our ability to target therapeutic nodes accurately to achieve greater seizure control.
Keywords
Refractory epilepsy, Posterior quadrant epilepsy, Responsive neurostimulation, Precuneus
Abbreviations
EEG: Electroencephalogram; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; IAP: Intracarotid Amobarbital Procedure; RNS: Responsive Neurostimulation System; MEG: Magnetoencephalography; FIAS: Focal Onset with Impaired Awareness Seizures; FTBTC: Focal Onset to Bilateral Tonic Clonic seizures; PDMS: Patient Data Management System; DBS: Deep Brain Stimulation
Introduction
Refractory epilepsy patients are defined as those patients that fail to achieve adequate seizure control despite the use of at least two adequately selected and doses anti-seizure medications, and account for about one-third of the epilepsy patient population [1]. The precuneus is a complex and highly connected structure in the parieto-occipital region with functional and anatomical connections to a variety of different parts of the brain and involvement in multiple neural pathways. Precuneus onset epilepsy is extremely uncommon, given that parietal onset epilepsy is only felt to be about 6% of all focal onset epilepsies and precuneus onset seizures are a subset of that, and knowledge about its prevalence and definitive treatment remains limited [2]. Manifestations and diagnosis of precuneus onset epilepsy are diverse and confusing and often missed, making this a rare and difficult condition to recognize and treat. Clear guidelines for treatment of this condition and similar epilepsies are lacking and expert opinion is diverse and often conflicting. RNS is a relatively newer modality of treatment for refractory epilepsy that involves placing a cerebral neurostimulator to electrically modify seizure networks in the brain. It has been used to directly target seizure onset zones or to modify seizure networks by targeting important transitional junctions in the seizure networks. The use of RNS to treat and control refractory epilepsy has been proven many times over and it is now being used to modulate seizure networks in poorly understood or poorly defined seizure networks in epilepsy patients as well. We present the case of a patient who underwent extensive evaluation of her precuneus onset epilepsy and had RNS implantation with significant seizure reduction as a result. We hope that this case report contributes to our understanding of this rare epilepsy condition and improves management guidelines. This should help improve our understanding of precuneus onset epilepsy and greatly facilitate development of effective and innovative treatment approaches using neuromodulation to treat such complex and refractory epilepsy patients.
Results
A 28-year-old woman presented to our epilepsy clinic for evaluation of her refractory epilepsy. She had seizure onset at the age of 16 years and had seen multiple general neurologists over the years and had failed more than 3 medications. She was on levetiracetam 3000 mg twice daily, lamotrigine 400 mg twice daily, and lacosamide 200 mg twice daily, and continued to have frequent seizures. She described two types of seizures: 1) Focal onset with impaired awareness seizures (FIAS) – aura of feeling weird followed by staring, loss of consciousness and manual automatisms affecting both hands (5-6 FIAS seizures per month) 2) Focal onset to bilateral tonic clonic seizures (FTBTC) – seizure onset with symptoms described in seizure type 1 progressing to generalized tonic clonic seizures / convulsions (1-2 FTBTC per month). She was presumed to have focal onset epilepsy of yet undetermined onset with rapid generalization, manifesting with 2 seizure types as described above.
Magnetic resonance imaging (MRI) imaging of her brain was unremarkable. PET scans revealed globally decreased metabolic pattern with multifocal regions of moderate metabolic defects, most pronounced in the left precuneus. Neuropsychological testing revealed low normal intelligence and mild cognitive deficits across multiple domains with greater involvement of the dominant hemisphere. Wada / intracarotid amobarbital procedure (IAP) revealed left hemispheric dominance for language and memory. MEG revealed frequent epileptiform discharges from both precuneus regions, left more than right (Figure 1). Scalp EEG revealed seizures with independent onset from the left anterior (1 seizure) and right posterior (3 seizures) regions (Figure 2). Interictal bifrontal epileptiform discharges were also seen occasionally.

Figure 1: MEG showing epileptogenic dipole activity over both precuneus regions, left greater than right.
