Abstract
Chronic stress exposure is linked to health and performance deficits including mental disorders, chronic diseases, and metabolic conditions. Stress management is an active process of coping with internal or external stressors to prevent or dampen biological and psychological strain. To enrich analysis of the causes and the effects of stress, the process of transitioning between “stressed” and “not stressed” is quantified here with a novel, 4-item instrument that prompts participants to self-report on the temporal nature of stress emergence and dissipation. Participants use visual analog scales (VAS) to record stress intensity (with responses recorded through a rating scale represented as a thermometer icon), stress frequency (as a calendar icon), speed of onset of stress (as a speedometer icon), and speed of recovery from stress (as a speedometer icon). This longitudinal study of six crewmembers during an 8-month simulated Mars mission has shown that the self-reported stress intensity is correlated to the perception of how quickly stress emerges and how slowly stress dissipates. These results indicate that stress is perceived as most intense when it emerges suddenly and then lingers on, slowly dissipating. This novel instrument is named SIFOR which is pronounced as “Sigh-Four” as a reminder for participants to take a moment to sigh then evaluate the four items of this self-report instrument. SIFOR results have shown agreement with the validated 10-item Perceived Stress Scale, demonstrating the potential for using SIFOR as both a research and clinical tool for stress monitoring. Self-reported data from SIFOR of how stress emerges and dissipates is useful for comparing the impact of various stressor types, evaluating stress coping strategies, and promoting resilient responses.
Introduction
An instrument called SIFOR is presented here as a novel approach to measuring how perceived stress intensity, or the impact of stressors, is related to the temporal nature of how stress emerges and dissipates. Participants are prompted to self-report on Stress Intensity (SI), Frequency (F), the speed of Onset of stress (O), and the speed of Recovery (R) from stress. Stress management is an active process for mitigating stressors that are threatening to induce biological or psychological strain. Resilience in the face of stressors relies on timely recognition and coping to prevent strain from accumulating beyond the elastic bounds of recovery. Simple, reliable methods of stress monitoring are needed to detect when stress is reaching critical levels and to assess the efficacy of coping strategies that can help mitigate and prevent health and performance deficits.
Prior longitudinal research on the time course of stress includes measurement of biomarkers and personality traits [1] and of biometrics on a short-term basis, such as heart rate and respiration rate recovery after artificiallyinducing stressors in laboratory settings [2-4]. Biomarker and biometric methods are advantageous for gathering quantitative, objective results; however, at the current state of technology, such methods are not scalable and become infeasible for monitoring stress over long periods of time in real-world settings or complex work environments. Biosampling requires time-intensive processing by laboratory staff with associated costs for materials and equipment; though someday in the future, if there will be miniaturized, low-cost laboratory equipment, such as portable mass spectrometers, then biosampling will become more accessible. Similarly, biometric recordings are typically conducted in clinical or laboratory settings, and these data require expertise for advanced data processing. In contrast, we propose a quick, easy-to-use 4-item instrument for analyzing stress levels over a set time period (i.e. weekly analysis) to study the impact of various types of stressors.
The SIFOR instrument is demonstrated with a longitudinal analysis of six crewmembers who self reported on stress levels while isolated and confined during a 240-day simulated Mars mission on Mauna Loa volcano in Hawaii. This Mars analog mission’s concept of operations required crew to have a high level of autonomy in performing mission tasks and self-organizing their schedules and routines [5]. Crew experienced stressors such as lack of privacy, limited communication with family and friends over a Mars-like 20-min latency or delay, shelf-stable food ingredients, restricted resource usage (water and power), and small team dynamics.
We compare the self-reported stress results of these crewmembers who provided responses weekly to both the SIFOR instrument and the 10-item Perceived Stress Scale (PSS) questionnaire which has been validated in adult and adolescent populations [6,7]. The SIFOR instrument uses VAS, or visuals, eliminating the time and skill required for reading comprehension of verbal statements in standard questionnaires such as the PSS. This research is motivated by the results of an earlier study of isolation and confinement, a 520-day simulated Mars mission in Russia, in which visual analog scales (VAS) were key to showing statistical differences in participant mood and behavior over time, whereas the validated questionnaires did not have the same outcome of finding significant differences over time [8]. This could be indicating that VAS are more sensitive to detecting longitudinal differences compared to questionnaires that implement likert responses to statements. The SIFOR instrument utilizes VAS to obtain perceived stress levels with the goal of longitudinal stress monitoring.
