Abstract
MicroRNAs are small, noncoding pieces of nucleic acid with the potential to control mRNA translation. These sequences participate in the regulation of cell dynamic growth and differentiation. In this study, the expression of miR-15a and miR-124 was monitored in adipose-derived tissue stem cells committed to pancreatic β cells in vitro over 28 days. In the current experiment, adipose-derived mesenchymal stem cells were incubated in an induction medium to accelerate differentiation toward the endocrine pancreatic lineage for 28 days with a three-stage protocol. To confirm the efficient trans-differentiation of stem cells into pancreatic β cells, we performed a Dithizone staining, a zinc chelating agent, and pancreas-specific hormones (insulin and C peptide) examination via electrochemiluminescence. Real-time PCR analysis was done to assess the expression of miR-15a and miR-124. Dithizone staining confirmed a successful orientation of adipose-derived mesenchymal stem cells into pancreatic β cells indicating a red to strong brown (crimson-red) appearance compared to negative control stem cells, indicating insulin positivity in differentiating cells. These effects were prominent over time and reached a maximum level on day 28. Concurrently, the expression of both miR-15a and miR-124 was induced and reached a peak expression level at the end stage of the experiment compared to the undifferentiated AD-MSCs (p<0.05).
Conclusion: The dynamic of distinct miRNAs, in particular, miR-15a and miR-124 was induced during pancreatic β cells derivation of adipose-derived mesenchymal stem cells.
Keywords
Adipose-derived mesenchymal stem cells, Differentiation, Insulin-producing cells, microRNAs, Stem cells
Introduction
Diabetes mellitus (DM) is a hyperglycemic condition originating from cellular insulin resistance and/or defects in the dynamics of pancreatic β cells. According to the statistics released by the American Diabetes Association and the WHO, type 1 DM (T1DM) is classified into two main categories such as autoimmune dysfunction and idiopathic forms [1]. In addition to pharmacological and conventional approaches, transplantation of cells from pancreatic islets seems to be an appropriate strategy for the alleviation of T1DM-related complications. However, this modality requires lifelong immunosuppression and coincides with remarkable morbidity and negligible mortality [2].
In recent decades, the use of stem/progenitor cells has increased to restore pancreatic function and elevation of insulin content in the systemic blood system. In line with this progress, regenerative medicine exploits developmental and molecular biology, and stem cell biology as a critical point to address the fate of stem cells after transplantation [3]. Considering the remarkable ability of stem cells to proliferate on a large scale and trans-differentiate into various cell lineages, these cells are at the center of attention to generate β-cells in diabetic subjects. Thus, β-cells differentiation of stem cells would provide promising unlimited biological sources for reducing diabetes-related complications [4].
Based on a great body of documents, various stem cell sources have been examined to give rise to insulin-producing cells (IPCs) from stem cells [5], induced pluripotent stem cells (iPSCs) [6], and mesenchymal stem cells (MSCs) [7]. Considering the lack of ethical issues related to the application of MSCs, these cells are the proper choice to restore pancreatic function compared to embryonic stem cells (ESCs) [8]. MSCs can be isolated from different human tissues such as bone marrow [9], adipose tissue [10], and umbilical cord [11]. MSCs have the potential to be easily expanded with potent bioactivity to transform into various cell lines [12]. Compared to other sources, adipose-derived stem cells (AD-MSCs) have the highest proliferation capacity and even retain multipotentiality after consequent passages [13].
MicroRNAs (miRNAs) are a class of small single-stranded and noncoding RNAs with 17 to 25 nucleotides [14]. miRNAs play a fundamental role in the regulation of stem cell differentiation and self-renewal through signaling pathways [15,16] and the suppression of mRNA translation [17].
