Mini Review
The hepatitis C virus (HCV) genome was isolated during the late 1980s using molecular cloning techniques. It is recognized as the cause of most cases of percutaneously transmitted non-A, non-B hepatitis. It is estimated, that up to 200 million people worldwide are infected with the hepatitis C virus (HCV), more than 3% of the world population [1]. The predominant risk factors for HCV are intravenous drug use, tattoos, exposure to blood products, occupational risk and ethnicity.
After identification of HCV, first treatment approaches included interferon-α (IFN) in different doses and with different therapy durations. First studies reported therapy duration from 12 weeks to 48 weeks with doses from 1 MU 3 times per week to 3 MU 3 times per week. Therapy response was defined as normalization of the transaminases under therapy and after 24 weeks.
With the introduction of the polymerase chain reaction (PCR) the virological response with eradication of HCV became the most important aim of treatment. This let to compare the different studies with an objective parameter. On the other hand, different genotypes could be identified. The genotype and viral load became with the development of different treatment regimes of interest.
Sustained virological response (SVR) rates increased in parallel with the development of more effective treatment regimes.
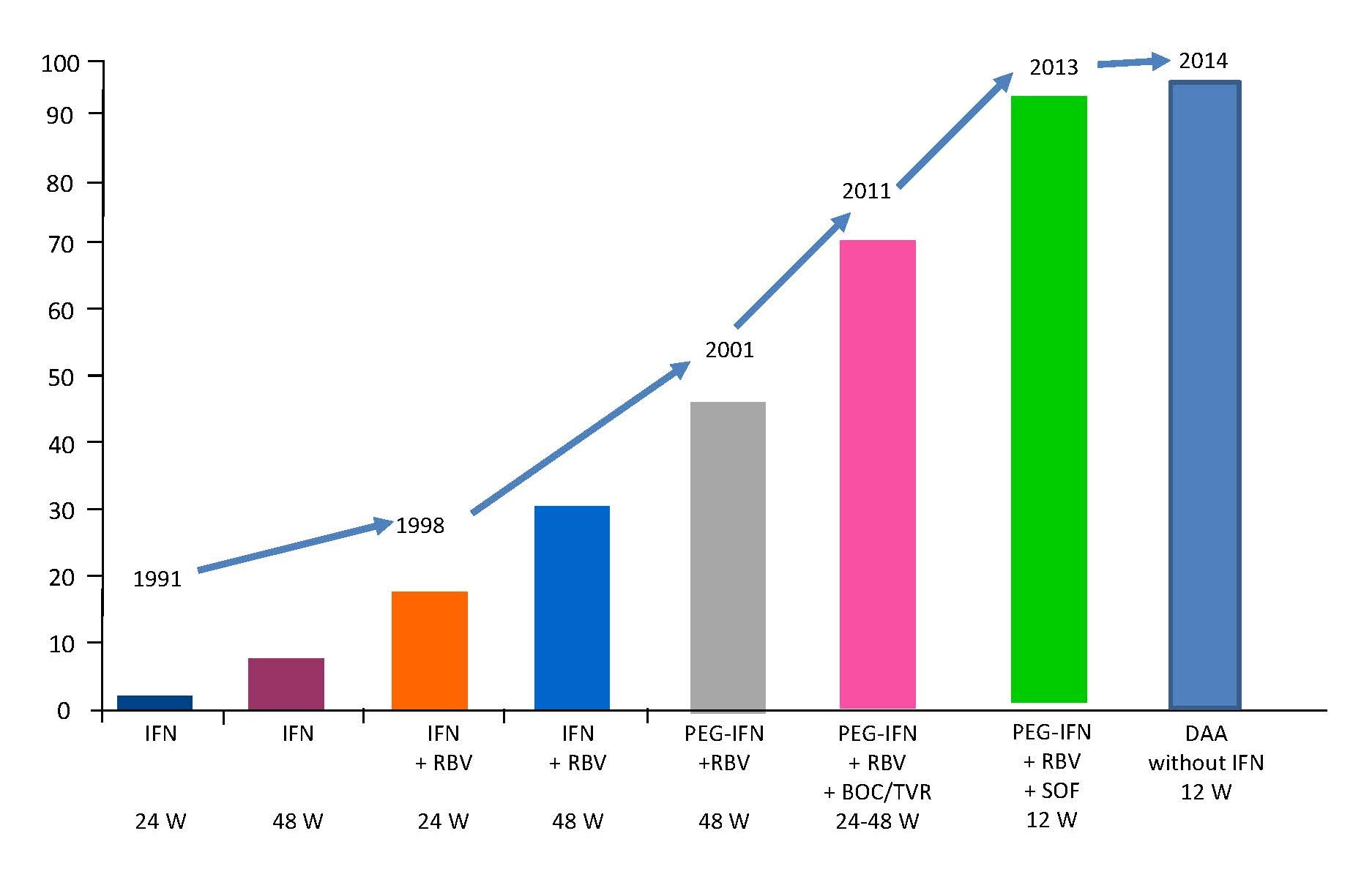
An end of treatment response of 29% could be achieved with a monotherapy of 3 MU 3 per week with therapy duration of 24 weeks over all genotypes. A SVR could be detected only in 6%. With an extension of the therapy duration to 48 weeks, the SVR rate increased to 13% [2]. SVR rates were significantly lower in patients infected with HCV genotype 1b treated with IFN (2% vs 16% in patients treated for 24 weeks; 7% vs 29% in patients treated for 48 weeks) [2].