The ease of use of this novel instrument enables stress monitoring in complex work environments, such as those experienced by astronaut crews, hospital staff, deployed military teams, or other busy professionals, to improve health and performance both during operations in complex work environments and while re-integrating after returning home. This instrument may also be helpful as a recovery tool for patients with impaired cognitive functioning, such as neurodegenerative or traumatic brain injury patients, who need to monitor and adjust their arousal levels to have enough stress to promote development but not overwhelm them. Patients with posttraumatic stress disorder (PTSD), depression, anxiety, or chronic diseases using the instrument for daily or weekly monitoring will have data-driven information to support their self-awareness and stress management abilities which will help with controlling their primary medical issues.
Methods
Participants responded on a weekly basis using Purdue University’s Qualtrics system to access the SIFOR instrument for self-reporting on Stress Intensity, Frequency, Onset, and Recovery. These four-items are answered with VAS to quickly identify how stress events were experienced each week as shown in Figure 1. To compare SIFOR with an existing, validated method for self-report of stress, the Perceived Stress Scale (PSS) was also hosted on Qualtrics for weekly self-reporting (Figure 2).
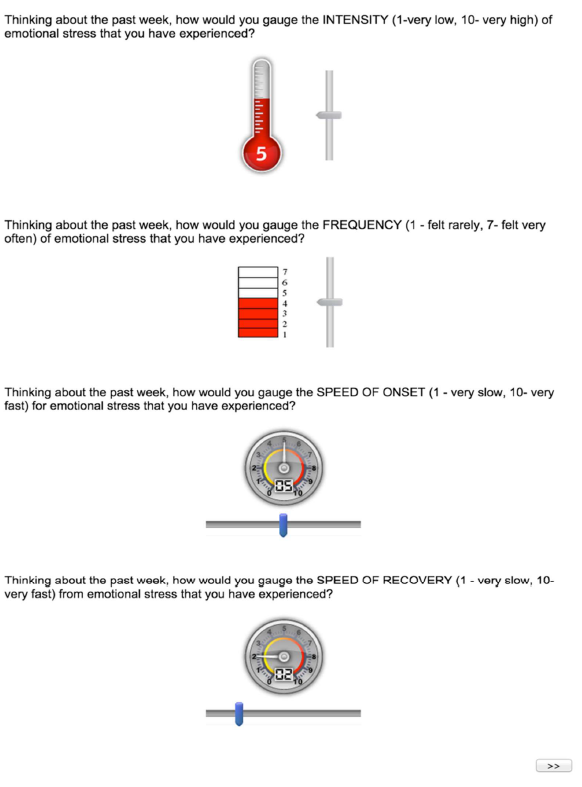
Figure 1. SIFOR: 4-item self-report instrument for measuring perceived stress; participants are told to move the sliders to respond to these rating scales.
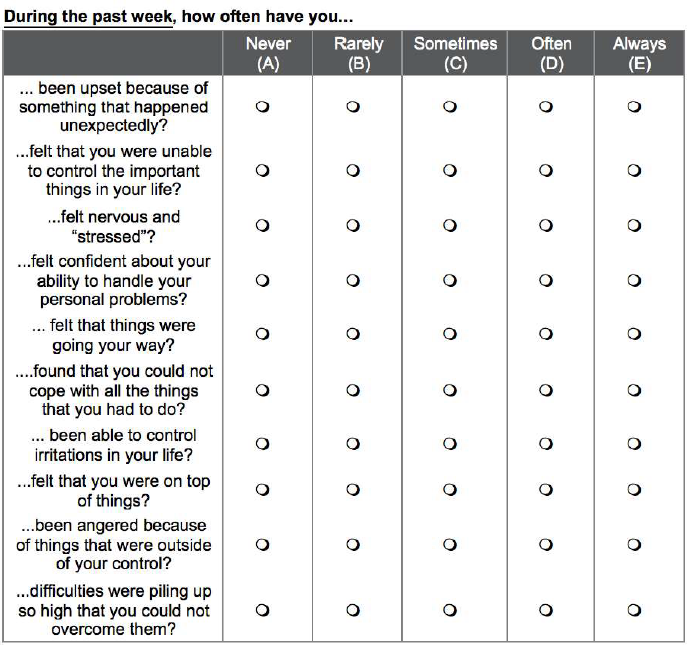
Figure 2. The 10-item PSS validated questionnaire for comparing with SIFOR.