There is a limited number of studies that have confirmed the regulatory role of miRNAs during the differentiation of MSCs to different cell lineages, especially IPCs. For instance, it has been shown that miR-124 can induce neural differentiation of adipose-derived mesenchymal stem cells (A-MSCs) via inhibiting specificity protein 1 expression [18]. Also, Zhang and his co-workers have illustrated that miR-124-5p can positively regulate the differentiation of ESCs to IPCs through suppression of Nkx6.1 which is pivotal for pancreatic [19].
Also, our previous work has shown that the expression of miR-101a and 107 slightly upregulated during IPCs differentiation of AD-MSCs [20].
According to recent studies, the regulatory role of miR-15a and miR-124 was evident in insulin biosynthesis and β-cell bioactivity [21,22]. Under high glucose conditions, miR-15a along with miR-424, miR-497, and miR-185 overexpressed and directly triggered insulin secretion [23].
Here, we investigate the possible expression of miRNAs (miR-15a and miR-124) during AD-MSCs differentiation into IPCs. To our knowledge, not enough data indicates the expression of miRNAs during the differentiation of MSCs-to-IPCs. In this study, we found a change in the transcription of miR?124 and miR-15a during the differentiation of AD-MSCs to IPCs.
Methods and Materials
Cell culture and expansion
The cryopreserved AD-MSCs cell line (5 ´ 105 cells) was obtained from Royan Institute (Tehran, Iran). After arriving, cells were thawed using a DMEM complete medium containing 10% FBS and seed in a T25 cm2 culture flask. Cells were expanded in low-glucose content Dulbecco’s modified Eagle’s medium (DMEM/LG; Gibco) enriched with 15% fetal bovine serum (FBS, Invitrogen, Carlsbad, USA) and 1% penicillin-streptomycin (Gibco) in a humidified atmosphere at 37°C incubator with 5% CO2. Cells at 70-80% confluence were trypsinized using 0.25% trypsin-EDTA (Gibco) and enzyme activity was neutralized by FBS. AD-MSCs at passages 3-6 were subjected to the subsequent analyses.
Cell differentiation into insulin-producing cells
For this propose, the 3.5´105 AD-MSCs were cultured in 6 well plates and after reaching 80% confluency, cells were pre-treated with three different media; (I) cells were cultivated in DMEM/LG medium containing 5% FBS, 0.5 mM β-mercaptoethanol (Cat no: M6250; Sigma-Aldrich, USA) and 10 mM nicotinamide (Cat no: N0636 Sigma-Aldrich, USA) for 2 days; (II) cells were induced in high-glucose DMEM (DMEM/HG; Gibco, UK) enriched with 2.5% FBS, 0.5 mM β-mercaptoethanol and 10 mM nicotinamide for next 10 days, and eventually differentiating cells were kept in DMEM/HG medium with 1.5% FBS, 0.5 mM β-mercaptoethanol and 10 mM nicotinamide and 10 nM exendin-4 (Cat no: E7144 Sigma-Aldrich, USA) until day 28. The differentiating media was replenished every 2-3 days.
Real time-PCR analysis
In the current experiment, the transcription of miR?124 and miR-15a was monitored during IPCs differentiation of AD-MSCs. To this end, an initial number of 3.5 × 105 AD-MSCs were plated in each well of 6-well plates (n=3) and allowed to reach 70-80% confluency. Thereafter, 3 ml of induction medium was poured into each well and maintained over 28 days. Following the completion of the induction period, total RNA and miRNA contents were isolated from the AD-MSCs on days 0, 7, 14, 21, and 28 by using TRIzol reagent (Cat no: T9424; Sigma-Aldrich, Germany). The quality of extracted RNAs was assessed by using Nanodrop® (Thermo Scientific). cDNA synthesis was conducted with the cDNA synthesis kit (Exiqon, Vedbaek, Denmark) using 50 ng RNA. The miRNAs primers are designed as follows: miR-15a, forward: 5′?CAGTAGCAGCACATAATGGT?3′ and reverse: 5′?GGTCCAGTTTTTTTTTTTTTTTCACA?3′, and for miR-124, and for miR-124, forward: 5′-AGCATCTTACCGGACAGT-3′ and reverse 5′-CCAGTTTTTTTTTTTTTTTCCAGCA-3′ . Real-time PCR analysis was carried out using BioMolecular Systems (Model: mic). Gene expression data were normalized with U6 housekeeping RNA and analyzed with the 2-??Ct method. Analysis was performed in triplicate (n=3).
