Abstract
Cancer stem cells, a family expressed by all cancers, are necessary for the origin and critical for the growth of all cancers. In the latter tissue the cancer stem cells are concentrated and operative in small areas called niches, analogous to the stromas of non-cancer tissues. In addition to cancer stem cells, the niches contain normal cancer cells and various types of cooperative non-cancer cells. Most important among the latter are the mesechymal stem cells, analogous to those expressed by non-cancer tissues, that are important, and in some cases necessary for the development of several cancers. Various effects dependent on the already mentioned, two types of stem cells are induced not directly by them, but via their secreted extracellular vesicles, the exosomes and ectosomes. Additional cancer-cooperative cells, concentrated within the niches, include fibroblasts and immune cells that participate in the development of some cancer-relevant processes including angiogenesis. Cancer stem cells govern many critical processes of cancer life, from initiation and progression, including generation and distribution of metastases, therapy resistance, and cancer-relapse, as well as additional severe tissue problems such as efflux of anti-cancer drugs, autophagy, and evasion of immune surveillance. Without their stem cells specific cancers cannot develop. In case of defect, the cancer stem cells are induced by transformation of either normal cancer cells or mesenchymal stem cells. The present knowledge about cancer stem cells and their key effects are of critical importance in the development of therapeutic processes addressed against these cells.
Keywords
Mesenchymal stem cells, Cancer stem cells, Extracellular vesicles, Niches, Cancer differentiation, Cancer initiation, Progression and relapse, Metastasis, Cancer microenvironment, Therapy targets
Introduction
Until the end of last century, the origin and development of cancers were mostly attributed to the proliferation of their active cells, operative in collaboration with non-cancer cells of the patients. In particular, an important role was attributed to cells now recognized as mesenchymal stem cells (MSCs), first considered a few decades ago. Initially, however, the role attributed to these cells was only limited. After 1990, interest about MSCs increased progressively, starting upon their discovery to induce relevant functions, including tissue regeneration and therapy of diseases. Among therapies, a growing number was recognized to depend on MSCs, first including those of bone and cartilage followed by blood, heart, brain, liver, kidney, lung, and almost all the other organs [1-3]. Soon thereafter the effects of MSCs were found to be even larger, mediated by the secretion of their extracellular vesicles (EVs) including exosomes and ectosomes [4,5].
Based on the data summarized so far, MSCs and their EVs were considered as the cells and vesicles active in all diseases. Such definitions, however, appeared short to other major classes of cells and vesicles, those of cancer. Cells somewhat analogous to MSCs were shown to be expressed by cancers, initially by those of brain and breast [6,7]. When the interpretation of these cells was extended to other types of cancers, they were appropriately named cancer stem cells, CSCs.