The participants in this study were encountering stress while living and working together in an 8-month simulated Mars mission called HI-SEAS (Hawaii Space Exploration Analog and Simulation). HI-SEAS immerses 6-person crews of astronaut-like individuals in a Mars-like habitat on Mauna Loa volcano, isolated from the rest of humanity for a long duration, confined to a 1000-square-foot living space, except for spacewalks wearing mock spacesuits, while living on limited energy, water, and food resources, and relying on delayed communications with “Earth” and mission support.
HI-SEAS is a NASA-funded study characterizing the behavioral health and performance (BHP) risks for long-duration human spaceflight missions. This stress questionnaire is an opportunistic research project that complemented the BHP study during HI-SEAS mission III and was conducted in accordance with the NASA Institutional Review Board (IRB) approved protocol for participant privacy protection. While the HI-SEAS mission began in mid-October 2014, this stress questionnaire was approved and added in December 2014 and the questionnaire continued until mission end in mid-June 2015, which is 6.5 months of the 8-month HI-SEAS mission.
Theory / calculations
Computing the perceived stress score of this novel instrument only requires simple arithmetic. From the responses to the four items, two factors (SIF and SOR) are calculated, each factor is transformed by min-max scaling to a relative scale for that participant, and then the two relative factors are averaged to compute the SIFOR score.
The first factor (SIF) is taking the result of item 1, stress intensity, and multiplying by the result of item 2, frequency. This factor, shown in Equation 1, is providing an overall picture of stress. For the longitudinal study results presented here, SIF is the stress level experienced in a given week by the crewmembers. Consider that only one day of low intensity stress during the week would be the lowest stress case, whereas seven days of high intensity stress would be the highest stress case. Then, min-max scaling of the factor is shown in Equation 1b which scales the magnitude of the factor to range from 0 (minimum) to 1 (maximum).
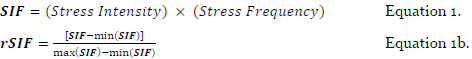
The second factor (SOR) is the speed of onset, which is item 3, minus the speed of recovery, which is item 4 (Equation 2). This factor describes the temporal nature of how stress was experienced. Consider that the highest case would be if stress emerged very quickly then lingered on, dissipating very slowly. The lowest case of stress for this factor is when stress has emerged slowly, then dissipated very quickly. Min-max scaling of this factor is shown in Equation 2b.
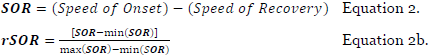
The overall score: SIFOR (Equation 3) is an average of relative SIF and SOR factors.
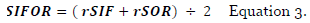
Results
Stress intensity results for each of the six crewmembers are shown in (Figure 3) plotting relative SIF (Equation 1b), relative SOR (Equation 2b), and the average of these two factors SIFOR (Equation 3). These results are reported on a relative scale from 0 to 1 representing the range of stress that was reported by each participant from lowest to highest stress during the mission.
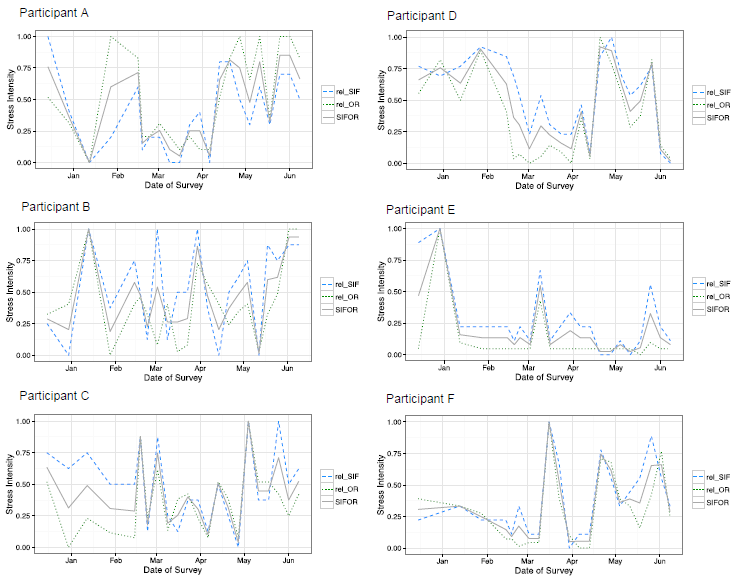
Figure 3. The results of the weekly questionnaire are shown here by plotting SIF (dashed line), SOR (dotted line), and the average of the two factors SIFOR (solid line) on a relative scale from 0 (lowest stress) to 1 (highest stress) for each participant.