Dithizone staining
To confirm the successful differentiation of AD-MSCs to IPCs, we performed Dithizone (DTZ) staining. At the respective time points, DTZ powder (Cat no: D5130; Sigma Aldrich, USA) was liquefied in dimethyl sulfoxide (DMSO) and then sterilized by using 0.2-μm pore size micro-filters. After that, DTZ solution (1% w/v) was added to each well containing differentiated and undifferentiated cells and incubated for 30 minutes at 37°C. The cells were then precisely washed three times with phosphate buffer saline (PBS). Crimson red-stained IPCs were explored under an inverted phase contrast microscope.
Measuring the level of insulin and peptide C by electrochemiluminescence assay
To confirm the functional behavior of IPCs originating from AD-MSCs, we measured the level of insulin and peptide C in the supernatant (n=3). After the completion of the differentiation protocol, supernatants were collected, and the level of insulin and peptide C were measured by Siemens 06602443 Immulite automated bioanalyzer and Roche insulin and C peptide examination kit according to the manufactory’s instructions.
Statistical analysis
Data are presented in mean ± SD. Statistical analyses were performed by using One-Way ANOVA. P-values<0.05 were considered to be statistically significant.
Results
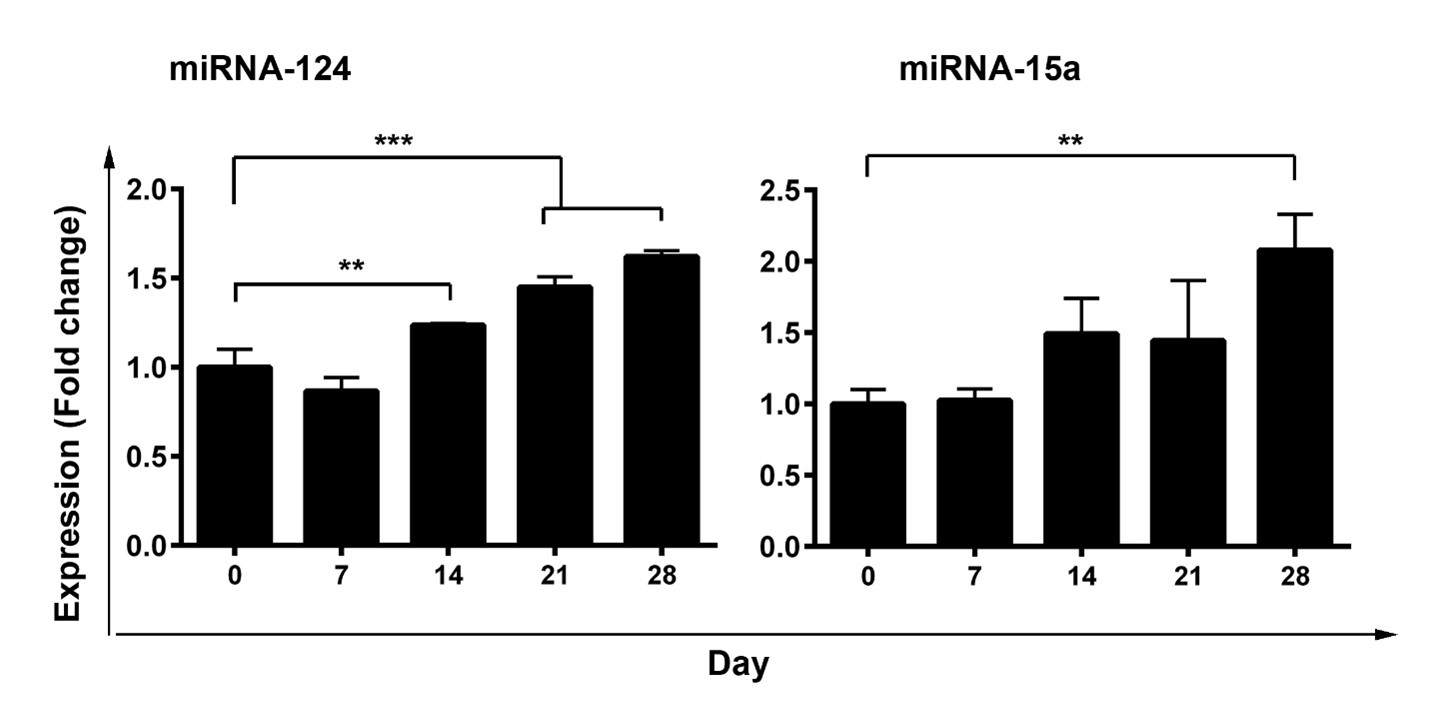
miR-124 and miR-15a were up-regulated in AD-MSCs differentiating into IPCs
We used a three-step protocol to direct pancreatic differentiation from the AD-MSCs cell line. Based on real-time PCR analysis, we found that the expression of both miR-124 and miR-15a was increased in AD-MSCs differentiating into IPCs over 28 days compared to the control group (Figure 1). We found that the first week of incubation with the differentiation medium did not change the transcription level of miR-124 