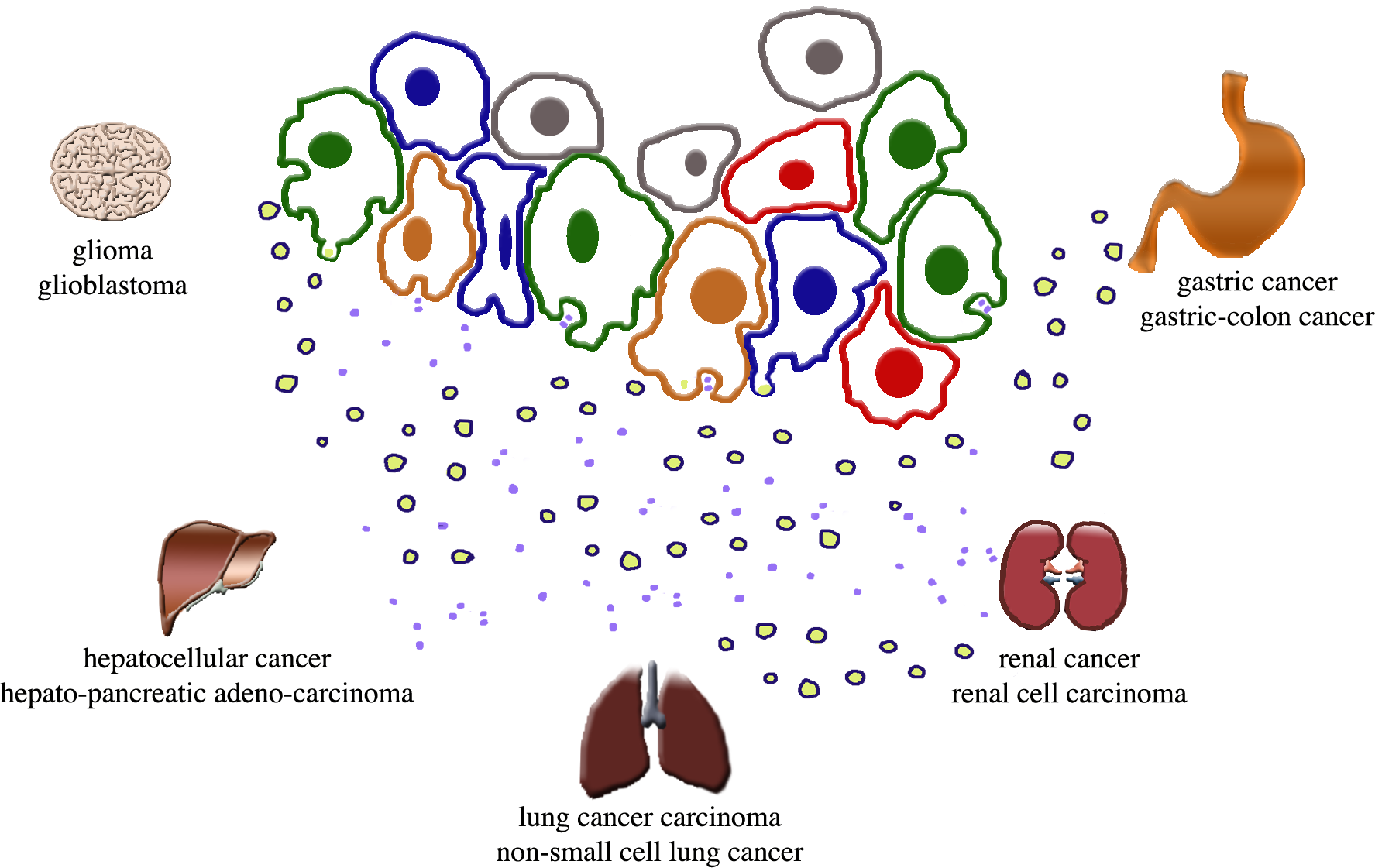
Increasing studies of CSCs, carried out during the following years, lead to their functional and pathological characterization. Subpopulations of heterogeneous CSC revealed their capacity of self-renewal and aberrant differentiation for divergent lineages of cancer cells [6-8]. CSC concentrations were found to occur within small tissue volumes, the niches, analogous to the stromas of non-cancer cells [9,10]. Niches are the sites of high degree CSC plasticity dependent on transitions from slowly cycling quiescent phases to actively proliferative phenotypes [10-12]. Within niches, stem cells and their EVs are accompanied by other cells and their EVs: normal cancer cells, fibroblasts, immune (macrophages, lymphocytes) and other cells, all cooperative to cancer function (Figure 1) [12-18]. Some of the general properties of CSCs and their vesicles were found to depend on the cooperation with MSCs and the other cell types [13,14]. The tumor microenvironment (TME), different from the non-cancer microenvironments, includes in space the niches and other areas of cancer development and growth [19,20]. Finally, studies about CSCs and their associated cells/structures have been developed in areas of potential interest for therapeutic development [10]. Some of these studies have already reached the field of clinical medicine [21,22]. So far, most published reviews and articles in the field were focused on single or a few specific cancers and their peculiar properties. The authors attempt is a short presentation of general cancer developments and progress, with special interest for recently published studies and their interpretation.
Figure 1. CSCs and associated cells of a niche are shown together with their released EVs. The names of the severe cancers governed by CSCs are reported near the images of their organs.
MSCs and their EVs in cancer
In agreement with a general criterion anticipated in the Introduction, present Section is focused on a family of cells and their EVs present in all diseases including cancers where they interact with CSCs, however with various effects. In some cancers, such as glioma and breast cancer, their results resemble those of non-cancer diseases, i.e. they induce reductions of cell proliferations. In other cancers, such as multiple myeloma and gastric cancer MSCs induce no reduction, but stimulation of cancer proliferation. In a third type of cancer, the effects of MSCs change depending on the parental cells. For example, the MSCs and their EVs from bone marrow and umbilical cord are protective, whereas those from adipose tissue increase cell proliferation [23-26]. Another factor participating in the MSC effects are miRNAs expressed by the EVs [27]. Interestingly, in tumor-positive results obtained in the niches of the cancer body were not confirmed on the metastatic developments, where responses were all negative. In this case, therefore, the mechanisms of stem cell function evolved in distinct processes can be different [19]. Additional examples of MSC effects in favor or against cancer growth have been reported in the literature.
