Abstract
Background: Borreliosis infection and post-treatment Lyme disease syndrome are associated with nonspecific, multisystem symptoms, and there is currently no effective direct test for Lyme disease. The seroprevalence of Borrelia miyamotoi among individuals with suspected tick-borne illnesses and persistent symptoms is not well documented. Although bacterial extracellular vesicles (BEVs) have been suggested as potential sources of disease biomarkers, evidence supporting their role in Borrelia infection is lacking.
Methods: Patients (N=66) with symptoms and with clinical suspicion of a tick-borne illness were enrolled in the study and followed longitudinally over the course of 1 year (min 1 visit, max 4 visits). The General Symptom Questionnaire (GSQ-30) was administered to the patients at each visit to measure symptom burden. Lyme and Borrelia miyamotoi serologies were conducted at LabCorp or Quest, under the standard of care. A mass spectrometry proteomic assay measuring Borrelia-specific peptides was conducted in the urine of the patients.
Results: Among the analyzed patients, 65% had positive Lyme serology and 33% had positive B. miyamotoi serology with partial overlap (20%). Urinary Borrelia-specific peptides correlated with the GSQ-30 score after correcting for age, Lyme serology, and B. miyamotoi serology (coefficient = 0.57, t-test p-value < 0.00001). Urinary extracellular vesicles had positive reactivity in a p66 immunoassay, a known marker of Borrelia BEVs. Urinary peptides included glycerophosphodiester phosphodiesterase, variable small and large outer membrane proteins, the multicopy lipoprotein family, and Borrelia direct repeat proteins, which are also known markers of Borrelia BEVs.
Conclusions: The B. miyamotoi seroprevalence (33%) in a symptomatic population with clinical suspicion of a tick-borne illness was higher than the seroprevalence estimated from previous studies. As it provides an objective measurement correlated with a standardized symptom burden score, urinary Borrelia peptide detection offers a promising avenue for future assay development. This proteomic analysis supported the presence of Borrelia BEVs in the urine of symptomatic patients with suspected tick-borne illnesses.
Keywords
Borrelia miyamotoi, Infection, Lyme disease, Peptide
Introduction
Lyme disease, or Lyme Borreliosis, is the most prevalent tick-borne illness in the Northern Hemisphere. It is caused by bacteria in the Borrelia burgdorferi sensu lato (s.l.) complex, which are transmitted to humans through the bite of infected hard bodied ticks, or Ixodes ticks [1-3]. Post-treatment Lyme disease syndrome (PTLDS) is a significant complication of Lyme disease, characterized by persistent or recurrent symptoms, such as fatigue, musculoskeletal pain, and cognitive issues, which can lead to functional decline. The complex, multifactorial, and non-specific symptoms of the disease often mimic those of other conditions, such as inflammatory disorders and autoimmune endocrine diseases, which complicate clinical assessment. Conditions such as fibromyalgia, chronic fatigue syndrome, rheumatoid arthritis, hypothyroidism, or adrenal insufficiency present with symptoms that may overlap with those of Lyme disease and its complications. This overlap, combined with the absence of reliable laboratory tests, presents significant challenges for the accurate diagnosis of Lyme disease [1]. The two-tier serology test is the recommended laboratory method for diagnosing borreliosis in both Europe and the US [2]. However, it has a low sensitivity at disease onset (approximately 50%) and variable sensitivity at later stages, ranging from 50% to 97% [2]. The only available direct test, PCR testing for Borrelia, is primarily limited to synovial fluid for diagnosing Lyme arthritis and skin biopsy samples for detecting acrodermatitis chronica atrophicans [2]. In other contexts, PCR shows very low and highly variable sensitivity, depending on the disease stage and the type of biospecimen used [2].
