Abstract
Tumor node metastasis (TNM) staging system is the most useful method in predicting prognosis of colorectal cancer (CRC), the third most common cause of death worldwide, even if other biological markers are currently under evaluation to assess their role in affecting CRC outcome and planning the best tailored therapeutic approach. Several molecular factors are being demonstrated to be effective in influencing both overall survival (OS) and disease-free survival (DFS) in CRC, acting on different aspects of tumor promoting and progression.
Patient inflammatory and nutritional status evaluation, strictly correlated with immune-surveillance system, plays a crucial role in affecting CRC outcome.
The Authors, analyzing data of 562 consecutives CRC patients, suggest a new prognostic model, called Naples Prognostic Score (NPS), for an exhaustive assessment of both inflammatory and nutritional status through the determination of several routine blood parameters. NPS is an independent factor in affecting outcome of CRC patients undergoing surgery with a power almost similar to TNM pathological system.
NPS results by albumin and total cholesterol serum levels, neutrophils to lymphocyte ratio (NRL) and lymphocyte to monocytes ratio (LMR); the score stratifies CRC patients in three homogeneous groups that are significantly different in terms of advanced stages, postoperative complication rate, DFS and OS.
Further studies with higher numbers of patients are necessary to standardize NPS routine use to become a reference model to modified management and integrated treatment of CRC patients.
Keywords
Tumor node metastasis (TNM), Overall survival (OS), Disease-free survival (DFS), Colorectal cancer (CRC)
Main Body
Despite the advances in diagnostic and therapeutic field [1], colorectal cancer (CRC) still remains the third most common cause of death worldwide, with more than 600,000 cancer-related deaths per year [2].
TNM system is currently the most useful method both in evaluating CRC prognosis and in planning its treatment [3]. Nevertheless, a different outcome in the same TNM stage is commonly observed [4], moreover in early-stage cancers [5], in which no other treatment than surgery is required, so that some other factors could affect the clinical history of CRC.
The assessment of further categories of high-risk patients, other than advanced TNM stages, is a major challenge nowadays in improving the outcome of the disease, moreover in such cases where adjuvant therapy is not currently indicated (I and II stages) [6].
Due to the molecular heterogeneity of CRC [7,8], many biomarkers are now being investigated [9], even if a wide accepted consensus about them is not reached yet.
Further studies will be necessary to set a standardized panel of these prognostic factors and their own cutoff values for the evaluation of the best therapeutic choice for each CRC patient. In the meantime, it could be useful to adopt simpler indicators that are currently effective in planning follow-up controls and therapeutic determinations, in the future.
There is a growing evidence that inflammatory and nutritional status, since they are strictly related with the immuno-surveillance system, affect the outcome of several tumors [10]. In the past decade, the strong relation between peripheral lymphocytes/monocyte count and prognosis of several tumors, including CRC, has been investigated [11,12]. Cell-mediated immunoresponse against tumors, mostly related to lymphocytes activity, is associated with a good prognosis in terms of OS and DFS, and a consistent lymphocyte infiltration in tumoral tissue is predictive of a better outcome.
Tumoral microenvironment is one of the most interesting topics in this field, since promoting and suppressing factors may affect tumor behavior [10,11,13]. Particularly the host reaction against the tumor growth, via the systemic inflammation, seems to affect the CRC prognosis [14].
The neutrophil to lymphocyte ratio (NLR) an easy to achieve inexpensive biomarker, expresses the balance between systemic (neutrophils) and cell-mediated (lymphocytes) immunity: the higher its value the more the host immune response is shifted towards a systemic not effective reaction against the tumor. A significant NLR increase is commonly observed in advanced stages of the diseases, elderly patients, non-curative surgery, and postoperative complicated cases. Furthermore, among early (node negative) CRC, a high NLR is significantly associated with a worse DFS and a higher recurrence rate (RR). In conclusion, NLR is useful in identifying a highrisk subgroup that should be considered for a further tailored therapy. Similarly, LMR is independently related to CRC prognosis, since monocytes, by differentiating into macrophages, promote tumor progression and metastases.
Controversial data are reported about the macrophage role in determining a favorable microenvironment for the tumor: it seems that specific subsets of these cells are involved in DFS rather than in OS [13]. Also, platelet count seems to be related to progression of malignant tumors [15] and many reports indicate Systemic Immune- Inflammation Index (SII), the proportion between peripheral platelet, neutrophil and lymphocyte count, as effective in predicting malignant tumors outcome [16-18].
In addition, the evaluation of patient nutritional status [19-21], through albumin and cholesterol serum levels, might offer a further help in achieving a better patient setting, showing its importance in affecting CRC outcome.
These parameters, organized in several score systems [22-24], are currently used in peroperative settings in order to predict the patient outcome. Score systems as CONUT, PNI and SIS are easy to achieve, based on data normally drawn by a simple blood sample, and easy to fit each patient. Nevertheless, nutritional and immunological status are evaluated separately in each of these scores.