The group of cells of the center illustrates the general structure of a cancer niche that includes many types of cells distinguished here by the color of their nucleus: green for CSCs, orange for MSCs, red for normal cancer cells, blue for examples on non-cancer cells, such as immune cells, that participate in cancer function. The dots spread below the cells are the two types of EVs secreted by all cells of the group: the small exosomes released upon exocytosis of the multi-vesicular bodies, the endocytic vacuoles that contain them; the larger ectosomes assembled at mini-rafts of the plasma membranes and then released by shedding of cell surface mini-expansions [5,10]. Various factors (interleukins, cytokines, growth factors, not shown) are secreted by all these cells. Based on the key role of CSCs in the processes governing cancer life and activity, their intense cooperation with the other cells is important for the activity of the niche.
Cancer Stem Cells: Most Important for Cancer Initiation, Specificity and Actions
CSCs, identified a few years after 2000, account for significant fractions of the cells concentrated within cancer niches together with normal cancer cells, MSCs and various other collaborative, non-cancer cells, that participate in cancer functions (Figure 1). Functionally, however, the critical properties of CSCs are original [10-13,16,28,29]. Shared with MSCs can be other properties, such as broad proliferation capacity, signaling and expression of stem cell markers. At variance to the cells cooperating with MSCs, many immune modulations of CSCs induce evasion of their surveillances. In other words, cancer growth is stimulated by the CSC resistance to immunotherapy [14,16,17]. Another property concerns differentiation. CSCs show multi-lineage differentiation properties leading to the generation of distinct cancer subtypes [12,16]. Moreover, CSCs govern many critical processes of cancer life, from initiation and progression to formation of metastases, therapy resistance, cancer-relapse [10,16,28,29]. Based on their unique support to critical cancer processes, CSCs are now recognized as potential key targets of anti-cancer therapy.
The origin of CSCs has been debated. Differentiation from normal cancer cells have been confirmed by experiments showing that, upon their elimination, CSCs are replaced by surrounding cancer cells, differentiated by changes and acquisition of peculiar properties [23,28]. Conversion into CSCs has been reported also from MSCs [28]. The sites of CSC generation and differentiation are distributed within small niches, present in all organs. The function of CSCs depends on their secretion of both soluble factors (interleukins, cytokines, growth factors) and EVs [9,23] (Figure 1). Concomitantly, CSCs receive soluble signaling factors and EVs released by cancer and other cells distributed at TMEs. Such bi-directional and also multi-directional exchanges of signals are important for preserving the activity and specificity of CSCs and other cells involved. Most important interactions, however, are mediated by their secreted EVs [12,13,29-31].
Up to now, consistent distinctions between EVs secreted by CSCs and MSCs have not been reported. Therefore, CSC-dependent vesicles are simply indicated as EVs, characterized by their interactions with cells distributed in TMEs and by their strong effects. During tumor progression the molecular profiling of circulating EVs provides non-invasive means to diagnose, monitor, and predict the course of cancers [9,23,24,30,31]. The main role of EVs is intra-tumoral cell-to-cell communication that includes fusion of the EVs from CSC and MSC with macrophages and other immune cells [15,32]. In addition, the EVs from CSCs are able to control cancer cell proliferation by proteins and miRNAs released from their luminal cargoes, believed to originate or participate in specific signaling cascades. Examples of miRNAs of CSC origin have been reported recently. Examples are the stimulation by miR-200c of metastatic traits of the colorectal cancer; the in vitro and in vivo attenuation of the human liver cancer by miR-145 and miR-200; the inhibition of lung metastasis in osteosarcoma by miR-101 [33-35].