We previously developed an unbiased proteomics approach to identify tick-borne pathogen peptides in the urine of patients with suspected tick-borne illnesses at various stages. In a published clinical study, the assay detected two pathogen-derived peptides in nine out of ten acute erythema migraines (EM) cases, with no false positives among 250 asymptomatic and symptomatic controls [4]. The presence of urinary peptides derived from a tick-borne pathogen was detected in 40% of patients with PTLDS and those under clinical evaluation for tick-borne diseases [4]. Identified Borrelia-specific proteins participated in pathways related to biosynthesis, cell wall organization and biogenesis, cell cycle, chemotaxis, immune evasion, metabolism, signal transduction, transcription/translation, and transmembrane transport. Thus, proteomics provides functional insights into pathogen physiology during interactions with human hosts [4].
Given the complexity of symptomatology in people with Lyme disease and PTLDS, the General Symptom Questionnaire-30 (GSQ-30) was developed and validated as a tool for assessing its multifactorial symptom burden [5]. The GSQ-30 is a self-report instrument comprising 30 items that evaluates the presence and severity of symptoms over a two-week period. Respondents indicate how much they have been affected by each symptom using a five-point scale: "not at all" (0), "a little bit" (1), "somewhat" (2), "quite a bit" (3), and "very much" [4]. The total score, represents the cumulative symptom burden, ranged from 0 to 120 [5]. The GSQ-30 has demonstrated excellent internal consistency, with a Cronbach's alpha of 0.95, indicating that the items consistently measured the intended construct. Factor analysis revealed four core domains within the questionnaire: pain/fatigue, neuropsychiatric, neurologic, and flu-like symptoms. These domains reflect the common symptom clusters reported by patients with Lyme disease and PTLD [5]. Studies have shown that patients with PTLDS exhibit significantly higher GSQ-30 total scores than those with early Lyme disease (erythema migrans) thus mirroring the increase in patient symptom burden over time. Additionally, GSQ-30 scores correlated strongly with measures of functional impairment, suggesting that higher symptom burden scores are associated with greater functional decline [5]. GSQ-30 has also been shown to be effective in detecting post-treatment changes. In a study assessing patients with erythema migrans, the questionnaire identified significant reductions in symptom burden following antibiotic therapy, correlating with improvements in functional status [5].
Borrelia miyamotoi, a bacterium genetically closer to relapsing fever spirochetes than to those causing Lyme disease, has emerged as a growing concern in tick-borne disease endemic regions [6]. B. miyamotoi is transmitted by the same Ixodes species of hard-bodied ticks that serve as vectors for Borrelia burgdorferi [6]. B. miyamotoi infection presents with clinical symptoms somewhat similar to Lyme disease, including fever, fatigue, headache, myalgia, chills, nausea and possible significant neurologic complications [6]. Unlike B. burgdorferi, though, B. miyamotoi does not produce the distinctive erythema migrans rash, making diagnosis based on clinical observation alone challenging and increasing the risk of misdiagnosis or delayed treatment [3]. Additionally, the effectiveness of standard treatments against B. miyamotoi has not been thoroughly documented, adding uncertainty to treatment strategies. More epidemiological research is therefore needed, given the significant healthcare implications of unrecognized or improperly managed cases [7].
The potential of bacterial extracellular vesicles (BEVs) as biomarkers for Lyme disease and other bacterial infections has garnered increasing interest in recent years [8]. These vesicles are lipid bilayer-enclosed particles released by bacteria that play crucial roles in pathogenesis, intercellular communication, and immune system modulation [8]. The molecular cargo of BEVs, including proteins, nucleic acids, and lipids, can facilitate the spread of infection, modulate host responses, and show potential for diagnostic applications [8]. However, while the utility of BEVs as biomarkers in other diseases [8,9] is being investigated, BEV presence, composition, and diagnostic value in Borrelia infections remains unknown. Once validated in patient and animal models, BEVs could provide a more sensitive, specific, and noninvasive source of diagnostic material for detecting Lyme disease, supporting early diagnosis and tracking disease progression [9].
