Abstract
Objective: To describe the evolution of Utero-Vesical Fistula (UVF) of one patient who received conservative treatment using intravenous (IV) infusion of 120x106 Adipose tissue Mesenchymal Stem Cells (ADMSCs). Clinical case report: A 36-year-old female presented with postcesarean hematuria 3 hours after an emergency c-section was performed consequently to placental abruption during labor. The primary measure was to use a transurethral Silastic 18F Foley catheter to prevent bladder expansion. The catheter remained for 21 days. Evaluation through Computerized Tomography Cystography (Cysto-CT) revealed failure to close the fistula. The decision to inject ADMSCs as an IV single dose was done. Three weeks after this intervention no blood was seen in the urine drainage bag. Complete resolution of UVF was confirmed with a control CT urogram. Result: Total closure of UVF. Conclusions: The IV infusion of ADMSCs proved to be a plausible and effective conservative treatment of UVF.
Introduction
An abnormal communication between uterus and urinary bladder, related with surgical trauma may result in Utero-Vesical Fistula (UVF) formation. The primary symptom of patients with these fistulas is usually urinary incontinence. However, patients may present with cyclic hematuria and amenorrhea. They represent 0.5 to 10% of all urogenital fistulae. Currently, its prevalence is higher due to the increase in cesarean sections [1,2]. UVF can have several etiologies: 1) Obstetric causes: prolonged labor, cesarean section/hysterectomy, instrumental maneuver during childbirth, traditional practices, embryotomy in abortions and symphysiotomy, 2) non-obstetric causes: trauma (intercourse, sexual violence, accident and sexual mutilation); infections (tuberculous granulomatous and HIV); as well as: pelvic surgery, radiotherapy and tumors [1,3].
The main sign of UVF is urine loss and can be confirmed through vaginal hysterosonography with cervical instillation of methylene blue. In recent years, cystography with computerized axial tomography (Cysto-CT) is also used [2,4].
Currently, the treatment of UVF can be done conservatively. After a cesarean section or other pelvic intervention, prolonged bladder catheterization is used for 4 to 6 weeks in order to allow the injured area to heal [3]. If the fistula is already formed, tube therapy offers little chance of spontaneous closure and instead increases inflammation and the risk of infection [3]. Despite the fact that in many cases the fistulas are already formed, doctors rather choose conservative treatment over surgical treatment [3]. The main reasons are the complications that may arise as a consequence of surgical repair (infections or urinary incontinence) and the complexity of the recovery process. As a consequence of this, less invasive alternatives are being sought in order to improve the success rates of UVF repair. Mesenchymal Stem Cells (MSCs) are being proposed in a diversity of studies as an alternative treatment [5,6]. Due to their high proliferation rate, regeneration capacity and their increased capability of cell differentiation into different cell types through asymmetric divisions [7,8]. Adipose tissue Mesenchymal Stem Cells (ADMSCs) have been considered, in recent years, as an innovative option to be used in regenerative medicine. ADMSCs are obtained from embryonic and extra-embryonic tissue, as well as from adult organs such as: bone marrow, peripheral blood, umbilical cord, and adipose tissue, among others. In addition, they can be autologous or donor (allogeneic). Its application —in clinical research— has been carried out as cell therapy for degenerative diseases such as Alzheimer's, Amyotrophic Lateral Sclerosis, Huntington's disease, Parkinson's disease, brain and myocardial infarctions, spinal cord damage, immune disorders, osteoarthritis and restoration of ovarian function. It has been also shown animal studies that MSCs increase sperm motility and the number of spermatogonia. In addition, they release a wide selection of cytokines, chemokines, and growth factors, with antiapoptotic, anti-inflammatory, proangiogenic, and immunomodulatory characteristics, making them highly attractive for a variety of medical applications [9-15].
Therefore, the objective of this study was to describe the case report of a patient who’s UVF was repaired with conservative treatment using ADMSCs IV injection, as an adjuvant.
Case Presentation
A 36-year-old woman with a history of cesarean section in 2003 (38 weeks pregnant) due to entangled umbilical cord and cesarean section in 2017 (40 weeks pregnant) due to placental abruption. She was admitted with active labor in the Labor, Delivery and Recovery (LPR) guard of the Bité Médica Hospital at 40.2 weeks pregnancy. Cervical dilation was confirmed to be 5 cm by her medical practitioner, Fetal Heart Rate was 140 bpm. Whilst spontaneous rupture of membranes during active uterine contractions presented, blood-stained amniotic fluid was seen and placental abruption was diagnosed. Fetal tachycardia was also observed. It was decided to perform an emergency cesarean section. Live and healthy newborn (3.975 grams weight, 53 cm length and APGAR score of 9/10).
