Abstract
Bacterial communication via quorum sensing (QS) molecules, as well as toxin-antitoxin (TA) gene modules located on bacterial chromosomes are well-studied mechanisms. Escherichia coli mazEF is a stress-induced TA system mediating cell death requiring a QS extracellular death factor (EDF), the pentapeptide NNWNN. MazF is an endoribonuclease specific for ACA sites. During adverse conditions, the activated MazF generates a stress induced translation machinery, composed of MazF-processed mRNAs and selective ribosomes that specifically translate these processed mRNAs. Moreover, we identified the molecular mechanism underlying the formation of EDF from the zwf mRNA that involves distinct steps comprising the activity of MazF, the trans-translation system as well as the protease ClpPX.
Bacterial trans-translation is generally known as a quality control process that rescues stalled translation complexes at the 3’-terminus of non-stop mRNAs. Our results indicate that trans-translation has a similar role in EDF generation from zwf mRNA. However, our data reveal that the trans-translation system may also provide a regulatory mechanism to attenuate EDF generation in the single cells. Thereby, the required threshold of EDF molecules is only achieved by the entire bacterial population, as expected for a genuine QS process.
Commentary
Bacteria communicate via quorum sensing (QS) signaling molecules [1-8]. QS allows bacteria to monitor and quantify the number of their kin present in their environment. By this means, they ensure the modulation of gene expression with respect to population density. Thereby, bacterial populations can behave like a multicellular organism [1-8]. Over the last years, a great deal of attention has also been focused on the abundance of toxin-antitoxin (TA) gene modules located in chromosomes of most bacteria (reviewed in [9,10]. E. coli mazEF is the first discovered chromosomal TA module [11]. The sequence-specific endoribonuclease toxin MazF preferentially cleaves single-stranded mRNAs at the 3’ or at the 5’ side of the first A in the ACA sequences [12,13]. When E. coli encounters stressful conditions the antitoxin MazE is proteolytically degraded thereby triggering MazF activity [14]. Under such conditions, the active MazF causes the generation of leaderless mRNAs by the removal of the 5’-UTR of some specific mRNAs by cleavage upstream of AUG start codons [15]. In addition, MazF cleaves the 16S rRNA at the decoding center of the ribosome resulting in its 3’-terminal truncations at position 1500 [15]. The removed 43 nucleotides comprise the anti- Shine-Dalgarno (aSD) region, which is essential for translation initiation on canonical mRNAs but is dispensable for the recognition of AUG start codons located close to the mRNA 5’-terminus. Thus, the aSD-deficient ribosomes exclusively translate leaderless mRNAs [15]. Taken together, the concerted action of MazF on ribosomes and mRNA transcripts engenders a stress-induced translation machinery (STM) [15,16], which allows the specific synthesis of a pool of proteins that affects cell viability within the bacterial population [17,18]. Moreover, the post-transcriptional autoregulatory circuit of mazF results in cell-to-cell translation and growth heterogeneity in isogenic populations [19,20].
We have also uncovered the first QS peptide in E. coli namely the pentapeptide NNWNN, termed extracellular death factor (EDF). As anticipated for a QS molecule, the pentapeptide is taken up by the cells after the concentration of EDF reaches a threshold level in the medium. In the cytoplasm, it binds to the MazE-binding pocket on MazF, thereby stimulating the activation of MazF. In this respect, EDF acts as signaling molecule linking the mazEF-mediated stress response to the population density in E. coli [21,22]. Our experiments further revealed that when E. coli encounters stressful conditions, activation of MazF results in the generation of the QS EDF factor employing the product of the zwf gene [21,22], which encodes the 491–amino acids long enzyme glucose-6- phosphate dehydrogenase involved in the central carbon metabolism [23].