In this study, 66 symptomatic patients with clinical suspicion of a tick-borne illness were enrolled under informed consent and followed longitudinally over the course of 1 year (min 1 visits; max 4 visits). Lyme and B. miyamotoi serology tests were conducted on patients under standard of care procedures. At each visit, the GSQ-30 questionnaire was delivered, and urine was collected. Urine was analyzed using an unbiased proteomic approach that identifies Borrelia-specific peptides, and urinary extracellular vesicles were analyzed using an immunoassay specific for Borrelia p66, a known marker of Borrelia BEVs.
Materials and Methods
Patient sample
This study included 66 patients with suspected tick-borne illness and persistent symptoms (Dr. Hope McIntyre, Lyme Hope LLC, MD). This study was approved by GMU IRB (IRBNet number 869592). A subset of these patients met the case-defined criteria for Post-Treatment Lyme Disease Syndrome (PTLDS) [10]. Patients were considered PTLDS if (a) they were previously diagnosed with Lyme disease (based on EM rash and positive two-tier serology) more than 6 months before the urine sample was collected, (b) if Lyme Borreliosis symptoms ameliorated following antibiotic treatment, and (c) if patients presented with fatigue, musculoskeletal pain, or cognitive impairment according to the IDSA criteria at the time of urine collection. The symptoms were judged by the physician to be functionally disabling. Blood samples for all patients were sent to Quest Diagnostics or LabCorp for Lyme disease and B. miyamotoi serological testing. To assess the burden of multi-system symptoms, the General Symptom Questionnaire-30 (GSQ-30) [5], a standardized questionnaire designed to measure symptom burden associated with Lyme disease. Symptoms included headaches, fatigue, brain fog, heart failure, dizziness, arthralgia, seizures, vertigo (Supplementary Table 1).
Urine collection
Urine collection kits comprising sterile urine cups, zip-loc bags, handling instructions, ice gel packs, and a Styrofoam box were sent to the doctor’s office. The kits were handed to the participants with instructions to collect the first-morning urine and send back the kit to George Mason University through overnight shipment. Urine samples were frozen at -80°C upon receival at George Mason University.
Affinity particle processing of biofluids from patient subjects
Urine samples (42 mL minimum) were thawed overnight at 4°C, analyzed using a Multistix 10 SG reagent strip, and centrifuged at 3700×g for 15 min to eliminate debris. After decanting, the pH was adjusted to 5.5 using 1 M hydrochloric acid or 1 M sodium hydroxide solutions. Urine samples were mixed with affinity hydrogel particles [4], to concentrate, partially purify, and preserve low-abundance, low-molecular-weight proteins present in urine, as described elsewhere [11,12]. Briefly, urine samples (40 mL) were incubated with 200 µL of affinity particles (10 mg/mL) for 30 min at RT. The particles were separated by centrifugation at 19,000×g (Beckman Avanti JXN-26 Centrifuge) for 45 min, and the supernatant was discarded. The particle pellet was washed twice by vigorous resuspension in 1 mL of 18 MΩ-cm water, followed by centrifugation at 16,100×g for 20 min. The supernatant was discarded, and the pellet was resuspended in 20 µL elution buffer solution (4% sodium dodecyl sulfate (SDS) in 50 mM ammonium bicarbonate) and incubated for 20 min at room temperature. The samples were centrifuged at 16,100×g for 20 min. Eluates were saved, transferred into new tubes, and processed for mass spectrometry, as described further.