Cancer Microenvironment
As already mentioned in previous Sections, within the niches CSCs are not distributed alone. Rather, they are co-localized and co-interactive with many normal cancer and non-cancer cells (Figure 1). In particular, the EVs secreted by the various niche cells undergo autocrine and paracrine fusions that often contribute their progress to cancer development. Both these and other processes are all sustained by the activity of non-cancer cell, such as those active on circadian clocks [18], that participate in the regulation of many cancer processes via direct and indirect effects [36]. The latter, that occur with niches and wider TMEs, include the inhibition of immune cells from the so-called immune escape process, sustained by the CSC interaction with macrophages [37]. An ensuing effect includes the development and dissemination of tumor-associated cells, promoting the immune escape of tumors via the immunosuppressive reprogramming of lymphatic vessels [17,35].
In addition to its transition processes already mentioned, TMEs includes angiogenesis by which new blood vessels grow from pre-existing vessels of tumors. Angiogenesis depends on EVs, favoring release of positive factors, including vascular endothelial growth factors and matrix metalloproteinases together with another factor inhibiting the hypoxia-inducible factor [31]. The tumor-derived EVs activate also the proliferation of endothelial cells, necessary for the ensuing formation of new vessels [18,38].
Therapy
The therapy by MSCs and their EVs was initially discovered from their positive effects against many non-cancer diseases [1-3]. The effects of CSCs are largely opposite, inducing strengthened cancer progress by activation of events such as efflux of anti-cancer drugs, autophagy, immunosuppression [28,32,36]. Investigation of EVs secreted by MSCs and CSCs opened possibilities against processes of cancer stimulation such as the transition from mesenchymal stromal cells into cancer-associated fibroblasts [39]. Specific therapeutic processes, aimed to eradicate tumors by preventing their main processes, such as metastases, tumor relapses and drug resistance, can be induced by reverse responses, such as increased drug efficacy [40,41]. Improved results have been obtained by new strategies of appropriate CSC lesion targeting. Nanomedicine strategies, initiated over 10 years ago, have reinforced the potential of anti-cancer drug strategies by the inclusion of various tools, such drugs and genes, qualified by specific targeting and combinational delivery. By this approach the prognosis observed in various types of cancers treated with typical drugs, potentially appropriate but little efficient, has been greatly improved [20,42]. The drugs employed in nanomedicine and EV therapy are encapsulated by an engineering technique developed according to a GMP technique [21,43]. Such encapsulation succeeds to target cancers, obtaining therapy effects much stronger than those of drugs administered alone [20,42,44-46].
Conclusions
At present, stem cells are attracting great interest, and innovative properties are reported every year about these cells. In their stroma of origin, MSCs undergo various types of differentiation that contribute peculiar properties and functions of their target cells. The various MSCs, including those employed in research, are not homogeneous. Yet, based on many common properties, most of them are indicated by the same MSC nomenclature.
This however is not the case of cancers. As emphasized in this review, the stem cell system of cancer cells is more complex with respect to the other cells. In particular, it does include not a single type but two distinct types of stem cells. Together with MSCs, all cancers express specific CSCs, known since 2005 based on their initially discovered properties [47,48]. Subsequent intense studies in recent years, reported here by two recent reviews [49,50], have led to the identification of many specific properties of CSCs and their EVs [9,12,13,28-31]. In cancer niches CSCs coexist not only with cells of the MSC family, but also with normal cancer cells and various non-cancer cells, such as fibroblasts, macrophages, and immune cells, participating in specific steps of cancer development. A main property of the cancer system is that of CSCs, cooperative however with MSCs and other types of cells.
The role of CSCs depends on critical cancer processes. CSCs show multi-lineage differentiation properties leading to distinct cancer subtypes [12,13,29]. The bidirectional exchange of signals between CSCs and the other cells of the niches protects the activity and the specificity of the various cells involved [10-13]. The role of CSC, including events such as efflux of anticancer drugs, autophagy and immune suppressions, can be envisaged from key steps of its actions: cancer initiation, its progression up to the formation of metastatic processes; therapy resistance and cancer relapse [9,10,23]. Such steps are highly important. Without CSCs, therefore, cancer diseases would not exist. From all these properties, CSCs are expected to become targets of innovative therapies, induced not only by scientific studies but also by clinical medicine.
References
2. Devine SM. Mesenchymal stem cells: will they have a role in the clinic?. Journal of Cellular Biochemistry. 2002;85(S38):73-9.
3. Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell‐derived extracellular vesicles. Proteomics. 2013 May;13(10-11):1637-53.