The combination therapy of IFN (3 MU 3 times per week) and ribavirin (1 000 to 1 200 mg daily) for 24 to 48 weeks yielded SVR rates between 36% and 47% [2,3]. The influence on the treatment response of the HCV genotype could be observed.
The introduction of pegylated-IFN (PEG-IFN) showed in clinical trials using PEG-IFN and ribavirin induced SVR rates of 54% to 56%, which are significantly higher than SVR rates induced with standard IFN in combination with ribavirin [4,5]. The IFN based therapy regimes were associated with side effects like fever, headache, bone pain, hair loss, depression, leukopenia, thrombopenia. These side effects let to abruption of the therapy in up to 20% [2].
The next step in the therapy of chronic HCV infection was the introduction of the direct acting antivirals (DAA). Boceprevir is a slow binding reversible HCV-NS3 protease inhibitor. In combination with PEG-IFN and ribavirin SVR rates of 67% could be reached in treatment of naïve patients infected with HCV genotype 1 [6]. The second DAA was telaprevir, a linear peptidomimetic HCV NS3/4A serine protease inhibitor. SVR rates up to 75% could be observed in patients treated with telaprevir in combination wit PEG-IFN and ribavirin [7]. Anemia, pruritis, and diarrhea were more common in patients receiving DAA compared to patients without DAA. The third DAA was sofosbuvir, a nucleotide analogue HCV NS5B polymerase inhibitor. SVR rates up to 97% could be reached in combination with PEG-IFN and ribavirin (Figure 1) [8].
Figure 1. SVR rates in therapy naïve patients infected with HCV genotype 1 during the last 20 years. IFN: Interferon-α; RBV: Ribavirin; W: Weeks; BOC: Boceprevir; TVR: Telaprivir; SOF: Sofosbuvir; DAA: Direct Acting Antivirals.
Based on the fact that treatment regimes including interferon let to abruption of the therapy caused by side effects, the aim was to establish interferon free treatment regimes with comparable SVR rates.
Different interferon free regimes with SVR rates up to 100% are available with a significant lower rate of side effects. Most treatment regimes are available for all HCV genotypes with a duration of 8 or 12 weeks.
In conclusion, treatment of chronic HCV infection developed very fast from the first treatment approaches and a SRV rate up to 100%. While the effort to develop effective treatment regimes was in foreground in the last two decades, the focus has to change to an effective screening system now if we want to eradicate the virus. If all infected patients would undergo therapy, a complete worldwide eradication of HCV infection seems possible.
References
2. McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. The New England Journal of Medicine 1998;339:1485-1492.
3. Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomized trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 1998;352:1426-1432.
4. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. The New England Journal of Medicine 2002;347:975-982.
5. Lindsay KL, Trepo C, Heintges T, Mitchell L, Shiffman MS, Stuart C, Gordon SC, et al. A randomized, doubleblind trial comparing Peginterferon Alfa-2b to Interferon Alfa-2b as initial treatment for chronic hepatitis C.Hepatology 2001;34:395-403.
6. Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. The New England Journal of Medicine 2011; 364:1195-206.
7. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for Previously Untreated Chronic Hepatitis C Virus Infection. The New England Journal of Medicine 2011; 364:2405-2416.
8. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres D, Hassanein T, Gordon SC, et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. The New England Journal of Medicine 2013; 368:1878-1887.