Mass spectrometry analysis
Mobile phases A (The eluates were reduced using 200 mM dithiothreitol at room temperature for 15 min and alkylated using 50 mM iodoacetamide at room temperature in the dark for 20 min. The enzymatic digestion ran overnight with 2 µL of (0.5 µg/µL) of sequencing grade trypsin (Promega, V5113) in 50 mM ammonium bicarbonate pH 8 at 37 °C. The digestion was stopped by adding 2 µL of 100% trifluoroacetic acid (TFA). Digested samples were desalted using C-18 spin columns (Pierce, #89870). The final eluates were dried using a nitrogen evaporator (Microvap 118, Organisation Associates, Inc.). The samples were reconstituted in 10 µL 0.1% formic acid. Liquid chromatography tandem mass spectrometry (LC–MS/MS) was performed using an Orbitrap Fusion Tribrid Mass Spectrometer (Thermo Scientific) coupled with a nano-spray EASY-nLC 1200 UHPLC. Reversed-phase chromatography separation of the peptide mixture was performed using a PepMap RSLC 75 μm i.d. × 15 cm long with a 2 μm C18 resin LC column (ThermoFisher). 0.1% formic acid) and B (0.1% formic acid and 80% acetonitrile as mobile phase B were used. Peptides were eluted using a linear gradient of 5% mobile phase B to 50% mobile phase B for 90 min at 300 nL/min, and then to 100% mobile phase B for an additional 5 min. A Thermo Orbitrap Fusion Tribrid Mass Spectrometer (Thermo Scientific) was operated in data-dependent mode, in which each full MS scan was followed by TopN MS/MS scans of the most abundant molecular ions with charge states from 2+ to 4+ were dynamically selected for collision-induced dissociation (CID) using a normalized collision energy of 35%. Tandem mass spectra were searched against Borrelia and Homo sapiens NCBI databases using Proteome Discoverer 2.1 software using tryptic cleavage constraints. A peptide identification and authentication algorithm were used to ensure accurate peptide attribution to Borrelia, with minimal false positives. Peptide spectrum matching: Stringent criteria included a peptide false discovery rate (FDR) of <1%; Xcorr values >2.0, 3.0, and 4.0, for 2+, 3+, and 4+ precursor ions; q-value <0.05; and precursor/fragment ion mass tolerances of <2 ppm and <0.5 Da. For triply charged ions, additional checks, such as the presence of basic residues and doubly charged precursor ions, were applied. Microorganism attribution: Peptides were verified using BLAST against the NCBI RefSeq database. Peptides identical to sequences from non-target organisms were discarded, and those shorter than 7 amino acids were excluded to minimize random attributions. Protein database validation: Proteins to which the peptides were attributed were aligned with homologous proteins in closely related species, with a threshold of >60% identity for validation. Peptides were unambiguously attributed to the Borrelia genus or species if they had 100% identity over 100% query sequences.
Borrelia culture
Borrelia burgdorferi B31 was purchased from ATCC (cat # 35210), the stock was expanded in 20 mL of BSK-II medium, and aliquots were frozen in large 1 mL and small 250 mL aliquots. Large aliquots were used for 1-1.5 mL BSK-II inoculation; after 5 days to a week when reaching countable density, cell suspensions were placed in a larger volume, while small aliquots were thawed and directly inoculated into 10 mL batches of BSK-II, allowing no more than 10% void volume in tubes. Spirochetes were visualized using FITC conjugated rabbit polyclonal anti Borrelia antibodies (Abcam ab69252). Spirochetes were counted by methanol fixation and trypan blue staining using a chemocytometer. Briefly, 100-500 mL of Borrelia culture was spun down at 2,300 rcf for 5 minutes and washed twice with phosphate buffered saline (PBS). The pellet was resuspended in a volume of ice-cold methanol ten times larger than the pellet volume, followed by incubation for 10 minutes at -20°C. This step simultaneously fixes and permeabilizes the spirochetes. After incubation, equal volumes of Trypan blue dye (0.4%w/v, Corning) were added to the permeabilized spirochete suspension, and allowed to incubate for 5 minutes, and the cells were counted using a chemocytometer (QuadCount, Accuris Instruments).
