Commentary
It was recently demonstrated that modulations in resting-state networks (RSNs) can be introduced via the stimulation of the vestibular inner ear by a strong magnetic field (>1 tesla) as used in magnetic resonance imaging (MRI) [1,2]. This magnetic vestibular stimulation (MVS) of the inner ear leads to a state of vestibular imbalance and can be verified behaviorally via nystagmus eye-movement [3-11].
We have now demonstrated how these MVS-induced RSN modulations can be addressed in fMRI analysis on the group-level [12]. We propose a parameter, derived from high-resolution anatomical imaging of the inner ear, that can be used for regressing out significant parts of the variance in RS-fMRI fluctuations [12]. We acquired RS-fMRI, and high-resolution CISS anatomical images of the inner ear in a sample of N=60 healthy volunteers balanced for age, gender, and handedness. We created a subject-specific proxy for MVS, “pMVS”, that corresponds to the sinus of the angle between the individual horizontal semicircular canal and the static magnetic field direction (also called “z-direction” in MR imaging). We found that pMVS explained a considerable fraction of the total variance in RS-fMRI fluctuations (from 11% up to 36%). In addition to pMVS, we examined the angle of Reid’s plane, as determined from anatomical imaging, as an alternative and found that this angle (with the same sinustransformation as for pMVS) explained considerably less variance, e.g., from 2% to 16%.
Our results show that it is possible to account for significant parts of the modulations in RSNs due to MVS. This is noteworthy for RS-fMRI analysis as this variability would otherwise have been part of the error term in the group-level regression model. Therefore, we categorize our approach analogous to established nuisance regression methods for RS-fMRI, like motion or pulsation regression. In our analysis it was better to use the CISS-derived orientations of the inner ear than to use the orientation of Reid’s plane. We, therefore, recommend acquiring highresolution anatomical images (CISS or similar) of the inner ear in addition to RS-fMRI and use the described pMVS parameter in the group-level analysis to capture variance associated with MVS and vestibular imbalance.
We conclude this commentary with a short review of MVS and give an outlook on the potential of MVS for future vestibular research.
MVS can be demonstrated in healthy controls as well as in patients with unilateral vestibular failures, but was absent in patients with complete bilateral vestibular loss, indicating that the inner ear is the origin of the stimulation [3]. Studies with mammals (e.g., rats, mice, and humans) have revealed signs of vestibular imbalance and dizziness in magnetic fields above 1 tesla [13,14]. For rats and mice, neuronal activity in the brain stem due to the presence of ga magnetic field was indicated via immunohistochemistry labeling of c-fos (a protein associated with the generation of action potentials). It was shown that this signal was abolished by labyrinthectomy [15]. Furthermore, the distribution of c-fos in the vestibular nuclei of the rats was shown to depend on the angle of the rat’s inner ear relative to the field. This was also reflected in the behavior of the rats, i.e., they swam in circles (“circling behavior”) after 15 min exposure to the magnetic field [16]. If the mice were oriented 90° relative to the MR-field, no significant c-fos induction (relative to sham treatment group) could be shown, and these mice did not show circling behaviors. In contrast, orientation at 0° and 180° relative to the field produced significant c-fos induction in the vestibular nuclei with a left-right asymmetry. This asymmetry was reverse for 180° vs. 0° orientation and reflected in the circling behavior, which was either clockwise or counterclockwise, respectively [16].