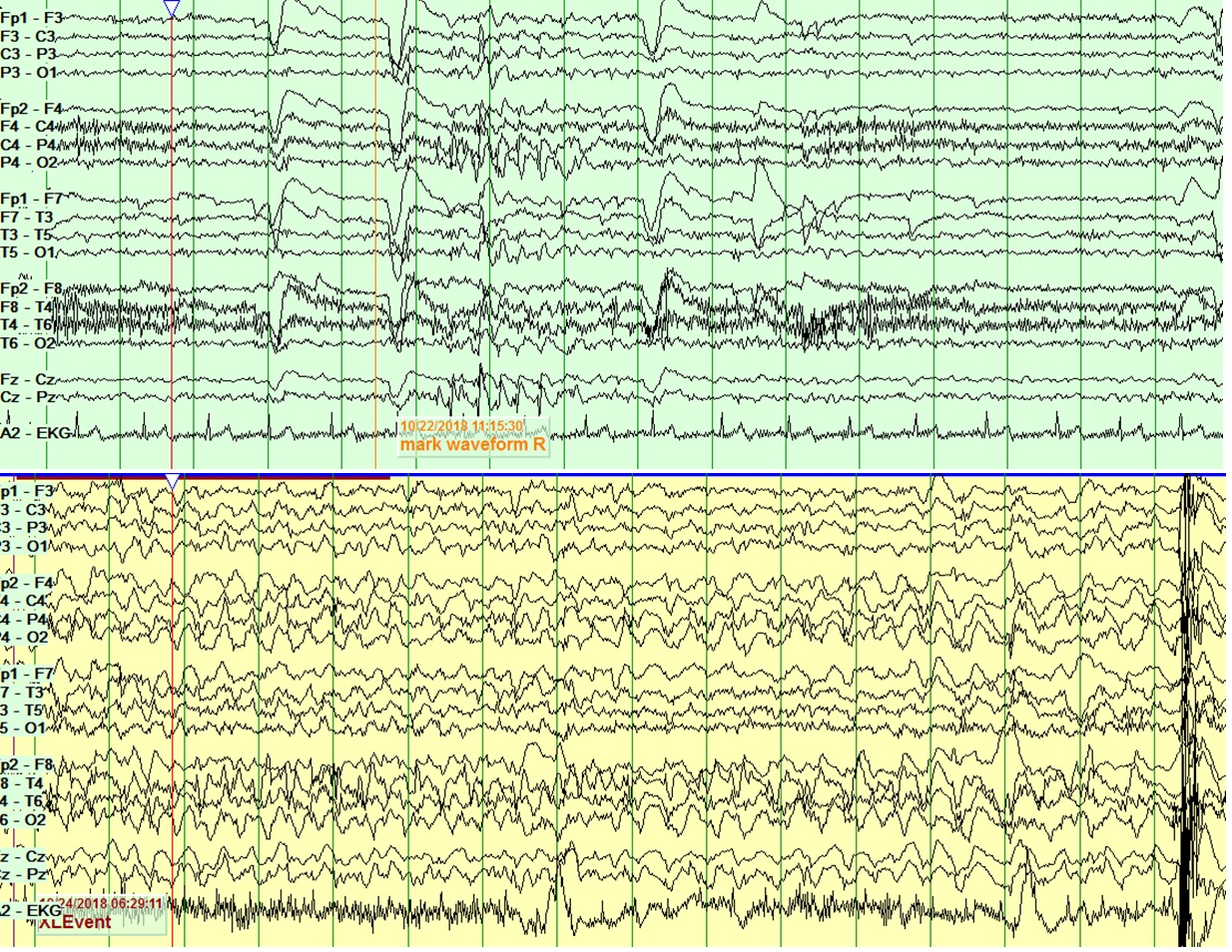
Figure 2: Interictal (top) and ictal (bottom) EEG screenshots showing posterior onset epileptiform discharges and right posterior onset seizures.
She underwent intracranial EEG evaluation using a combination of subdural strip electrodes and stereotactic depth electrodes (Figure 3). Intracranial EEG captured 3 seizures, two of them with left posterior onset with rapid spread to left frontal and right posterior regions, while one had a left anterior onset with rapid spread to left and right posterior head regions (Figure 3). Abundant interictal epileptiform discharges were seen in both posterior regions, left more than right.