Extracellular vesicle isolation
Extracellular vesicles were isolated from human urine and Borrelia culture supernatants. Borrelia culture was grown for 7-10 days in BSKII prepared using extracellular vesicle-free rabbit serum (the serum was spun at 100,000 rcf for 1 hour prior to including it into BSKII). Spirochete cultures (10 mL, total number of spirochetes 1.1x10^7) and human urine (10 mL) were pelleted at 500 rcf for 10 min, followed by centrifugation at 10,000 rcf for 30 min to remove cellular debris. BEV were obtained by high-speed ultracentrifugation at 100,000 rcf for 1 hour. BEV were washed twice with PBS via ultracentrifugation and resuspended in a final volume of 100-200 mL. BEVs were characterized and counted using a zeta view (ParticleMetrix).
Immunoblotting
Borrelia lysates, Borrelia BEVs and extracellular vesicles isolated from human urine using ultracentrifugation were analyzed with western blotting and dot blotting. To obtain the Borrelia lysate, spirochetes were spun down at 5,000 rcf at room temperature for 10 minutes and washed three times with PBS at the same acceleration. Lysates were obtained by sonication (Cole-Parmer) of 500 mL of spirochete suspension for 5 cycles (15 seconds on and 10 seconds off) at 30% power setting, while keeping the sample on ice. The lysate was spun at 7,000 rcf for 10 mins to eliminate cell debris. Samples were separated using 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS PAGE, Bio-Rad), transferred onto polyvinylidene difluoride membrane (Bio-Rad), and incubated overnight at 4°C with an anti-p66 mouse monoclonal primary antibody diluted 1:1,000 in PBS additioned with 2% tween 20 and 1% bovine serum albumin. The p66 monoclonal antibody was custom generated using a target peptide epitope conserved across multiple Borrelia species. The peptide was sent to SinoBiological for the generation of monoclonal antibodies using hybridoma technology. The dot blot was obtained by manually spotting 1 mL of sample on a nitrocellulose membrane (0.45 mm, BioRad). Specific immunoreactive signal was detected using the ChemiDoc XRS + Imaging System (Bio-Rad).
Statistical analysis
Linear regression, statistical tests, and scatterplots were obtained using Python (Stats models, and scikit-learn libraries) and R software. In the linear model, the GSQ-30 score was the dependent variable, and peptide hits, age, Lyme disease serology, and B. miyamotoi serology were independent variables.
Results
Patient cohort and standardized survey
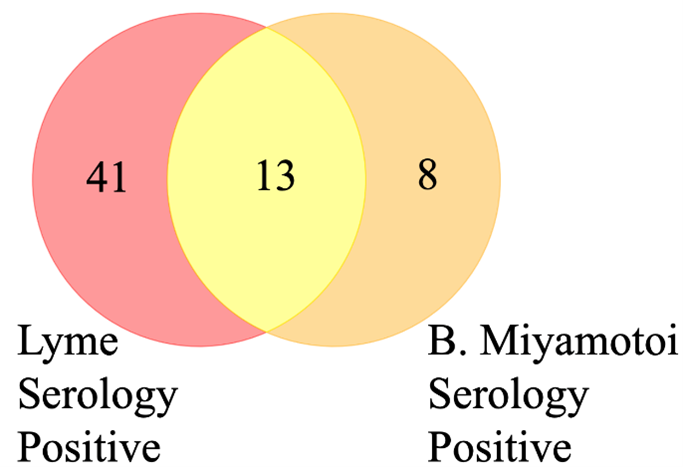
This study was conducted on 66 symptomatic patients with suspected tick-borne illnesses who were recruited in a specialized clinical practice, Lyme Hope LLC, MD. The mean age of the participants was 42.30 years (SD = 21.65). Most of the patients (53.85%) were female. The patients were monitored longitudinally over four or more visits. The General Symptom Questionnaire-30 (GSQ-30) is a standardized survey designed to assess the severity and frequency of symptoms in patients with post-treatment Lyme disease syndrome (PTLDS) and other illnesses. It provides a comprehensive evaluation of the multisystemic symptoms often associated with Lyme disease, helping to measure their impact on patients’ quality of life. The GSQ-30 survey was administered to participants at each visit, and their scores were recorded along with urine collection. The median GSQ-30 score was 36, which reflects a moderate symptom burden across the population, and a standard deviation of 22.62, which indicates considerable diversity in symptom experiences, with some individuals experiencing mild symptoms and others facing severe symptom burden. Serological testing for Lyme disease, conducted at LabCorp or Quest, showed that 65% of the patients tested positive. Additionally, B. miyamotoi serology results indicated that 33% of the patients were seropositive (Table 1, Figure 1).