4. Askenase PW. Ancient evolutionary origin and properties of universally produced natural exosomes contribute to their therapeutic superiority compared to artificial nanoparticles. International Journal of Molecular Sciences. 2021 Jan;22(3):1429.
5. Racchetti G, Meldolesi J. Extracellular vesicles of mesenchymal stem cells: Therapeutic properties discovered with extraordinary success. Biomedicines. 2021 Jun;9(6):667.
6. Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Research. 2003 Sep 15;63(18):5821-8.
7. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences. 2003 Apr 1;100(7):3983-8.
8. Luo HT, Zheng YY, Tang J, Shao LJ, Mao YH, Yang W, et al. Dissecting the multi-omics atlas of the exosomes released by human lung adenocarcinoma stem-like cells. NPJ Genomic Medicine. 2021 Jun 14;6(1):1-4.
9. Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumor Biology. 2014 May;35(5):3945-51.
10. Meldolesi J. Cancer Stem Cells and Their Vesicles, Together with Other Stem and Non-Stem Cells, Govern Critical Cancer Processes: Perspectives for Medical Development. International Journal of Molecular Sciences. 2022 Jan;23(2):625.
11. Fukushi D, Shibuya‐Takahashi R, Mochizuki M, Fujimori H, Kogure T, Sugai T, et al. BEX2 is required for maintaining dormant cancer stem cell in hepatocellular carcinoma. Cancer Science. 2021 Nov;112(11):4580.
12. Lindoso RS, Collino F, Vieyra A. Extracellular vesicles as regulators of tumor fate: crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells. Stem Cell Investigation. 2017;4.
13. Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Developmental cell. 2016 May 23;37(4):301-9.
14. Dou D, Ren X, Han M, Xu X, Ge X, Gu Y, Wang X. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Frontiers in Immunology. 2020:2026.
15. Aramini B, Masciale V, Grisendi G, Banchelli F, D’Amico R, Maiorana A, et al. Cancer stem cells and macrophages: molecular connections and future perspectives against cancer. Oncotarget. 2021 Feb 2;12(3):230.
16. Castagnoli L, De Santis F, Volpari T, Vernieri C, Tagliabue E, Di Nicola M, Pupa SM. Cancer Stem Cells: Devil or Savior—Looking behind the Scenes of Immunotherapy Failure. Cells. 2020 Mar;9(3):555.
17. Ferguson LP, Diaz E, Reya T. The role of the microenvironment and immune system in regulating stem cell fate in cancer. Trends in Cancer. 2021 Jul 1;7(7):624-34.
18. Ludwig N, Rubenich DS, Zaręba Ł, Siewiera J, Pieper J, Braganhol E, et al. Potential roles of tumor cell-and stroma cell-derived small extracellular vesicles in promoting a pro-angiogenic tumor microenvironment. Cancers. 2020 Dec;12(12):3599.
19. Attar-Schneider O, Dabbah M, Drucker L, Gottfried M. Niche origin of mesenchymal stem cells derived microvesicles determines opposing effects on NSCLC: primary versus metastatic. Cellular Signalling. 2020 Jan 1;65:109456.
20. Gener P, Gonzalez Callejo P, Seras-Franzoso J, Andrade F, Rafael D, Abasolo I, et al. The potential of nanomedicine to alter cancer stem cell dynamics: The impact of extracellular vesicles. Nanomedicine. 2020 Oct;15(29):2785-800.
21. Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu-Chi Medical Journal. 2020 Apr;32(2):113.
22. Koshkin SA, Anatskaya OV, Vinogradov AE, Uversky VN, Dayhoff GW, Bystriakova MA, et al. Isolation and Characterization of Human Colon Adenocarcinoma Stem-Like Cells Based on the Endogenous Expression of the Stem Markers. International Journal of Molecular Sciences. 2021 Jan;22(9):4682.
23. López de Andrés J, Griñán-Lisón C, Jiménez G, Marchal JA. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. Journal of Hematology & Oncology. 2020 Dec;13(1):1-22.
24. Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Letters. 2012 Feb 1;315(1):28-37.
25. Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opinion on Biological Therapy. 2015 Apr 3;15(4):495-504.
26. Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015 Aug 3;14(15):2473-83.