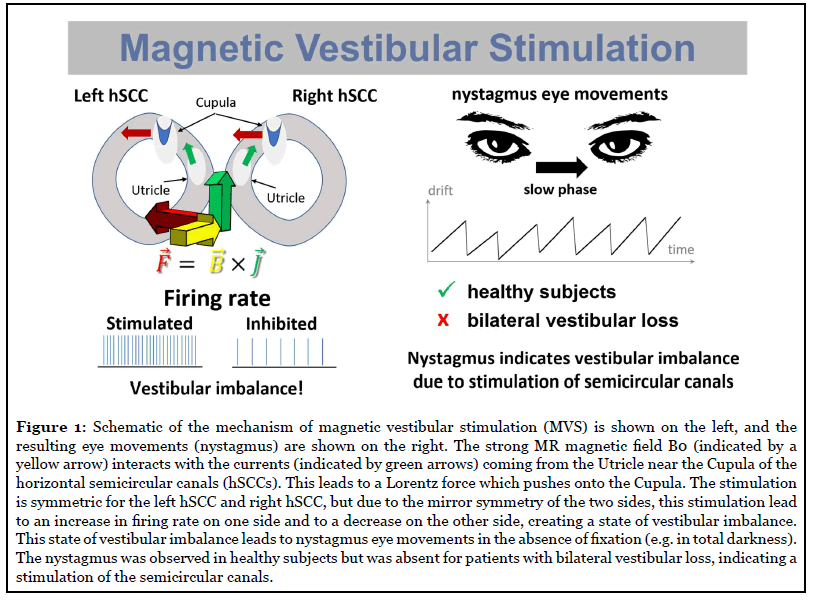
A Lorentz force model was proposed for MVS, that explains not only the occurrence, direction, and persistence of the observed nystagmus (and the circling behavior of the rats) but also its dependence on the head positions within the static magnetic field and the strength of the magnetic field itself [3,5]. Based on eye-tracking data in healthy controls and patients, it is assumed that the MVS acts most dominantly on the semicircular canals [9,10]. The vestibular stimulation is most likely caused by ionic currents coming from the hair cells or the utriculus, which due to a Lorentz-force creates a pressure onto the cupula of (mainly) the horizontal and (to a lesser extend) the anterior canal [3-6,9-11,16-18]. As MVS is a byproduct of MR-scanners, it was unknowingly applied to thousands of subjects and patients over the last decades. It can, therefore, be concluded that MVS used over multiple hours is safe and does not have any long-lasting adverse effects [6,18,19].
We suggest that MVS should be seen as a new way of manipulating networks that either process vestibular information or show vestibular interactions, by using strong magnetic fields ≥1 tesla. MVS can be studied behaviorally by examining the associated nystagmus and in imaging experiments using resting-state fMRI. For fMRI, a modulation is necessary to reveal the MVS modulation as fMRI does not provide an absolute signal. This can be done, either by repeating the same experiment at scanners with different field strengths, different field directions (south pole at foot end vs. south pole at the head end of the MRI, e.g., Siemens vs. Philips) or by varying the head positions across different sessions [1,2,12]. It is crucial to keep in mind that the effect of MVS is not like the constant acoustic noise stimulation during fMRI. MVS induces an imbalance state with a directional preponderance, i.e., has a signed difference effect, unlike audible noise that can be supposed to be equal and balanced for the auditory network and its connections. The potential of modulating or inducing vestibular imbalances can be used to create states that are similar to the diseased state in healthy controls, but without peripheral or central lesions that patients have. In patients, this will allow to extend or reduce vestibular imbalances.
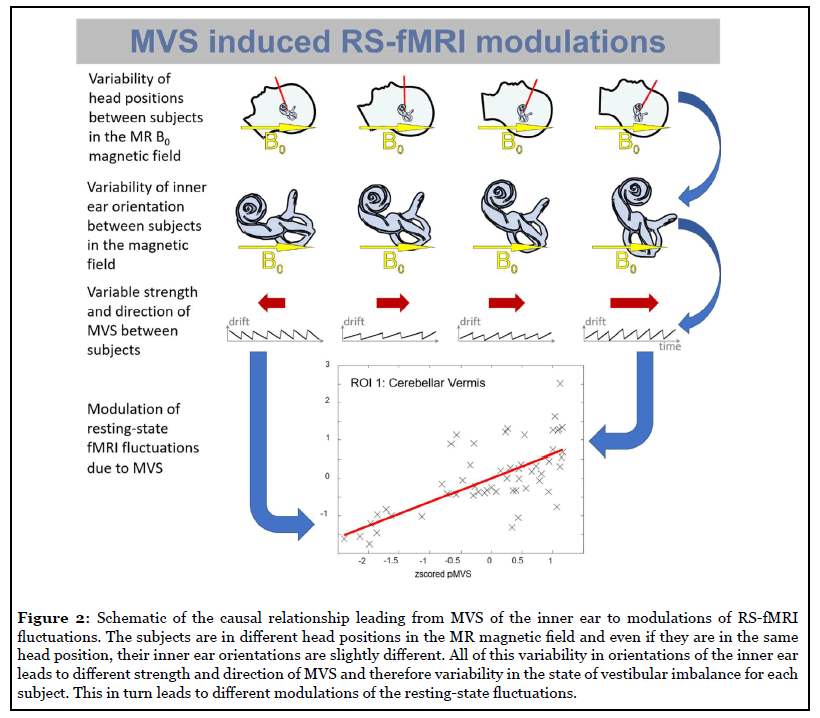
Recent studies examining patients with vestibular deficits using resting-state fMRI showed widespread changes in various networks similar to our findings for MVS [20-22]. Our results suggest caution when interpreting such studies. In the case of patients with bilateral vestibular loss, it should be noted that the patients will not show an MVS influence, but the healthy control group will be under the influence of MVS [1-3,12,20]. This might then lead to changes in the comparison of differences between the two groups as examined with fMRI that are not expected to appear in imaging methods without the use of strong magnetic fields, e.g., PET (for a methods review see [19]). In this case, the healthy controls might be more akin to patients with acute unilateral vestibular neuritis, given that such patients also show a directional imbalance with horizontal nystagmus, not unlike that evoked by MVS [1-3,5,12]. For studies of vestibular neuritis patients versus healthy controls (e.g. [21,22]), MVS effects should be expected for both the patients and the healthy controls [1-3,5,12]. However, MVS will affect patients with unilateral vestibular deficits differently than healthy controls, which will then further obscure the real differences between the two groups [5]. This means that the reported differences at every time interval during the compensation