|
Age: mean (SD) |
42.30 (21.65) |
|
Sex: %Female, %Male |
53.8%, 46.2% |
|
GSQ-30 scores: Median, max - min |
36, 3 - 98 |
|
Lyme Serology: (%positive, number of not available) |
65%, 2 |
|
Borrelia Miyamotoi Serology (% positive, number of not available) |
33%, 2 |
Figure 1. Lyme disease and Borrelia miyamotoi seropositivity according to standard of care serology methods. 41 patients tested positive exclusively for Lyme disease, whereas eight tested positive only for B. miyamotoi. Thirteen patients were seropositive for both infections.
Urinary protein analysis revealed essential metabolic functions and proteins associated with Borrelia BEVs
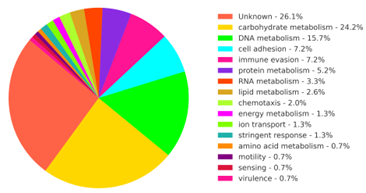
Mass spectrometry analysis of the urine of participants identified 105 unique peptides unambiguously attributed to pathogenic Borrelia species. The peptides were attributed to 62 proteins that contribute to the following biological processes and functions: phospholipid synthesis and catabolism [13], isoprenoid synthesis, amino acid metabolism, peptidoglycan biosynthesis, complex carbohydrate degradation, glycolysis, DNA and RNA processing, protein cleavage, protein phosphorylation, nucleic acid methylation, transmembrane ion active transport, copper tolerance, stringent response, immune evasion, antigenic variation, adhesion, chemotaxis, and motility (Figure 2, Supplementary Table 1).
Figure 2. Biological processes carried out by the Borrelia-derived proteins identified in the patient samples.
Among the identified urinary Borrelia proteins are glycerophosphodiester phosphodiesterase and outer membrane lipoproteins, including variable outer membrane proteins, variable small proteins, variable large proteins, Mlp family lipoproteins, and bdr proteins [14], which were identified in Borrelia BEVs in previous studies [15].
Borrelia BEV presence in the urine was confirmed by antibody-mediated detection of p66, a Borrelia BEV marker
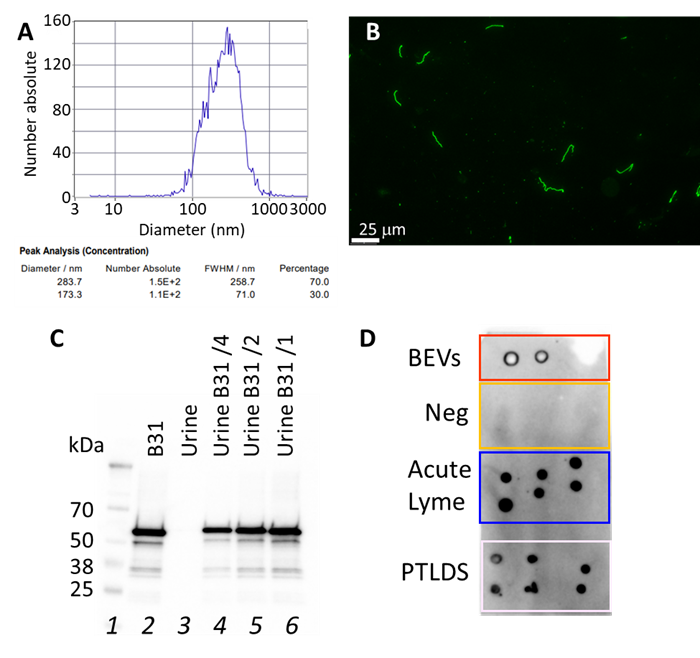