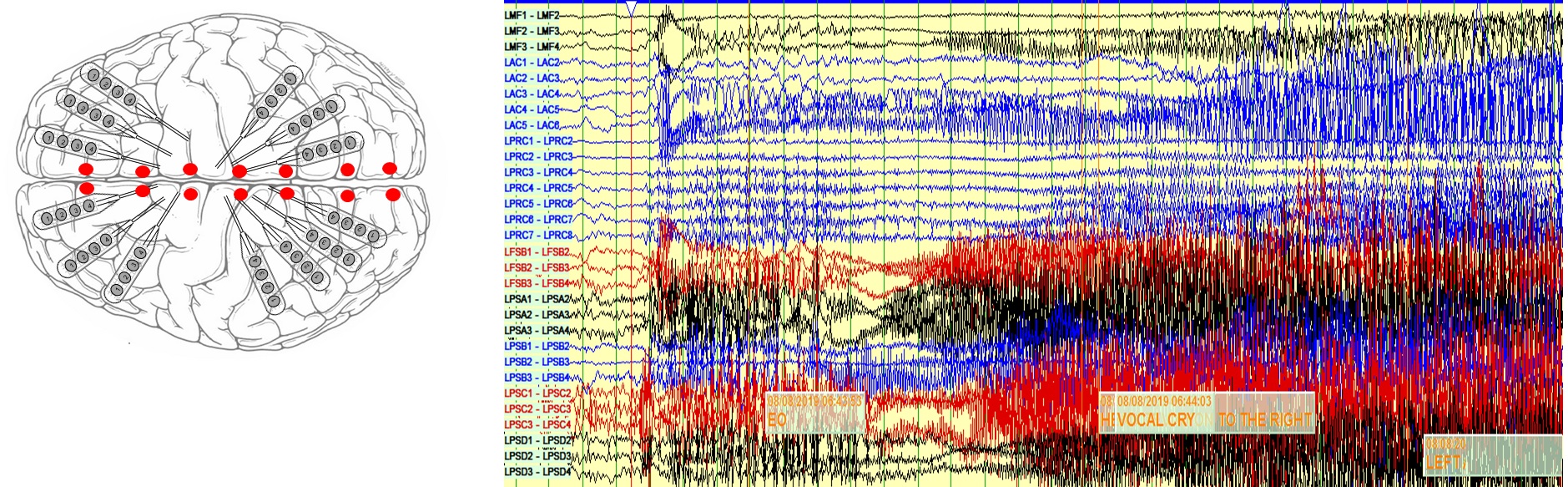
Figure 3: LEFT) Intracranial EEG implantation schematic plan consisting of surface strip electrodes and depth electrodes. RIGHT) Screenshots from intracranial EEG showing independent seizure onset from left posterior and left anterior regions with rapid spread to other parts of the brain (limited montage only shown).
She received an RNS implantation with 1 left frontal electrode, 2 left posterior electrodes and 1 right posterior electrode (Figure 4). One of the left posterior electrodes and the left frontal electrode were turned on for recording and stimulation as they showed the most activity and earliest involvement on intracranial EEG and to permit greater coverage of her seizure network. Our surgeons were not comfortable with resection due to risk of deficits, and thalamic insertion of electrodes for targeting was felt to be too experimental at the time and not having enough data to support it. Our proposed approach was felt to be safest and most likely to target the seizure network effectively. She remains on 2 anti-seizure medications at present (levetiracetam 1500 mg twice daily and lacosamide 100 mg twice daily) and has had 90 percent seizure reduction thus far with 1 FIAS per month on average (subclinical) and 1 FTBTC every 3-4 months. Patient data management system (PDMS) data from the RNS device has shown good capture of seizure data after implantation (Figure 4).