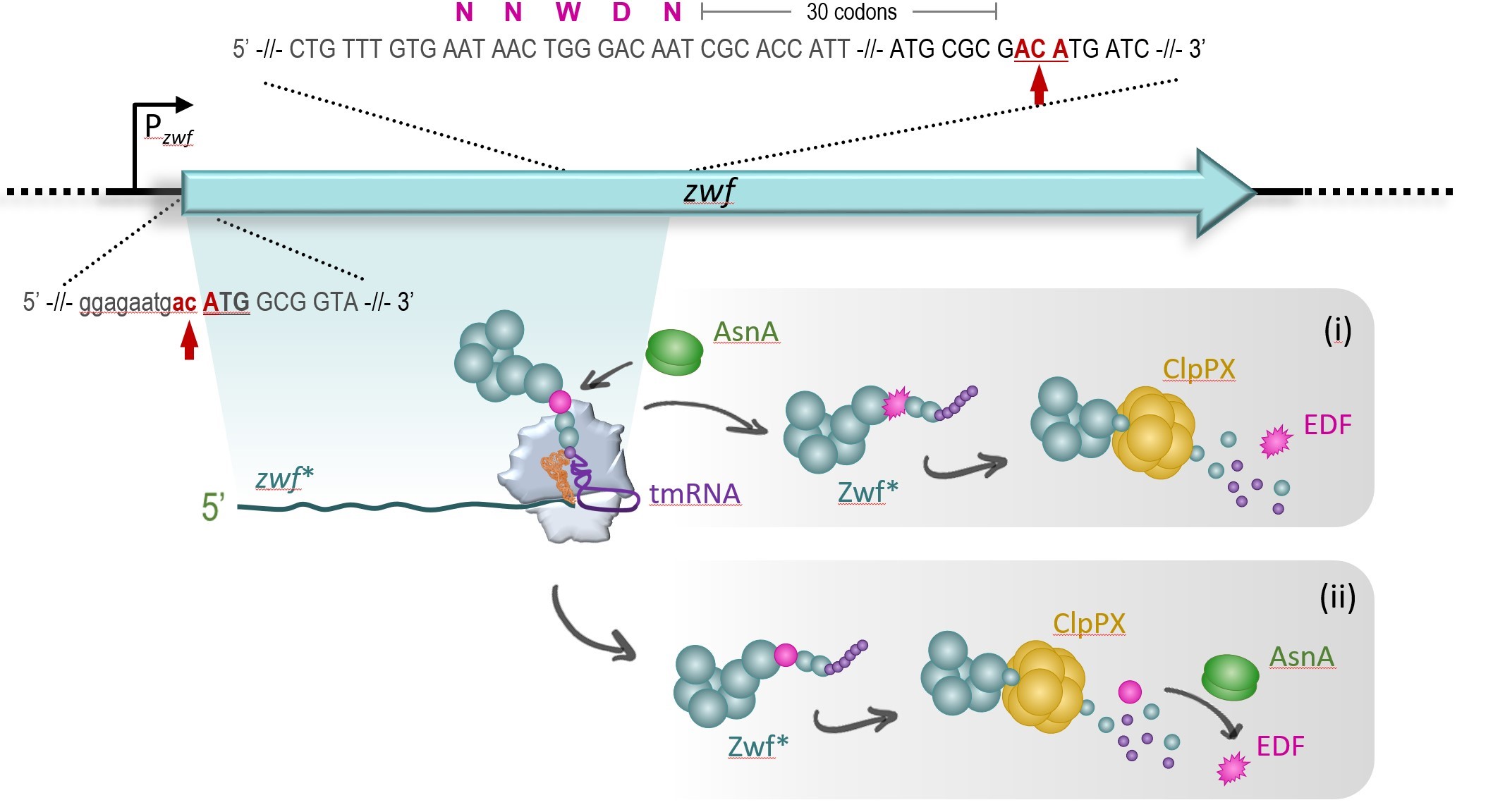
More recently, we studied the MazF-dependent mechanisms underlying the generation of the pentapeptide EDF from the zwf gene [24]. We uncovered several distinct molecular steps that are surprisingly involved under stress in the generation of EDF. In particular, we showed that under stress the endoribonuclease MazF cleaves the zwf mRNA at specific positions (Figure 1). Thereby, a leaderless zwf mRNA variant is engendered that is truncated 30 codons after the EDFencoding region, a length corresponding to the ribosome nascent peptide exit tunnel. As the absence of a stop codon at the 3’-terminus of the truncated zwf* mRNA results in stalling of translating ribosomes, the trans-translation machinery is recruited to release the truncated Zwf protein (Zwf*) equipped with the C-terminal degradation tag encoded by the tmRNA. Consequently, the protease ClpPX is likewise involved in and essential for the maturation of the EDF pentapeptide (Figure 1).