The absolute values for stress intensity reported by each participant varied; for an interested reader, the range of SIFOR values that is represented by the relative scale for each crewmember is the following: from 1 to 19 for subplot 1 (top left), from 0.5 to 33 for subplot 2 (top right), from 0.5 to 22.5 for subplot 3 (middle left), from -3.5 to 11 for subplot 4 (middle right), from 5.5 to 33.5 for subplot 5 (bottom left), and from 0.5 to 39 for subplot 6 (bottom right).
To compare the novel metric of Stress Intensifty, Frequencey, Onset, and Recovery (SIFOR) with the Perceived Stress Scale (PSS) questionanire, which is a validated, gold-standard method for obtaining self- reports of perceived stress, both metrics are plotted for each crewmember in (Figure 4). These results are reported on a relative scale from 0 to 1 representing the range of self-reported stress for each participant from lowest to highest. For an interested reader, the range of PSS values represented by the relative scale for each crewmember is the following: from 10 to 16 for subplot 1 (top left), from 9 to 20 for subplot 2 (top right), from 13 to 26 for subplot 3 (middle left), from 10 to 15 for subplot 4 (middle right), from 12 to 24 for subplot 5 (bottom left), and from 8 to 26 for subplot 6 (bottom right).
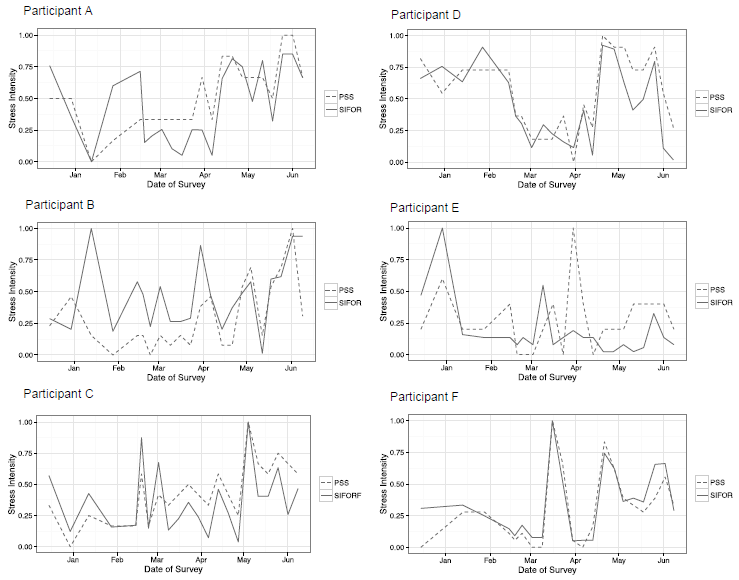
Figure 4. Comparing results of the novel metric SIFOR (solid line) with the gold-standard PSS (dotted line) results during the mission on a relative scale from 0 (lowest stress) to 1 (highest stress). Note that this relative scale differs for each measure, as the average values with PSS are from 10 to 21, whereas SIFOR ranges from 1 to 26.
After combining the results for all six participants to compare PSS and SIFOR, in (Figure 5) it shown that there is relative agreement between these two methods of measuring stress. The two methods are generally correlation, though there are notable outliers in subplot 3 (middle left, Participant B) in mid-January and subplot 4 (middle right, Participant E) in early April.
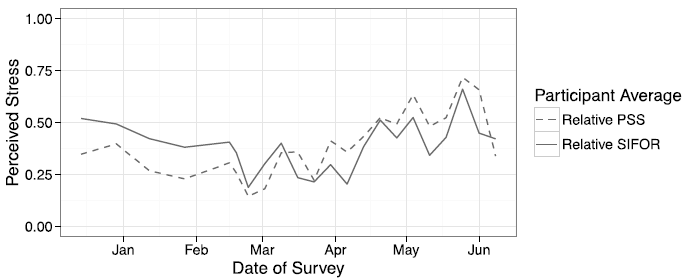
Figure 5. Average PSS and SIFOR values for the group of six participants, indicating that the SIFOR metric is a valid metric for assessing perceived stress of crewmembers.