NPS [25], the combined contemporary evaluation both of inflammatory and nutritional status, is able to better predict the long-term outcome of patients undergoing surgery for gastrointestinal malignancies [26].
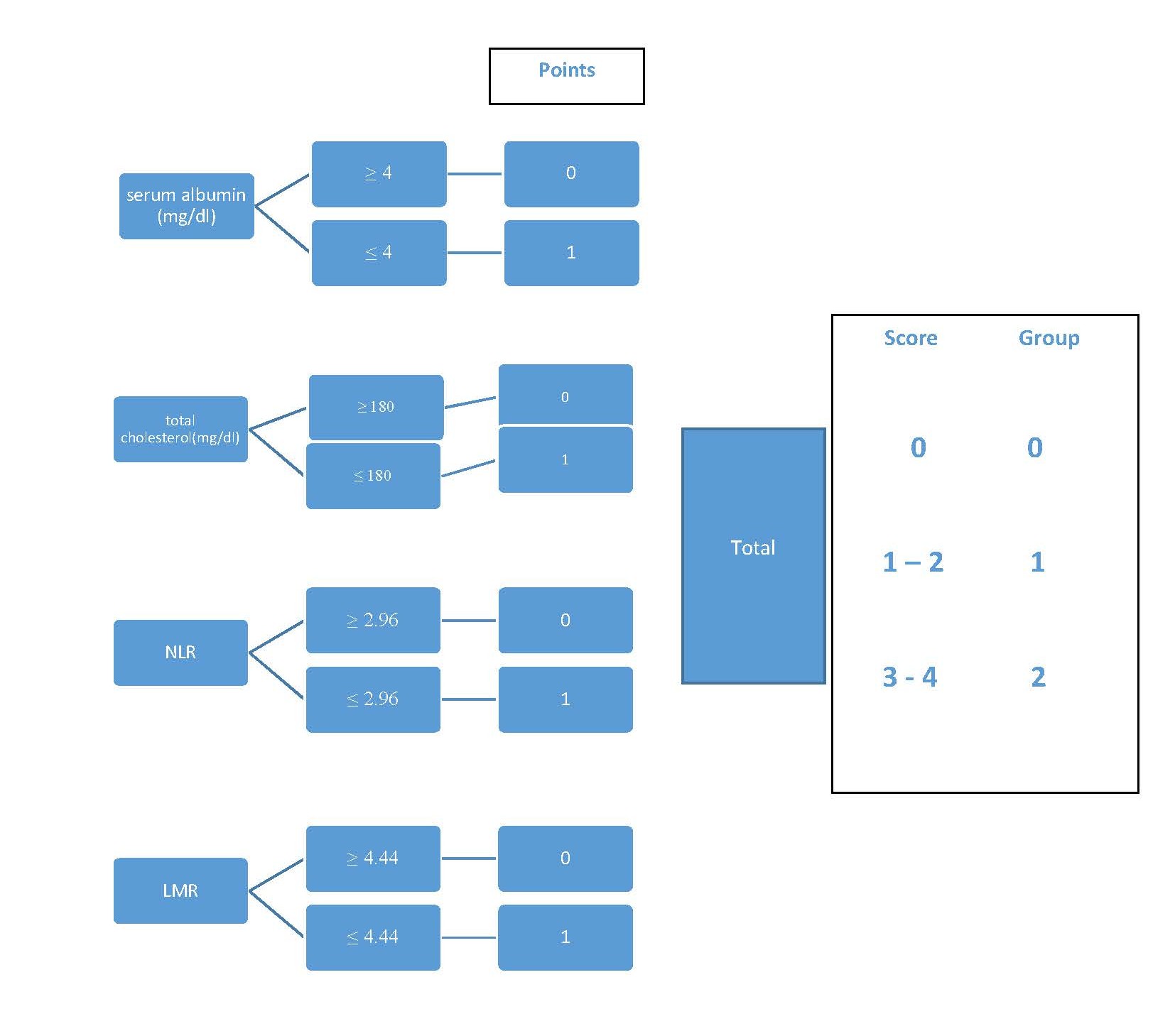
NPS (Figure 1) is a widely applicable prognostic model, using routinely detection values, such as albumin and total cholesterol serum levels, NLR and LMR; in this way, NPS simultaneously investigates nutritional and immunological status. It is noteworthy that hypoalbuminemia, due to a low assumption and a low hepatic production, is a significative malnutrition sign; in neoplastic patients, it may be also linked to increased cytokine levels, that represent the systemic inflammatory state. On the other hand, hypocholesterolemia, often present in CRC patient, by changing cell membrane composition, reduces the ability of the immune system to detect the cancer. Likewise, lymphocytopenia indicates the lack of cell mediated response in fighting cancer and macrophages seem to be involved in tumoral microenvironment by promoting neoangiogenesis and tumor progression.