and miR-15a. However, the miR-124 expression significantly increased on day 14 (p<0.01), day 21(p<0.001), and day 28 after differentiation in comparison with the control group (day 0) (p<0.001). On the other hand, miR-15a expression reached a maximum level 28 days after treatment (p<0.01) (Figure 1). These data demonstrated an upward trend in the level of miR-124 and miR-15a during the differentiation of AD-MSCs toward IPCs.
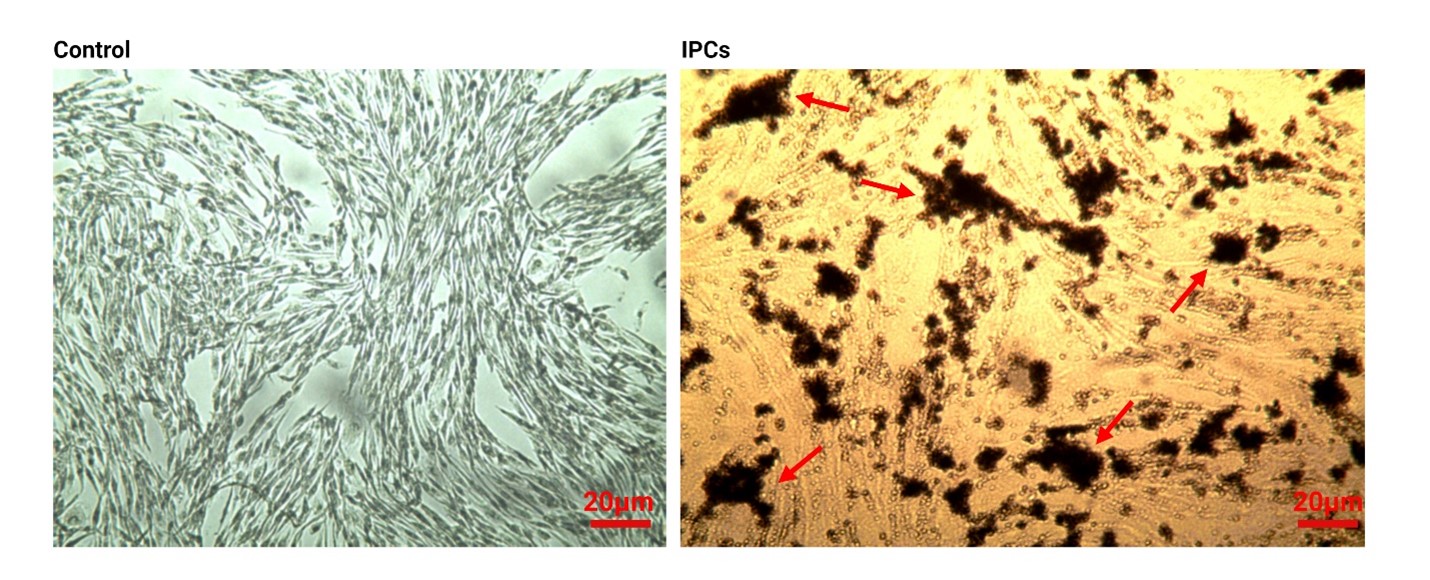
DTZ staining confirmed AD-MSC-IPCs differentiation
Pancreatic islets located at the endocrine part contain a large amount of zinc components in comparison with other tissues. DTZ is used as a zinc-binding substance to discover the existence of these compounds inside IPCs, showing the level of trans-differentiation (Figure 2). Bright-field microscopic imaging showed the crimson-red stained clusters in differentiating AD-MSCs after 28 days compared to the non-treated control AD-MSCs (Figure 2). These data showed that 28-day incubation of AD-MSCs with differentiation medium caused accumulation of zinc components inside IPCs, showing the successful phenotype acquisition.
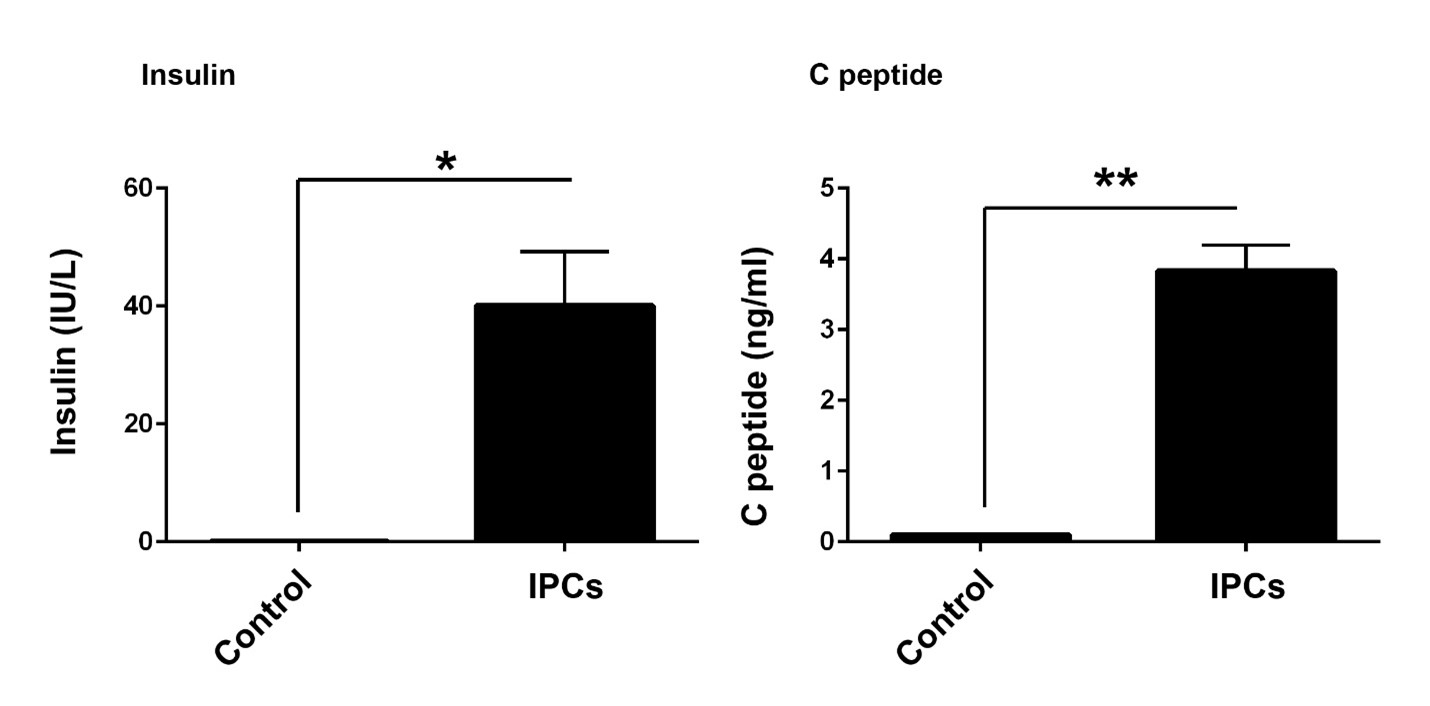
IPCs had the potency to secret insulin and C peptide
The ability of IPCs to secrete C peptide and insulin could show the functional behavior of target cells after induction of phenotype acquisition. Results from the electrochemiluminescence panel showed an increase of insulin (p<0.05) and C peptide (p<0.01) in the supernatant of differentiating cells compared to the control group (Figure 3). These results showed the functional behavior of differentiated cells from AD-MSCs sources.
Discussion