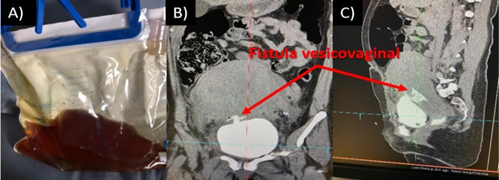
Three hours after cesarean delivery, macroscopic hematuria was observed. Permanent transurethral catheterization with a 18F Silastic Foley catheter was indicated (Figure 1). Hematuric urine was still seen in spite of lessening stress on the bladder. Evaluation through Cysto-CT was performed [Emotion16(2010) CT 78883, Somaris/5 syngo CT 2014A]. A defect was detected in the fundus of urine bladder, confirming abnormal communication between uterus and bladder, of approximately 7.5 mm (Figure 2). Conservative treatment was chosen.
Figure 1. A) Foley catheter in which hematuria could be observed, B and C) Cysto- CT showed the passage of contrast from the bladder to the uterus, through a 7.5 mm fistula.
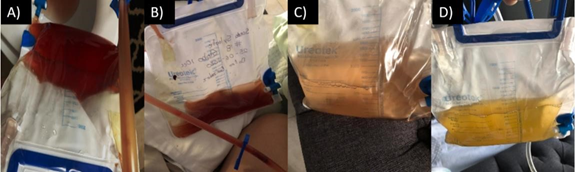
Figure 2. A lessening of hematuria concentration can be observed: A) One day after, B) One week after, C) Two weeks after and D) Three weeks after application of ADMSCs.
Three weeks after starting treatment, hematuria was still observed and no decrease in UVF was observed. Therefore, intravenous injection of Adipose Tissue Stem Cells (ADMSCs) was proposed to the patient and her partner. The positive results that we have obtained using ADMSCs as an adjuvant for the repair of placental hematomas was explained to them, and thus, the plausibility of this measure. After the patient had accepted the treatment, the application of 120 million ADMSCs (homologous) was injected as an intravenous infusion.
After the application of ADMSCs, outpatient daily follow up using photographs of urine drainage bag was carried out for three weeks. Progressive clearing of urine color was taken as evidence of UVF narrowing and complete repair of the fistula was confirmed with Cysto CT control image (Figure 2 and 3).
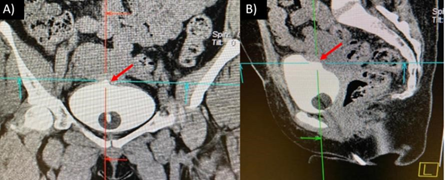
Figure 3. Cysto CT image of a bump in the fundus of bladder was observed in the area of uterovesical fistula, demonstrating closure and repair of the fistulous tract (red arrows).
Discussion
Conservative treatment of UVF has been followed up for years and various methods have been detailed that allow a fistula to be spontaneously repaired. Such is the case of continuous bladder drainage, catheterization with fulguration of the fistula tract, glue injection, platelet-rich plasma, and the administration of anticholinergics [16]. Most conservative methods have a reported success rate ranging from 3% to 100%. In addition, conservative methods should be used in carefully selected patients, since clinical scenarios vary. Therefore, the choice of a conservative method depends on the physician's experience, always bearing in mind that surgical intervention will be required if conservative treatment would fail [5]. Conservative treatment is even used as a strategy to give fistula closure an opportunity and as a measure to deflate the tissues prior to intervention, since in the immediate postsurgical or postpartum period, the structures are modified, frequently found friable and edematous. All of these implies the possibility of fistula recurrence if surgery is performed in this acute phase [17].
Regarding ADMSCs as an adjuvant treatment for UVF, there are studies in laboratory animals (such as the one carried out by Mardiyan et al., [5]), where they observed in NZW rabbits that the application of mesenchymal stem cells, obtained from amniotic membrane directly into the fistula, reduced TNF-α concentrations and inflammatory cell infiltration, enhancing the reduction of UVF. Similarly, in this case report, the application of 120 million ADMSCs intravenously was able to help reduce and fully repair a 7.5 mm UVF over the course of 3 weeks. In the case of humans [18], in a clinical trial that included 12 patients with Crohn's disease, they applied ADMSCs in a bioabsorbable matrix for perianal fistula repair, observing clinical repair in 83% of patient cases. In the investigation performed by Aho et al. [19], a graft seeded with autologous ADMSCs was used to repair recurrent bronchopleural fistula (BPF) and showed that, follow-up at 3, 6, and 18 months after application of Ad-MSCs did not present recurrence of BPF. The review by Borycka et al. [20], concluded that the application of directly injected ADMSCs could be a promising tool for the management of perianal fistulas resistant to treatment.
Currently, there are few clinical trials that evaluate the efficacy of ADMSCs as an adjuvant treatment for UVF; in addition, the studies that can be found have only been carried out in laboratory animals. In a systematic analysis in the Cochrane database, no related articles were found. ADMSCs may be an innovative, safer, therapeutic field of study for the treatment of fistulas, although it needs more clinical studies to fully understand the nature and therapeutic scope of ADMSCs.