Figure 1. Calculating NPS
Authors investigated the role of NPS in 562 consecutive CRC patients and found that NPS positively related to other nutritional and inflammatory score systems, such as CONUT and SIS, and inversely related to PNI; moreover, NPS worsened with advanced TNM stage and postoperative complication rate. NPS was significantly related with OS: among patients with all parameters within the normal range (score 0), five-year OS was 88%; in score 1 (1-2 altered values) 68%; in score 2 (3 -4 altered values) 34%, respectively. Also, DFS was strictly related to NPS showing a progressive worsening from score 0 to score 3.
Compared with other immunologic or nutritional score systems, such as SIS [16,17], PNI [19-21] and CONUT [22,23], NPS represents the best model to achieve homogeneity among groups with similar overall survival rate; moreover, in each group the five-year prognostic power is comparable to that of TNM staging system, currently considered the best one [26].
Furthermore, NPS can be useful also in analyzing patients outcome because it is independently correlated with DFS, risk of recurrence and post-operative complications. In addition, while TNM system analyzes only pathological stage, NPS intersects both the tumor stage and the patient’s state of competence in responding to the disease. In this way, the peroperative study of the patient, according to NPS, may help the physician to predict the risks of recurrence and early complications, and gives useful suggestions in terms of therapeutic regimen, as well as in the long-term prognostic judgment.
In conclusion, although NPS, as far as others, needs further validations in larger groups of patients. Nevertheless, it might allow an easy classification of CRC patients in order to better decide about their management and surgical treatment.
References
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015 Mar 1;136(5):E359-86.
3. Sobin LH, Gospodarowicz MK, Wittekind C.International Union against Cancer (UICC). TNM classification of malignant tumors. 7th ed New York NY: Wiley-Liss; 2010.
4. Brenner H, Kloor M. Pox cP. colorectal cancer. Lancet. 2014;383(9927):1490-502.
5. Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gills S, Woods R, et al. Adjuvant chemotherapy use and outcome of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015 Feb 15;121(4):527-34.
6. Coppedè F, Lopomo A, Spisni R, Migliore L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World journal of gastroenterology: WJG. 2014 Jan 28;20(4):943.
7. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759-67.
8. Zhou W, Goodman SN, Galizia G, Lieto E, Ferraraccio F, Pignatelli C, et al. Counting alleles to predict recurrence of early-stage colorectal cancers. The Lancet. 2002 Jan 19;359(9302):219-25.
9. Yiu AJ, Yiu CY. Biomarkers in colorectal cancer. Anticancer research. 2016 Mar 1;36(3):1093-102.
10. Koelzer VH, Dawson H, Andersson E, Karamitopoulou E, Masucci GV, Lugli A, et al. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Translational Research. 2015 Aug 1;166(2):207-17.
11. Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and metaanalysis. International Journal of Cancer. 2014 May 15;134(10):2403-13.
12. Lieto E, Galizia G, Auricchio A, Cardella F, Mabilia A, Basile N, et al. Preoperative neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio are prognostic factors in gastric cancers undergoing surgery. Journal of Gastrointestinal Surgery. 2017 Nov 1;21(11):1764-74.
13. Li J, Li L, Li Y, Long Y, Zhao Q, Ouyang Y, et al. Tumorassociated macrophage infiltration and prognosis in colorectal cancer: systematic review and meta-analysis. International Journal of Colorectal Disease. 2020 Apr 17:1-8.
14. Galizia G, Lieto E, Zamboli A, De Vita F, Castellano P, Romano C, et al. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: A propensity score-matched analysis. Surgery. 2015 Jul 1;158(1):112-20.
15. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nature Reviews Clinical Oncology. 2015 Oct;12(10):584.
16. Yatabe S, Eto K, Haruki K, Shiba H, Kosuge M, Ohkuma M, et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Age (years). 2020 May 8;66:58-74.
17. Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Scientific Reports. 2016 Dec 21;6:39482.
18. Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Annals of Surgery. 2019 Jul 1;270(1):139-46.
19. Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. Journal of Cancer Research and Clinical Oncology. 2014 Sep 1;140(9):1537-49.
20. Fruchtenicht AV, Poziomyck AK, Kabke GB, Loss SH, Antoniazzi JL, Steemburgo T, Moreira LF. Nutritional risk assessment in critically ill cancer patients: systematic review. Revista Brasileira de terapia intensiva. 2015 Jul;27(3):274.
21. Takagi K, Buettner S, Ijzermans JN. Prognostic Significance of the Controlling Nutritional Status (CONUT) Score in Patients with Colorectal Cancer: A Systematic Review and Meta-Analysis. International Journal of Surgery. 2020 Apr 23; 78: 91-96.
22. Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016 Dec;16(1):722.
23. Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, Sakurai K. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PloS one. 2015;10(7): e0132488.
24. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treatment Reviews. 2013 Aug 1;39(5):534-40.
25. Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Diseases of the Colon & Rectum. 2017 Dec 1;60(12):1273-84.
26. Galizia G, Auricchio A, de Vita F, Cardella F, Mabilia A, Basile N, Orditura M, Lieto E. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer. Validation of the Naples prognostic score. Annali italiani di chirurgia. 2019;90:404.