27. Shang S, Wang J, Chen S, Tian R, Zeng H, Wang L, et al. Exosomal miRNA‐1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Medicine. 2019 Dec;8(18):7728-40.
28. Afify SM, Seno M. Conversion of stem cells to cancer stem cells: undercurrent of cancer initiation. Cancers. 2019 Mar;11(3):345.
29. Afify SM, Hassan G, Yan T, Seno A, Seno M. Cancer stem cell initiation by tumor-derived extracellular vesicles.
30. Hernandez-Oller L, Seras-Franzoso J, Andrade F, Rafael D, Abasolo I, Gener P, et al. Extracellular vesicles as drug delivery systems in cancer. Pharmaceutics. 2020 Dec;12(12):1146.
31. Su C, Zhang J, Yarden Y, Fu L. The key roles of cancer stem cell-derived extracellular vesicles. Signal Transduction and Targeted Therapy. 2021 Mar 8;6(1):1-5.
32. Zheng P, Li W. Crosstalk between mesenchymal stromal cells and tumor-associated macrophages in gastric cancer. Frontiers in Oncology. 2020 Oct 9;10:2085.
33. Brossa A, Fonsato V, Grange C, Tritta S, Tapparo M, Calvetti R, et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell‐derived tumor growth in vitro and in vivo. International Journal of Cancer. 2020 Sep 15;147(6):1694-706.
34. Zhang K, Dong C, Chen M, Yang T, Wang X, Gao Y, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411.
35. Zhou C, Wei W, Ma J, Yang Y, Liang L, Zhang Y, et al. Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Molecular Therapy. 2021 Apr 7;29(4):1512-28.
36. Xuan W, Khan F, James CD, Heimberger AB, Lesniak MS, Chen P. Circadian regulation of cancer cell and tumor microenvironment crosstalk. Trends in Cell Biology. 2021 Nov 1;31(11):940-50.
37. Wang Y, Wang B, Xiao S, Li Y, Chen Q. miR‐125a/b inhibits tumor‐associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. Journal of Cellular Biochemistry. 2019 Mar;120(3):3046-55.
38. Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. International Journal of Molecular Sciences. 2020 Jan;21(16):5840.
39. Yang Y, Li J, Geng Y. Exosomes derived from chronic lymphocytic leukaemia cells transfer miR-146a to induce the transition of mesenchymal stromal cells into cancer-associated fibroblasts. The Journal of Biochemistry. 2020 Nov;168(5):491-8.
40. Zhang J, Song Q, Wu M, Zheng W. The emerging roles of exosomes in the chemoresistance of hepatocellular carcinoma. Current Medicinal Chemistry. 2021 Jan 1;28(1):93-109.
41. Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. Journal of cellular physiology. 2019 Jun;234(6):8381-95.
42. Xie FY, Xu WH, Yin C, Zhang GQ, Zhong YQ, Gao J. Nanomedicine strategies for sustained, controlled, and targeted treatment of cancer stem cells of the digestive system. World Journal of Gastrointestinal Oncology. 2016 Oct 15;8(10):735.
43. Wiest EF, Zubair AC. Challenges of manufacturing mesenchymal stromal cell–derived extracellular vesicles in regenerative medicine. Cytotherapy. 2020 Nov 1;22(11):606-12.
44. Zhao Q, Hai B, Kelly J, Wu S, Liu F. Extracellular vesicle mimics made from iPS cell-derived mesenchymal stem cells improve the treatment of metastatic prostate cancer. Stem Cell Research & Therapy. 2021 Dec;12(1):1-3.
45. Pinto A, Marangon I, Méreaux J, Nicolás-Boluda A, Lavieu G, Wilhelm C, et al. Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano. 2021 Jan 22;15(2):3251-63.
46. Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sciences. 2020 Nov 15;261:118369.
47. Liu BB, Qin LX, Liu YK. Adult stem cells and cancer stem cells: tie in or tear apart?. Journal of Cancer Research and Clinical Oncology. 2005 Oct;131(10):631-8.
48. Trosko JE. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomedicine & Pharmacotherapy. 2005 Oct 1;59:S326-31.
49. Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma—From origin to clinical implications. Nature Reviews Gastroenterology & Hepatology. 2021 Sep 9:1-9.
50. Huang B, Yan X, Li Y. Cancer Stem Cell for Tumor Therapy. Cancers. 2021 Jan;13(19):4814.