Conclusions
The intravenous injection of ADMSCs as an adjuvant in conservative treatment of UVF helped to its complete closure and repair.
Due to the aforementioned, we propose to carry out prospective, multicenter and randomized studies, using conservative management with the intravenous application of autologous MSCs as the first therapeutic option and perform detailed follow up during the next 3 weeks in addition to lifestyle medicine measures to reduce inflammation and promote the repairing activity of mesenchymal stem cells.
References
2. Bettez M, Breault G, Carr L. Early versus delayed repair of vesicouterine fistula. Canadian Urological Association Journal. 2011 Aug;5(4):E52-5.
3. Jilaveanu A, Socea B, Bohiltea R, Stiru O, Al Aloul A, Ursut B, et al. Uterovesical fistulas as obstetric complications: Diagnosis, management and prognosis. Experimental and Therapeutic Medicine. 2023 Mar 1;25(3):1-4.
4. Machado Junior RA, Machado Junior LC, Lourenço LL. Vesicouterine fistula (Youssef syndrome): case report and literature review. Revista Brasileira de Ginecologia e Obstetrícia. 2018;40:563-9.
5. Kurniawati EM, Rahmawati NA. The Role of Regenerative Medicine in Wound Healing in Cases of Vesicovaginal Fistulae. Journal of Drug Delivery and Therapeutics. 2021 Aug 15;11(4-S):5-8.
6. Buscail E, Le Cosquer G, Gross F, Lebrin M, Bugarel L, Deraison C, et al. Adipose-derived stem cells in the treatment of perianal fistulas in Crohn's disease: rationale, clinical results and perspectives. International Journal of Molecular Sciences. 2021 Sep 15;22(18):9967.
7. Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfusion Medicine and Hemotherapy. 2016;43(4):268-74.
8. Schweizer R, Tsuji W, Gorantla VS, Marra KG, Rubin JP, Plock JA. The role of adipose-derived stem cells in breast cancer progression and metastasis. Stem Cells International. 2015 Oct;6(3):312-21.
9. Edessy M, Hosni HN, Shady Y, Waf Y, Bakr S, Kamel M. Autologous stem cells therapy, the first baby of idiopathic premature ovarian failure. Acta Medica International. 2016 Jan 1;3(1):19-23.
10. Elfayomy AK, Almasry SM, El-Tarhouny SA, Eldomiaty MA. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: possible direct and indirect effects. Tissue and Cell. 2016 Aug 1;48(4):370-82.
11. Ghadami M, El-Demerdash E, Zhang D, Salama SA, Binhazim AA, Archibong AE, et al. Bone marrow transplantation restores follicular maturation and steroid hormones production in a mouse model for primary ovarian failure. PLoS One. 2012 Mar 7;7(3):e32462.
12. Shammaa R, El-Hakim A, Abusarah J, Rafei M. Mesenchymal Stem Cells Beyond Regenerative Medicine. Frontiers in Cell and Developmental Biology. 2020 Feb 18;8:72.
13. Afflerbach AK, Kiri MD, Detinis T, Maoz BM. Mesenchymal stem cells as a promising cell source for integration in novel in vitro models. Biomolecules. 2020 Sep 10;10(9):1306.
14. Hsiao CH, Ji AT, Chang CC, Chien MH, Lee LM, Ho JH. Mesenchymal stem cells restore the sperm motility from testicular torsion-detorsion injury by regulation of glucose metabolism in sperm. Stem Cell Research & Therapy. 2019 Dec;10:270.
15. Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iranian Journal of Reproductive Medicine. 2013 Jul;11(7):537-44.
16. Rajaian S, Pragatheeswarane M, Panda A. Vesicovaginal fistula: review and recent trends. Indian journal of urology: IJU: Journal of the Urological Society of India. 2019 Oct;35(4):250-8.
17. Kurniawati EM, Hastomo T, Santoso B, Rantam FA. Effect of amniotic membrane-derived mesenchymal stem cells on TNF-α expression and inflammatory cells infiltration during vesicovaginal fistule repair healing process. Indian Journal of Forensic Medicine & Toxicology. 2020;14(2):1145-9.
18. Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn's disease. Gastroenterology. 2017 Jul 1;153(1):59-62.
19. Aho JM, Dietz AB, Radel DJ, Butler GW, Thomas M, Nelson TJ, et al. Closure of a recurrent bronchopleural fistula using a matrix seeded with patient-derived mesenchymal stem cells. Stem Cells Translational Medicine. 2016 Oct;5(10):1375-9.
20. Borycka-Kiciak K, Pietrzak A, Kielar M, Tarnowski W. Mesenchymal stem cells for the treatment of complex perianal fistulas in patients with Crohn disease. Polski Przeglad Chirurgiczny. 2019 Jun 10;92(1):38-47.