Stem cells are known as natural cell resources for the regeneration of injured tissues. Compared to the current approaches, stem cell therapy paves the way with promising results to alleviate diabetes-related complications [24]. It was found that these cells have the potential to produce IPCs after administration to the pancreatic tissue [3]. According to a great body of documents, various stem cell types were used to produce IPCs such as ESCs, and MSCs [13,25]. Even, there was a report on the potency of induced pluripotent stem cells (IPS) in orientation toward IPCs [26]. In this regard, MSCs have been introduced as a suitable cell resource for application in different pathologies [27]. To acquire novel cell phenotype, different intracellular signaling pathways, effectors, and genetic elements are involved in favor of trans-differentiation.
However, there is no consolidated agreement about regulatory mechanisms that dictate stem cells’ trans-differentiation to various cell types, especially IPCs [28-30]. Considering the regulatory effect of miRNAs in cell bioactivity, the analysis of miRNA profile could shed light on giving optimum stem cell differentiation rate [31]. In support of this statement, experiments showed that the inhibition of Dicer 1, an RNAase endonuclease that synthesizes miRNAs, contributes to the modification of islet structure, decrease of β-cells, and insulin secretion in response to glucose [32]. The selection of appropriate miRNAs could be helpful in the detection, dynamics, and differentiation capacity of stem cells to IPCs. Previously, Wei and his colleagues explored expression patterns of miR-375, -7, -146a, and -34a during the ESCs differentiation into IPCs. Based on their results, a diverse expression pattern was found, showing the distinct effect of each miRNA in the dynamic of stem cells toward IPCs [5].