Discussion
This novel self-report instrument for describing the process of stress emergence and stress dissipation and quantifying the temporal nature of the stress event process has provided a bottom-up, data-driven approach to evaluating stress trajectories. It can also be used to assess various interventions and coping strategies. By providing a measure of the process of moving from “stressed” to “not stressed,” this novel metric can enable hypothesis testing of both vulnerability and resiliency to stress. Behavioral, psychological, or biological histories may promote resilience or alternatively, increase a person’s vulnerabilities to stressful situations [9,10]. While characteristics of resilient individuals are being analyzed in legacy studies of aged populations and of adolescents who beat-the-odds to reach success despite low access to resources, support, and opportunity in their communities [11-13], there is a need for assessing resilience to stressors in a longitudinal manner over time, rather than in a retrospective manner.
While chronic stress is known to be linked to poor health outcomes [14], research is needed to determine which types of chronic stressors are most detrimental for a given context (i.e. financial stress may be more damaging than social stress for some people and situations), and not all stressors are negative, as Yerkes-Dodson law states that some arousal is helpful for improving performance [15]. Many stressor types have been recorded in questionnaires or induced in laboratory research of humans and animals, including environmental toxins, aggression, subordination, financial insecurity, death of a loved one, isolation, confinement, or poor sleep hygiene [8,16-21].
Here, we consider the intensity of stressful experiences (such as dealing with small team dynamics while in isolation and confinement), how they are triggered, and how they resolve over time. For example, the sudden shock of a loved one getting diagnosed with a disease would be a quick onset stress, in the case of the news being surprising, not known nor suspected, and then would be a slow recovery due to ongoing stress of going through the health care treatment, including schedule and financial disruptions, versus the surprise of a work deadline or a teammate’s criticism that has come and gone quickly. The results presented here show that stress is perceived as intense when stress levels have increased quickly and then keep lingering on. Similarly, the theory of allostasis explains that when stress does not dissipate quickly and continues straining a living system beyond the elastic bounds of recovery, aging and disease progress as “wear and tear” from stress exposures accumulating over time [22,23]. Accumulation of allostatic load due to stress exposures has been linked to a plethora of health problems, including cardiovascular disease, PTSD, depression, anxiety, metabolic disorders, digestion problems, and neurodegenerative diseases [9,10].
Future studies with this instrument are recommended to evaluate patterns of stress that are most detrimental. For example, testing the hypothesis that stress events with high speed of onset and low speed of recovery have more lasting psychological or biological impacts, or studying if individual personality traits or characteristics of a given stressor type are related to various SIFOR responses. Developing predictors of biological or psychological impacts will provide information for promoting self-awareness and for improving stress management. Analyzing the emergence and dissipation of various stressors can enable improved development and assessment of stress management strategies, such as diaphragmatic breathing techniques as well as adequate sleep and exercise, and proper nutrition, or pharmacological solutions [24-26]. Coping strategies should be practiced, recorded, and assessed in a longitudinal manner to promote adaptive and resilient responses to stressful situations.
Conclusions
This study has developed empirical evidence that the intensity of stress is related to the temporal nature of stress, defined here as an individual’s perception of how quickly stress emerges and how slowly stress dissipates. In future work, we will validate perceived stress intensity with biological sampling (i.e. urine, hair, etc.) by comparing SIFOR results with catecholamine and cortisol levels [18,21].
Stressor types should be classified in a bottom-up approach, whereas most of the prior work in developing classifications for stress types has been developed in a topdown inductive approach, including important academic and clinical distinctions such as acute stress (short-term), chronic stress (long-term), eustress (good stress), distress (bad stress), and PTSD. Time-dependent and contextspecific classifications of stress can be developed in a data-driven manner to complement existing definitions of stress types in the literature induced from theoretical, conceptual, or lexicographical angles [27-29].
Preventing the development of unhealthy states, or mitigating the negative impacts of stress, requires knowledge of a person’s tolerance of stressors and current states of vulnerability, resilience, and adaptability or coping methods. The timing of stressors should be considered in future analysis of stress trajectories as feedback from earlier stress events that are slowly dissipating may create future vulnerabilities to stress emergence from other stressors. Depending on earlier events, a given stressor may provoke a different path of stress emergence and dissipation, and a given coping strategy may be more effective for a given scenario. This research has developed an instrument for studying the time-dependent nature of stress.
References
2. Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiology & Behavior. 1991 Nov 1;50(5):1039- 45.
3. Lu Y, Burykin A, Deem MW, Buchman TG. Predicting clinical physiology: a Markov chain model of heart rate recovery after spontaneous breathing trials in mechanically ventilated patients. Journal of Critical Care. 2009 Sep 1;24(3):347-61.