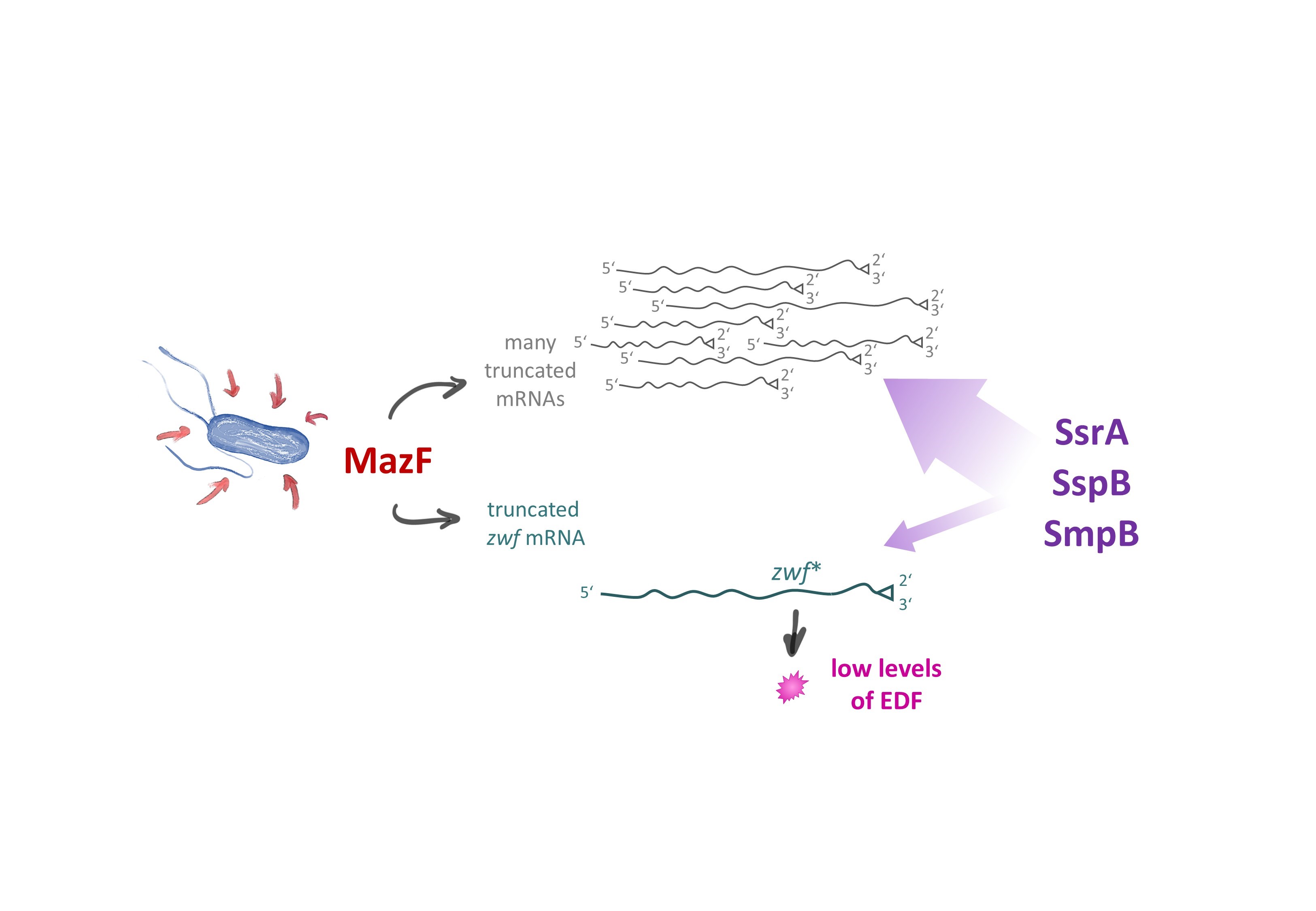
In general, bacterial trans-translation represents a quality control process that recognizes and rescues stalled ribosome complexes at the 3’-terminus of so-called non-stop mRNAs [25-27]. Taken together, our results indicate that transtranslation is essential for the formation of EDF employing the zwf gene (Figure 1). Intriguingly, based on our observations we hypothesize that for EDF synthesis the trans-translation system might further provide a negative feedback loop regulating EDF formation (Figure 2). As numerous mRNA molecules are truncated by active MazF, a plethora of stalled ribosome complexes are formed that recruit the majority of trans-translation components. Thus, only very few of those components are available for the generation of EDF. Collectively, our results support the notion that this process results in the attenuation of EDF generation in the single cell (Figure 2). Thereby, the required threshold of EDF molecules is only achieved by the entire bacterial population, as expected for a genuine quorum sensing (QS) process.
Figure 1. Mechanisms involved in the generation of EDF after activation of MazF during stress. The genetic locus of the zwf gene (in cyan) is shown. Active MazF cleaves the zwf mRNA at ACA sites (in red, marked by arrows) directly upstream of the start codon (capital letters, underlined) and 30 codons downstream of the codons that code for the pre-EDF pentapeptide NNWDN (in magenta). The resulting leaderless and 3’-terminally truncated zwf* mRNA (cyan) is translated by the specialized ribosomes that stall at the 3’ terminus. Here, the tmRNA (in purple) is essential to resolve the stalled ribosome complex by adding the degradation tag (purple spheres) to the N-terminal part of the Zwf Protein, Zwf* (cyan). The position of the pre-EDF pentapeptide NNWDN is indicated by a