To our knowledge, there are few reports related to the critical effects of miR-124 and miRNA-15a dynamics during AD-MSCs to IPCs orientation. Interestingly, there is conflict in the specific role of miR-124 during the insulin secretion process, for instance, while Hashimoto et al. have confirmed that miR-124a negatively regulates glucose-dependent insulin secretion, Zhang and his co-workers reported upregulation and positive regulation effect of miR-124 during IPCs differentiation of AD-MSCs [19,33].In line with this statement, we aimed to explore the expression of both miRNAs over 28 days. Our data showed that the expression of both miR-124 and miRNA-15a increased during IPCs differentiation of AD-MSCs which coincided with zinc elements and cell potency to deliver insulin and C peptide. Consistent with our results, Sebastiani et al. monitored the expression of eighteen miRNAs, especially miR-124a from the miRNA family, and noted the induction of this miRNA inside IPSCs committed to IPCs. They also revealed that the transcription of other miRNAs such as miR-135a, miR-138, miR-149, and miR-375 was induced while the miR-31, miR-127, miR-143, miR-373 profoundly suppressed [34]. Empirical studies found a close relationship between miR-15a and miR-124 with insulin biogenesis inside β cells. In a recent study conducted by Sun and co-workers, it was found that miR-15a could inhibit uncoupling protein-2 belonging to mitochondrial inner membrane carriers that prohibit insulin secretion [21]. The increase of miR-15a could decrease the inhibitory effect of uncoupling protein-2 on insulin secretion. On the other hand, the induction of miR-124 takes part in the development of pancreatic β-islets via engaging forkhead box protein A2 [22]. Consistent with our data, both miR-124 and miRNA-15a were increased during IPCs generation. The analysis of these miRNAs possibly could be monitored to address the successful differentiation toward IPCs.