period relative to the healthy control group will be obscured or biased by MVS. However, the trajectory of recovery of the patients and, therefore, the path of associated relative differences to the healthy controls might not be affected by MVS influence. Thus, the trend of the change of the disputes over the time intervals of compensation should be unaffected by MVS. This requires, however, that the subjects are imaged in, at least, very similar head positions and field strength at every time interval of compensation to stay comparable over the time intervals [1,2]. In the resting-state study on vestibular neuritis patients, it is interesting to note that no significant correlations were found for the caloric testing covariate. However, this is usually a good indicator of impairment or restoration of vestibular function [22]. One might speculate that MVS had obscured this correlation because MVS seems to share essential characteristics in terms of temporal dynamics with caloric stimulation [6,18].
In summary, in a sufficiently strong (e.g., ≥1 Tesla) magnetic field, MVS will induce a state of vestibular imbalance in each healthy subject. The variability between subjects will increase with field strength due to the multiplicative nature of the Lorentz-force. These effects may lead to biased results and reduced statistical significance. However, as we have shown, the variance can be reduced by using the pMVS parameter as an additional regressor in the group-level analysis [1,2,12].
There are essential limitations in the current research and future avenues for research to consider, that we want to highlight.
First, our approach of “regressing out a nuisance” might suffer from similar drawbacks like other nuisance regression methods, e.g., motion regression. By this we mean, that it is simply better to avoid the occurrence of MVS altogether, rather than to use regression of the associated parameter, just like for other nuisance processes. In other words, it is better to avoid MVS from occurring (just as it is better to prevent motion from occurring during imaging), than it is to regress these nuisances out of the data. If one wants to avoid the effects of MVS, this can be achieved by adjusting the head position of each subject until the “null-position”, where MVS is minimal or not present is found, as MVS depends on the orientation of the inner ear relative to the “z-direction” of the MR static magnetic field [3]. Eye-movements of the subject must be checked for the nystagmus resulting from to MVS, and the head position must then be changed iteratively until no nystagmus is measurable any longer. This would then also deal with any dynamic effects of MVS on RSNs, as our current regression method only deals with average effects, not with dynamic effects. There are two important drawbacks of this method for finding the “null-position”. The first is that it is cumbersome to measure eye movements while adjusting each subject before imaging. The second and more important drawback is that it might not be possible to change the head position of some participants as MRI head coils are rather small by design, in order to restrict motion and to achieve a good signal to noise ratio by bringing the coil close to the head. Maybe in the future, manufacturers could create MRI head coil systems that could be worn like a cap and allow positioning of the head such that avoidance of MVS and signal to noise ratio can be achieved in tandem. Generally, controlling for MVS should be seen as an effort to remove unwanted variance, i.e., as an effort to homogenize the group and achieve better statistical results due to less (uncontrolled) MVS interference that increases bias and variance with increasing field strength.