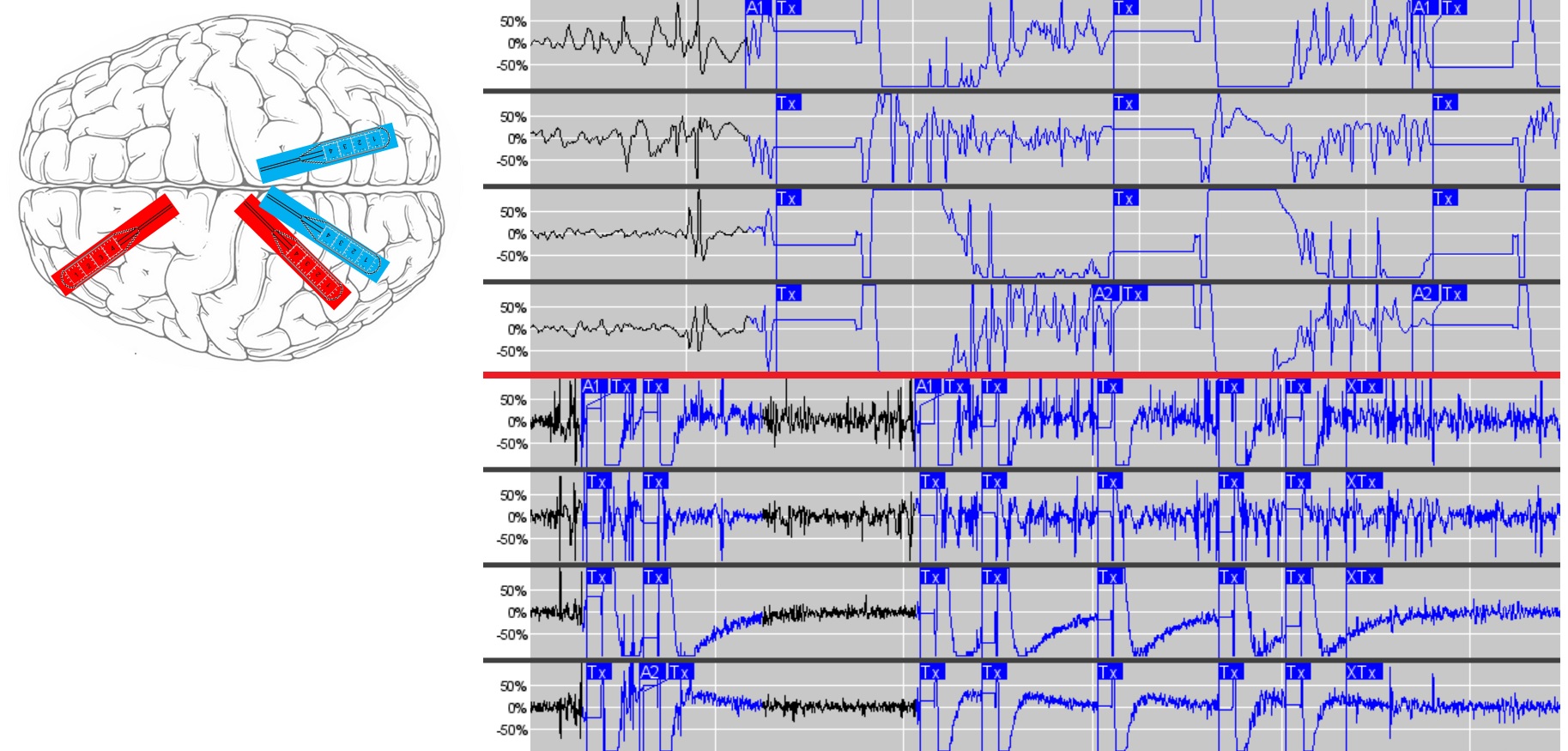
Figure 4: LEFT) RNS implantation schematic shown with 2 active strip electrodes over left anterior precuneus and left frontal regions in red and 2 inactive strip electrodes over left posterior precuneus and right precuneus regions shown in blue. RIGHT) Screenshots from PDMS showing good seizure capture from the implanted RNS device.