We are aware of some limitations regarding the current experiment. We suggest ongoing experiments be conducted to monitor the expression of multiple miRNAs at the same time over a prolonged period. It is mandatory to find the possible relationship between the above-mentioned miRNAs with pancreatic-specific factors.
Conflict of Interest
Authors declare that there is no conflict of interest.
References
2. Robertson RP, Davis C, Larsen J, Stratta R, Sutherland DE. Pancreas and islet transplantation in type 1 diabetes. Diabetes Care. 2006 Apr 1;29(4):935.
3. Kobayashi N, Yuasa T, Okitsu T. Regenerative medicine for diabetes mellitus. Cell Transplantation. 2009 May;18(5-6):491-6.
4. English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation?. Cell stem cell. 2010 Oct 8;7(4):431-42.
5. Wei R, Yang J, Liu GQ, Gao MJ, Hou WF, Zhang L, et al. Dynamic expression of microRNAs during the differentiation of human embryonic stem cells into insulin-producing cells. Gene. 2013 Apr 15;518(2):246-55.
6. Shaer A, Azarpira N, Karimi MH, Soleimani M, Dehghan S. Differentiation of human-induced pluripotent stem cells into insulin-producing clusters by microRNA-7. Exp Clin Transplant. 2016 Oct 1;14(5):555-63.
7. Jafarian A, Taghikani M, Abroun S, Allahverdi A, Lamei M, Lakpour N, et al. The generation of insulin producing cells from human mesenchymal stem cells by MiR-375 and anti-MiR-9. PLoS One. 2015 Jun 5;10(6):e0128650.
8. Bhonde RR, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. The international journal of biochemistry & cell biology. 2014 Jan 1;46:90-102.
9. Xin Y, Jiang X, Wang Y, Su X, Sun M, Zhang L rt al. Insulin-producing cells differentiated from human bone marrow mesenchymal stem cells in vitro ameliorate streptozotocin-induced diabetic hyperglycemia. PloS one. 2016 Jan 12;11(1):e0145838.
10. Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, et al,. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochemical and biophysical research communications. 2006 Mar 24;341(4):1135-40.
11. Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP, et al. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell transplantation. 2011 Apr;20(3):455-66.
12. Carnevale G, Riccio M, Pisciotta A, Beretti F, Maraldi T, Zavatti M, et al. In vitro differentiation into insulin-producing β-cells of stem cells isolated from human amniotic fluid and dental pulp. Digestive and Liver Disease. 2013 Aug 1;45(8):669-76.
13. Gabr MM, Zakaria MM, Refaie AF, Abdel-Rahman EA, Reda AM, Ali SS, et al. From human mesenchymal stem cells to insulin-producing cells: comparison between bone marrow-and adipose tissue-derived cells. BioMed Research International. 2017 Oct;2017:3854232.
14. Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003 Apr 4;113(1):25-36.
15. Ozeki N, Hase N, Hiyama T, Yamaguchi H, Kawai-Asano R, Nakata K, et al. RETRACTED: MicroRNA-211 and autophagy-related gene 14 signaling regulate osteoblast-like cell differentiation of human induced pluripotent stem cells. Experimental Cell Research. 2017;352(1):63-74.