Second, currently, we do not know how to measure current density in the inner ear. As current density is the second factor that can vary in the Lorentz force model besides the angle between the current and the magnetic field, it would be imperative to get a handle on this parameter. Were it possible to measure this current strength; this would be a great additional tool for the study of MVS and the vestibular periphery.
Third, although we seek a simple method to correct for the MVS influence like the inner ear anatomy, instead of having to measure the nystagmus eye movements, it would be essential to track and segment simultaneous eye-movements during RS-fMRI. As the nystagmus eyemovements due to MVS are only visible if the subject does not actively move her eyes, one could use the nystagmus as a label for “interoceptive” periods. More research is necessary to examine the relationship of “interoception”, “mind wandering” and periods of nystagmus vs active eye-movements for exploration. MVS could be a tool for monitoring state changes during RS-fMRI. This could also be an approach for future research into the dynamics of MVS in the individual subject as the slow-phase velocity of the nystagmus changes over time, reflecting set-point adaptation processes [6,18].
Fourth, in our current study, no conscious percept of the subjects, such as the strength of their vestibular percept and differences in alertness or how much they engaged in interoception, was assessed. A reporting system or questionnaire for assessing these values would be useful for further studies.
Fifths, we have not assessed the full morphometry of the inner ear. For example, the relationship of the utriculus position and orientation towards the horizontal and superior semicircular canals would be an exciting topic for future studies of MVS. We leave this and other assessments of the inner ear morphology to future research when higher-resolution imaging and detailed morphometrical modeling are available.
Sixth, there was no systematic variation of the time until the RS-fMRI sequence was started, i.e., how long the subjects were in the MRI before the RS-fMRI sequence started. However, all participants were in the MRI for several minutes previous to the RS-fMRI sequence, i.e., enough time for the MVS dynamic modulation effect to have stabilized [6,18]. The time difference was used as a regressor, and the rank-transformed version was also included; both regressors did not explain a significant fraction of the variance in this study [12]. It could be an interesting topic for future studies to examine dynamic changes over the RS-fMRI acquisition within individual subjects concerning the dynamic changes in MVS in the first few minutes upon entering the MRI.
Seventh, we have focused on between-subject variance, and, likely, our suggestion for the simple regression of inner ear morphology parameters is not sufficient to remove the MVS effect in single subject RS-fMRI. In order to measure the full variability due to MVS in restingstate fMRI for an individual participant, a study with subjects undergoing substantial head angle changes (and therefore change in the angle for the semicircular canals) would be needed. Simultaneous eye movement recordings and fMRI acquisition for many different head positions repeated in multiple MR field strengths produced by different MRI scanners would also be necessary. This could then allow measurement of the MVS modulation effect on the individual level. In other words, one would have to not only do an experiment with different MR magnetic field strengths but also simultaneously observe the modulation due to different head orientations for a subject in such a way that the current density intrinsic to the subject remains the only determinable factor. Besides, future studies are needed to examine the influence of both vestibular end organs with all their separate components, such as left and right, anterior, posterior and horizontal semicircular canals as well as utricle and saccule, on the nystagmus as well as the different vestibular brain areas in RS-fMRI studies.
All in all, we see an excellent future for MVS research and vestibular research in general.
Conflict of Interest
The authors declare they have no competing financial interests.
Acknowledgments
Partially funded by the Friedrich-Baur-Stiftung (FBS), the Graduate School of Systemic Neurosciences (GSN), the German Federal Ministry of Education and Research (German Center for Vertigo and Balance Disorders -IFBLMU, Grant code 01EO140) and the German Foundation for Neurology (Deutsche Stiftung Neurologie).
References
2. Boegle R, Ertl M, Stephan T, Dieterich M. Magnetic vestibular stimulation influences resting-state fluctuations and induces visual-vestibular biases. Journal of Neurology. 2017 May 1;264(5):999-1001.
3. Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS. MRI magnetic field stimulates rotational sensors of the brain. Current Biology. 2011 Oct 11;21(19):1635-40.
4. Antunes A, Glover PM, Li Y, Mian OS, Day BL. Magnetic field effects on the vestibular system: calculation of the pressure on the cupula due to ionic current-induced Lorentz force. Physics in Medicine & Biology. 2012 Jun 22;57(14):4477.
5. Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Magnetic vestibular stimulation in subjects with unilateral labyrinthine disorders. Frontiers in Neurology. 2014 Mar 13;5:28.
6. Glover PM, Li Y, Antunes A, Mian OS, Day BL. A dynamic model of the eye nystagmus response to high magnetic fields. Physics in Medicine & Biology. 2014 Jan 17;59(3):631.
7. Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Vestibular stimulation by magnetic fields. Annals of the New York Academy of Sciences. 2015 Apr;1343(1):69.
8. Mian OS, Glover PM, Day BL. Reconciling magnetically induced vertigo and nystagmus. Frontiers in Neurology. 2015 Sep 15;6:201.
9. Mian OS, Li Y, Antunes A, Glover PM, Day BL. Effect of head pitch and roll orientations on magnetically induced vertigo. The Journal of physiology. 2016 Feb 15;594(4):1051-67.
10. Otero-Millan J, Zee DS, Schubert MC, Roberts DC, Ward BK. Three-dimensional eye movement recordings during magnetic vestibular stimulation. Journal of Neurology. 2017 Oct 1;264(1):7-12.
11. Ward BK, Otero-Millan J, Jareonsettasin P, Schubert MC, Roberts DC, Zee DS. Magnetic vestibular stimulation (MVS) as a technique for understanding the normal and diseased labyrinth. Frontiers in Neurology. 2017 Apr 5;8:122
12. Boegle R, Kirsch V, Gerb J, Dieterich M. Modulatory effects of magnetic vestibular stimulation on resting-state networks can be explained by subject-specific orientation of inner-ear anatomy in the MR static magnetic field. Journal of Neurology. 2020 Jun 11:1-3.
13. Weiss J, Herrick RC, Taber KH, Contant C, Plishker GA. Bio-effects of high magnetic fields: a study using a simple animal model. Magnetic Resonance Imaging. 1992 Jan 1;10(4):689-94.
14. Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Annals of the New York Academy of Sciences. 1992 Mar;649(1):285-301.
15. Cason AM, Kwon B, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiology & Behavior. 2009 Apr 20;97(1):36-43.
16. Houpt TA, Kwon B, Houpt CE, Neth B, Smith JC. Orientation within a high magnetic field determines swimming direction and laterality of c-Fos induction in mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2013 Oct 1;305(7):R793-803.
17. Ward BK, Tan GX, Roberts DC, Della Santina CC, Zee DS, Carey JP. Strong static magnetic fields elicit swimming behaviors consistent with direct vestibular stimulation in adult zebrafish. PloS one. 2014 Mar 19;9(3):e92109
18. Jareonsettasin P, Otero-Millan J, Ward BK, Roberts DC, Schubert MC, Zee DS. Multiple time courses of vestibular set-point adaptation revealed by sustained magnetic field stimulation of the labyrinth. Current Biology. 2016 May 23;26(10):1359-66.
19. Ertl M, Boegle R. Investigating the vestibular system using modern imaging techniques—A review on the available stimulation and imaging methods. Journal of neuroscience methods. 2019 Oct 1;326:108363.
20. Göttlich M, Jandl NM, Wojak JF, Sprenger A, von der Gablentz J, Münte TF, et al. Altered resting-state functional connectivity in patients with chronic bilateral vestibular failure. NeuroImage: Clinical. 2014 Jan 1;4:488-99
21. Helmchen C, Ye Z, Sprenger A, Münte TF. Changes in resting-state fMRI in vestibular neuritis. Brain Structure and Function. 2014 Nov 1;219(6):1889-900.
22. Klingner CM, Volk GF, Brodoehl S, Witte OW, Guntinas-Lichius O. Disrupted functional connectivity of the default mode network due to acute vestibular deficit. NeuroImage: Clinical. 2014 Jan 1;6:109-14.