Discussion
Precuneus onset epilepsy remains uncommon with its exact prevalence unknown. It is often misdiagnosed or falsely localized due to its extensive connectivity, variable semiology, limitations of testing modalities and rarity of occurrence [2]. Diagnosis can be challenging in many cases, especially if non-lesional on MRI or other imaging modalities, as testing is often discordant or falsely localizing. Quantitative PET imaging made a significant difference in diagnosis in our case as it pointed us towards the precuneus as the major player in the patient’s seizure network. The value of PET imaging, especially with quantitative analyses, in the evaluation of a presurgical patient is high [3] and this was proven again in our patient. MEG remains an excellent investigative modality for surgical epilepsy [4] and has wonderful value in evaluating our patient and assisting in surgical decision making. We were able to use results from quantitative PET imaging and MEG to understand the patient’s seizure network better, especially due to discordance seen in scalp EEG and neuropsychological testing, making network localization more difficult.
Intracranial EEG remains the gold standard of seizure network localization and we sought to modify our approach accordingly. The precuneus shows high levels of connectivity due to its associations with cognitive functions like visuo-spatial imagery, episodic memory retrieval and self-processing operations, namely first-person perspective taking and an experience of agency [5]. Exploring such a large network using only subdural electrodes would be challenging and probably inaccurate, while performing stereotactic EEG only would limit our ability to cover cortical regions in the posterior quadrant bilaterally. We thus chose to use a hybrid approach consisting of depth electrodes placed along the midline from anterior to posterior along with cortical strips over both hemispheres, with greater left sided coverage. This approach gave us the ability to study a larger network and minimize surgical morbidity as well.
Our intracranial EEG evaluation did reveal that while the left precuneus was the major node in the seizure network, there was early and rapid involvement of the left frontal and right precuneus regions as well. We were unable to identify a single target that would help us achieve seizure freedom through resection. Surgical resections in the precuneus regions have been associated with visuospatial and cognitive impairment [2] and our surgeons were not keen on performing a left precuneus resection, especially as it was in the dominant hemisphere. Deep brain stimulation (DBS), while approved for the treatment of refractory epilepsy [6], was felt to be a newer modality and not favored by the surgeons or the patient either.
RNS was felt to be our best option going forward, due to its diagnostic and therapeutic utility. There have been a few studies demonstrating the utility of RNS in multifocal epilepsy with electrode placement in the anterior nucleus of the thalamus, although the number of patients in these studies are small (1-3 patients each) [7]. Other case series have also talked about the utility of targeting the pulvinar of the thalamus for epilepsy of posterior quadrant onset [8]. We felt that these numbers were small and not strong enough to warrant a similar intervention. We thus decided to implant four strip electrodes for the RNS system and activate one over the left precuneus and another over the left frontal region to enable maximum modulation of the seizure network. We have left the inactive electrodes in place over the posterior part of the left precuneus and right precuneus for a change in modulation parameters in future as needed.
Precuneus onset focal epilepsy is highly diverse and variable in its symptomatology and involvement of different cerebral regions in the seizure network. Misdiagnoses are common in this complex and poorly understood condition, often due to limitations in scalp EEG localization from unclear onset or rapid generalization or different appearance on EEG of different seizures in the same patient, or lack of clear localization of seizure onset zone on imaging like MRI or PET, or discordance between different imaging modalities due to large or variable seizure network involvement, or limitations in sampling with intracranial EEG using either subdural grid electrodes or stereotactic EEG due to poorly defined networks or surgical limitations or operator inexperience, or lack of utilization of quantitative analyses of imaging like PET or EEG. Diagnostic approaches and treatment guidelines for such conditions are lacking, due to limited number of properly diagnosed and studied cases, restrictive collaboration across different centers on such cases and relative novelty of newer treatments like RNS or DBS for such large and diverse seizure networks. Our case highlights the importance of a detailed and quantitatively analytical approach to such patients with poorly understood seizure networks and emphasizes the importance of a customized and innovative approach to properly define their seizure networks and develop an appropriate treatment plan maximizing the utility of the RNS device. The details of our case showcase the efficacy of intracranial EEG, especially using hybrid approaches, the necessity of quantitative analyses to ensure concordance and understanding of the network and the importance of correctly implementing the use of neuromodulation with the RNS with appropriate targeting to ensure good patient response and seizure control. It is our fervent desire that lessons learned from this case be used to reinforce the importance of advanced testing and analytical techniques in epilepsy surgery, imaging analyses and develop newer approaches and innovative treatments for complex epilepsy patients using newer neuromodulation techniques like RNS or DBS.