magenta sphere. After release the truncated Zwf* protein is subjected to the activity of the ClpP protease. Whether the conversion of the aspartic acid (D) to asparagine (N) by the asparagine synthetase AsnA (green) takes place at the ribosome (i) or after degradation by the ClpPX protease (ii) still remains to be elucidated.
Figure 2. Regulatory circuit ensuring the generation of only low levels of EDF. When E. coli cells encounter diverse environmental stress conditions (indicated by red arrows), MazF is activated and cleaves the zwf mRNA at the ACA sites indicated in Figure 1 leading to the formation of the zwf* mRNA (in cyan). Besides, MazF activity generates a large number of different truncated mRNAs (in gray). Thus, only a minor fraction of the components of the trans-translational machinery (in purple), namely SsrA, SspB, and SmpB, are available to resolve the stalled ribosome complex on the zwf* mRNA. Consequently, the titration of the trans-translational machinery by stalled complexes on all other truncated mRNAs ensures that only low levels of EDF are generated, as required for a genuine QS molecule.
References
2. Okada M, Sato I, Cho SJ, Iwata H, Nishio T, Dubnau D, et al. Structure of the Bacillus subtilis quorum-sensing peptide pheromone ComX. Nature Chemical Biology. 2005 Jun;1(1):23-4.
3. Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends in Microbiology. 2005 Jan 1;13(1):27-33.
4. Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 2005 Nov 10;21:319-46.
5. Bassler BL, Losick R. Bacterially speaking. Cell. 2006 Apr 21;125(2):237-46.
6. Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006 Feb 24;311(5764):1113-6.
7. Novick RP, Geisinger E. Quorum sensing in staphylococci. Annual Review of Genetics. 2008 Dec 1;42:541-64.
8. Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annual Review of Genetics. 2009 Dec 1;43:197-222.