4. de Geus EJ, Willemsen GH, Klaver CH, van Doornen LJ. Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biological Psychology. 1995 Nov 16;41(3):205-27.
5. Heinicke C, Poulet L, Dunn J, Meier A. Crew selforganization and group-living habits during three autonomous, long-duration Mars analog missions. Acta Astronautica. 2021 May 1;182:160-78.
6. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983 Dec 1:385-96.
7. Cohen S, Williamson G. Perceived Stress in a Probability Sample of the United States. Spacapan S, Oskamp S. The Social Psychology of Health. Newbury Park, CA: Sage, 1988.
8. Basner M, Dinges DF, Mollicone DJ, Savelev I, Ecker AJ, Di Antonio A, et al. Psychological and behavioral changes during confinement in a 520-day simulated interplanetary mission to mars. PloS One. 2014 Mar 27;9(3):e93298.
9. Oken BS, Chamine I, Wakeland W. A systems approach to stress, stressors and resilience in humans. Behavioural Brain Research. 2015 Apr 1;282:144-54.
10. Priyadarshini S, Aich P. Effects of psychological stress on innate immunity and metabolism in humans: a systematic analysis. PloS One. 2012 Sep 19;7(9):e43232.
11. Foebel AD, Pedersen NL. Genetic influences on functional capacities in aging. The Gerontologist. 2016 Apr 1;56(Suppl_2):S218-29.
12. Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of Personality and Social Psychology. 2006 Oct;91(4):730.
13. Fergus S, Zimmerman MA. Adolescent resilience: A framework for understanding healthy development in the face of risk. Annu. Rev. Public Health. 2005 Apr 21;26:399-419.
14. Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney Jr JM. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychology. 1998 May;17(3):214-23.
15. von Guntena A, Giannakopoulosa P, Bourasb C, Hofc PR. Neuropathological Changes in Visuospatial Systems in Alzheimer’s. Vision in Alzheimer’s disease. 2004;34:30.
16. Van der Meer E, Van Loo PL, Baumans V. Short-term effects of a disturbed light-dark cycle and environmental enrichment on aggression and stress-related parameters in male mice. Laboratoy Animals. 2004 Oct;38(4):376-83.
17. Haller J, Halasz J, Mikics E, Kruk MR. Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. Journal of Neuroendocrinology. 2004 Jun;16(6):550-7.
18. Faresjö Å, Theodorsson E, Chatziarzenis M, Sapouna V, Claesson HP, Koppner J, et al. Higher perceived stress but lower cortisol levels found among young Greek adults living in a stressful social environment in comparison with Swedish young adults. PloS One. 2013 Sep 16;8(9):e73828.
19. Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013 Aug 1;38(8):1220-35.
20. Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in the rat. Physiology & Behavior. 1974 Jan 1;12(1):1-3.
21. Bouhuys AL, Flentge F, Van den Hoofdakker RH. Effects of total sleep deprivation on urinary cortisol, selfrated arousal, and mood in depressed patients. Psychiatry Research. 1990 Nov 1;34(2):149-62.
22. Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Handbook of Life Stress, Cognition, and Health. Fisher S, Reason J (Editors). New York: John Wiley & Sons. 1988: pp 629-649.
23. McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of Aging. 2002 Sep 1;23(5):921-39.
24. Ertin E, Stohs N, Kumar S, Raij A, Al’Absi M, Shah S. AutoSense: unobtrusively wearable sensor suite for inferring the onset, causality, and consequences of stress in the field. InProceedings of the 9th ACM Conference on Embedded Networked Sensor Systems. Seattle, 2011 Nov 1 (pp. 274-287).
25. Varvogli L, Darviri C. Stress management techniques:Evidence-based procedures that reduce stress and promote health. Health Science Journal. 2011 Apr 1;5(2):74-89.
26. Davidson JR, Payne VM, Connor KM, Foa EB, Rothbaum BO, Hertzberg MA, et al. Trauma, resilience and saliostasis: effects of treatment in post-traumatic stress disorder. International Clinical Psychopharmacology. 2005 Jan 1;20(1):43-8.
27. Green BL. Defining trauma: Terminology and generic stressor dimensions 1. Journal of Applied Social Psychology. 1990 Nov;20(20):1632-42.
28. Hobfoll SE. Conservation of resources: a new attempt at conceptualizing stress. American Psychologist. 1989 Mar;44(3):513-24.
29. McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999 Dec;896(1):30-47.