The above case report does highlight some limitations. Many centers may not possess sufficient expertise or resources to offer a complex, hybrid intracranial EEG evaluation. Most centers offer subdural grids and stereotactic EEG in a sequential manner rather than as a hybrid approach, and this can often provide conflicting and confusing results. Our center was able to avoid this quagmire by using quantitative PET and MEG analyses to understand the seizure network better prior to intracranial EEG evaluation, a technology that is somewhat limited with very few experts available to implement and modify it, thus making it difficult for most centers nationwide to offer such diagnostic techniques to their patients. Our RNS implantation plan is clearly customized to the patient and was made in consultation with multiple experts and their expert opinions. While this highlights the absence of clear guidelines on management in such cases, it also points to differences in management of complex cases amongst experts. Many experts at other centers on reviewing our approach, in hindsight, stated that they would have used other treatment options like non-targeted RNS therapy or DBS or VNS or different targets like the thalamus or hippocampi or additional medications or possibly targeted resections as well. Finally, our case report represents our customized experience with a single patient only and can be used to develop approaches for other patients; but would require significant extrapolation, as no two patients are like and all of them require customized approaches. While we would have liked to describe our approaches to multiple patients with such epilepsies, this condition is extremely rare, and we have not had other patients with this condition at our center. This finding does highlight the need for greater collaborations across multiple centers to enable greater standardization of care and better dissemination of knowledge and expert opinion.
Conclusions
Posterior quadrant onset, and especially precuneus onset, refractory epilepsy remains challenging to diagnose, evaluate and treat. Quantitative analyses of imaging improve the diagnostic yield in identifying such cases and delineating the seizure network, especially in the event of discordant imaging or EEG. Stereotactic EEG remains the gold standard for studying the seizure network in such cases and permits exploration of extensive networks and anatomically distant regions of the brain. RNS remains a great choice of therapy in such patients, although more data is needed to determine targets for electrode placement to maximize efficacy of treatment. DBS may be an excellent option for such patients, but data in this regard is lacking. More research is needed to improve diagnostic accuracy and therapeutic efficiency in patients with these disorders.
Author Contribution
AS treated the patient, participated in writing the manuscript and performed literature reviews on the subject. There was no funding involved in this study.
Disclosure of Conflicts of Interest
The author does not have any relevant disclosures of conflicts of interest.
Ethical Publication Statement
The author has read the journal’s statement on ethical practices in research and confirms that the work published in this manuscript is consistent with those guidelines. The author confirms that this is an authentic and original piece of research and has not been published elsewhere or submitted elsewhere for publication. This patient’s case was presented as a poster publication at the Annual Meeting of the American Epilepsy Society in Dec 2020.
References
2. Harroud A, Boucher O, Tran TPY, Harris L, Hall J, Dubeau F, et al. Precuneal epilepsy: Clinical features and surgical outcome. Epilepsy Behav. 2017;73:77-82.
3. van't Klooster MA, Huiskamp G, Zijlmans M, Debets RM, Comans EF, Bouvard S, et al. Can we increase the yield of FDG-PET in the preoperative work-up for epilepsy surgery?. Epilepsy Res. 2014;108(6):1095-1105.
4. Bagić AI, Funke ME, Kirsch HE, Tenney JR, Zillgitt AJ, Burgess RC. The 10 Common Evidence-Supported Indications for MEG in Epilepsy Surgery: An Illustrated Compendium. J Clin Neurophysiol. 2020;37(6):483-497.
5. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564-583.
6. Salanova V. Deep brain stimulation for epilepsy. Epilepsy Behav. 2018;88S:21-24.
7. Elder C, Friedman D, Devinsky O, Doyle W, Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open. 2019;4(1):187-192.
8. Burdette D, Mirro EA, Lawrence M, Patra SE. Brain-responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: A case series. Epilepsia Open. 2021;6(3):611-617.