9. Harms A, Brodersen DE, Mitarai N, Gerdes K. Toxins, targets, and triggers: an overview of toxin-antitoxin biology. Molecular Cell. 2018 Jun 7;70(5):768-84.
10. Fraikin N, Goormaghtigh F, Van Melderen L. Type II toxinantitoxin systems: evolution and revolutions. Journal of Bacteriology. 2020 Mar 11;202(7):e00763-19.
11. Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal” addiction module” regulated by guanosine [corrected] 3’, 5’-bispyrophosphate: a model for programmed bacterial cell death. Proceedings of the National Academy of Sciences. 1996 Jun 11;93(12):6059-63.
12. Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Molecular Cell. 2003 Oct 1;12(4):913-23.
13. Zhang Y, Zhang J, Hara H, Kato I, Inouye M. Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase. Journal of Biological Chemistry. 2005 Feb 4;280(5):3143-50.
14. Hazan R, Engelberg-Kulka H. Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1. Molecular Genetics and Genomics. 2004 Sep;272(2):227-34.
15. Vesper O, Amitai S, Belitsky M, Byrgazov K, Kaberdina AC, Engelberg-Kulka H, et al. Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli. Cell. 2011 Sep 30;147(1):147-57.
16. Moll I, Engelberg-Kulka H. Selective translation during stress in Escherichia coli. Trends in Biochemical Sciences. 2012 Nov 1;37(11):493-8.
17. Amitai S, Kolodkin-Gal I, Hananya-Meltabashi M, Sacher A, Engelberg-Kulka H. Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genetics. 2009 Mar 13;5(3):e1000390.
18. Sauert M, Wolfinger MT, Vesper O, Müller C, Byrgazov K, Moll I. The MazF-regulon: a toolbox for the post-transcriptional stress response in Escherichia coli. Nucleic Acids Research. 2016 Aug 19;44(14):6660- 75.
19. Nikolic N, Bergmiller T, Vandervelde A, Albanese TG, Gelens L, Moll I. Autoregulation of mazEF expression underlies growth heterogeneity in bacterial populations. Nucleic Acids Research. 2018 Apr 6;46(6):2918-31.
20. Nikolic N, Didara Z, Moll I. MazF activation promotes translational heterogeneity of the grcA mRNA in Escherichia coli populations. PeerJ. 2017 Sep 21;5:e3830.
21. Kolodkin-Gal I, Hazan R, Gaathon A, Carmeli S, Engelberg-Kulka H. A linear pentapeptide is a quorum-sensing factor required for mazEF-mediated cell death in Escherichia coli. Science. 2007 Oct 26;318(5850):652-5.
22. Kolodkin-Gal I, Engelberg-Kulka H. The extracellular death factor: physiological and genetic factors influencing its production and response in Escherichia coli. Journal of Bacteriology. 2008 May 1;190(9):3169-75.
23. Peyru G, Fraenkel DG. Genetic mapping of loci for glucose-6-phosphate dehydrogenase, gluconate-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrase in Escherichia coli. Journal of Bacteriology. 1968 Apr;95(4):1272-8.
24. Kumar S, Kolodkin-Gal I, Vesper O, Alam N, Schueler-Furman O, Moll I, et al. Escherichia coli quorum-sensing EDF, a peptide generated by novel multiple distinct mechanisms and regulated by trans-translation. MBio. 2016 Jan 26;7(1):e02034-15.
25. Janssen BD, Hayes CS. The tmRNA ribosome-rescue system. Advances in Protein Chemistry and Structural Biology. 2012 Jan 1;86:151-91.
26. Keiler KC, Waller PR, Sauer RT. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996 Feb 16;271(5251):990-3.
27. Karzai AW, Roche ED, Sauer RT. The SsrA–SmpB system for protein tagging, directed degradation and ribosome rescue. Nature Structural Biology. 2000 Jun;7(6):449-55.