The presence of Borrelia BEVs in the urine, supported by the proteins detected by mass spectrometry (Table 2), was confirmed by isolating urinary extracellular vesicles (average diameter = 170–280 nm, Figure 3A) and subjecting them to an immunoassay directed against a known marker of Borrelia BEVs, p66. A novel mouse monoclonal antibody (mAb) was raised against a p66 epitope that is conserved among several species of Borrelia. To validate the antibody and produce BEVs, B. burgdorferi B31 cultures were conducted using extracellular vesicle-free medium (Figure 3B) and yielded an average of 10,000 BEVs per cell over 10 days. The mAb showed high specificity and sensitivity for detecting p66 in the Borrelia lysate (Figure 3C). Lab-grown B. burgdorferi BEV and extracellular vesicle isolated from the urine of acute and late-stage Lyme disease yielded a strong antibody signal. The absence of cross-reactivity was observed in negative controls (Figure 3D).
|
Proteins |
Found in Borrelia BEV |
|
Glycerophosphodiester Phosphodiesterase, BAN67553 |
[15] ( Shang et al., 1998 ) |
|
Variable Outer Membrane Protein, WP_012538010 |
[14] ( Templeton, 2004 ) |
|
Variable Small Protein, WP_015633341 |
[14] ( Templeton, 2004 ) |
|
Variable Large Protein, WP_025444237 |
[14] ( Templeton, 2004 ) |
|
Mlbp Family Lipoprotein, WP_119024480 |
[15] ( Shang et al., 1998 ) |
|
Bdr Protein, ACH93789 |
[14] ( Templeton, 2004 ) |
Figure 3. The presence of Borrelia BEVs in the urine of patients was confirmed by antibody-mediated detection of p66, a Borrelia BEV marker. (A) A representative report of Zeta View analysis of extracellular vesicles isolated from patient urine indicates particle diameter distribution peaks ranging from 173 to 284 nm. (B) Fluorescence microscopy image of Borrelia burgdorferi B31 cells stained with a FITC labelled anti-Borrelia rabbit polyclonal antibody. (C) A custom-produced mouse anti-p66 monoclonal antibody showed specific reactivity against p66 in Borrelia lysate and no cross-reactivity against human proteins. Lane 1: molecular weight ladder, lane 2: B. burgdorferi B31 lysate (1.4 mg), lane 3: human urine, lane 4: B. burgdorferi B31 lysate spiked in urine 0.34 mg, lane 5: B. burgdorferi B31 lysate spiked in urine 0.7 mg, lane 6: B. burgdorferi B31 lysate spiked in urine 1.4 mg. (D) Dot blot analysis showing anti-p66 mAb reactivity with laboratory grown Borrelia BEVs and urinary extracellular vesicles isolated from acute Lyme patients and PTLDS patients, but not Lyme negative controls.