16. Zhu YL, Wang S, Ding DG, Xu L, Zhu HT. miR 217 inhibits osteogenic differentiation of rat bone marrow derived mesenchymal stem cells by binding to Runx2. Molecular Medicine Reports. 2017 May 1;15(5):3271-7.
17. Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nature reviews Molecular cell biology. 2009 Feb;10(2):116-25.
18. Mondanizadeh M, Arefian E, Mosayebi G, Saidijam M, Khansarinejad B, Hashemi SM. MicroRNA‐124 regulates neuronal differentiation of mesenchymal stem cells by targeting Sp1 mRNA. Journal of Cellular Biochemistry. 2015 Jun;116(6):943-53.
19. Zhang X, Shao S, Zhao X, Zhang M, Wang J. Micro-RNA-124-5p promotes insulin producing cell differentiation through regulating transcriptional factor NKX6. 1. Biochemistry and Biophysics Reports. 2022 Jul 1;30:101273.
20. Rajabi H, Aslani S, Rahbarghazi R. Level of miR-101a and miR-107 in human adipose mesenchymal stem cells committed to insulin-producing cells. International Journal of Molecular and Cellular Medicine. 2021;10(1):68.
21. Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes research and clinical practice. 2011 Jan 1;91(1):94-100.
22. Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, et al. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic β-cell lines. Journal of Biological Chemistry. 2007 Jul 6;282(27):19575-88.
23. Lang H, Xiang Y, Lin N, Ai Z, You Z, Xiao J, et al. Identification of a panel of miRNAs as positive regulators of insulin release in pancreatic β-cells. Cellular Physiology and Biochemistry. 2018 Aug 23;48(1):185-93.
24. Soria B, Skoudy A, Martin F. From stem cells to beta cells: new strategies in cell therapy of diabetes mellitus. Diabetologia. 2001 Apr;44:407-15.
25. Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001 May 18;292(5520):1389-94.
26. Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: potential for regenerative medicine. Seminars in Cell & Developmental Biology. 2012 Aug 1; 23(6):701-10.
27. Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. International Journal of Medical Sciences. 2018;15(1):36.
28. Hu YH, Wu DQ, Feng G, Li GD, Zhang XC. Notch signaling: a novel regulating differentiation mechanism of human umbilical cord blood-derived mesenchymal stem cells into insulin-producing cellsin vitro. Chinese Medical Journal. 2010 Mar 1;123(5):606-14.
29. Dayer D, Tabar MH, Moghimipour E, Tabandeh MR, Ghadiri AA, Bakhshi EA, et al. Sonic hedgehog pathway suppression and reactivation accelerates differentiation of rat adipose-derived mesenchymal stromal cells toward insulin-producing cells. Cytotherapy. 2017 Aug 1;19(8):937-46.
30. Wang H, Ren Y, Hu X, Ma M, Wang X, Liang H, et al. Effect of Wnt signaling on the differentiation of islet β-cells from adipose-derived stem cells. BioMed Research International. 2017 Feb 20;2017:2501578.
31. Bai C, Gao Y, Li X, Wang K, Xiong H, Shan Z, et al. MicroRNAs can effectively induce formation of insulin‐producing cells from mesenchymal stem cells. Journal of tissue engineering and regenerative medicine. 2017 Dec;11(12):3457-68.
32. Kalis M, Bolmeson C, Esguerra JL, Gupta S, Edlund A, Tormo-Badia N, et al. Beta-cell specific deletion of Dicer1 leads to defective insulin secretion and diabetes mellitus. PloS one. 2011 Dec 27;6(12):e29166.
33. Hashimoto N, Tanaka T. Role of miRNAs in the pathogenesis and susceptibility of diabetes mellitus. Journal of Human Genetics. 2017 Feb;62(2):141-50.
34. Sebastiani G, Valentini M, Grieco GE, Ventriglia G, Nigi L, Mancarella F, et al. MicroRNA expression profiles of human iPSCs differentiation into insulin-producing cells. Acta Diabetologica. 2017 Mar;54:265-81.