Borrelia-derived peptide abundance correlates with the GSQ-30 symptom score
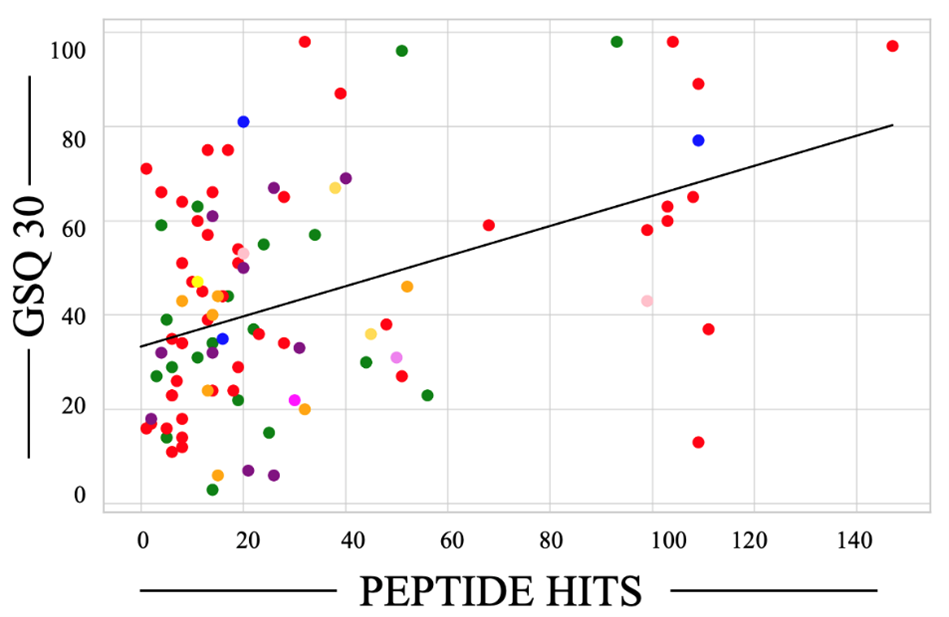
In a linear model of 143 observations, where the dependent variable was the GSQ-30 pain score and the independent variables were Borrelia peptide hits, Lyme serology, Borrelia miyamotoi serology, and age, the R-squared value was 0.203, the R-squared adjusted for the number of predictors was 0.180, and the overall F-statistic was 8.788, with p = 2.38e-06. The only significant predictor of the GSQ-30 pain score was Borrelia peptide hits (coefficient 0.31, p < 0.001), whereas the other predictors (Lyme serology, Borrelia miyamotoi serology, and age) did not show significant effects (coefficient -5.0, p = 0.174; coefficient -4.1, p = 0.412; coefficient 0.01, p = 0.928, respectively). A plot of the GSQ30 score as a function of peptide hits is reported in Figure 4.
Figure 4. Borrelia specific-peptide hits correlate with the General Symptom Questionnaire-30 (GSQ-30) scores. Dot colors were coded to represent protein biological processes. Green, unknown; red, metabolism; blue, chemotaxis; purple, cell adhesion; violet, sensing; mustard yellow, ion transport; pink, stringent response; orange, immune evasion; lemon yellow, motility; and magenta, virulence.
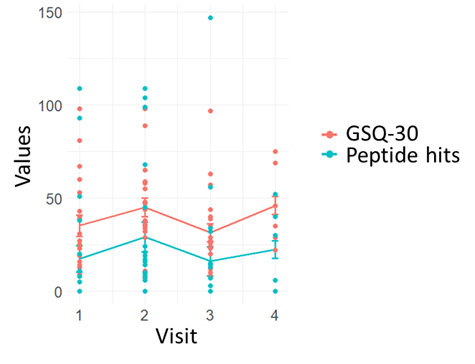
Figure 5 shows a scatterplot of GSQ-30 and peptide hits data in function of patient visits during the follow-up period. The data show that the GSQ-30 average across all patients trends higher from visit 1 to 2 (t = -1.34, p-value = 0.196) and then fluctuates (visit 2 versus visit 3 t = 2.38, p-value = 0.028, visit 3 to 4 t = -2.54, p-value = 0.05). Peptide hits follow a similar pattern (visit 1 versus visit 2 t = -1.2, p-value = 0.26, visit 2 versus visit 3 t = 1.4, p-value = 0.2, visit 3 versus visit 4 t = -2.7, p-value = 0.04) when monitored longitudinally over the follow up period.
Figure 5. Borrelia peptide hits mirror GSQ-30 trends during the longitudinal visits.
Discussion
This study enrolled 66 symptomatic patients with suspected tick-borne illnesses who were followed over the course of one year. At each visit (min 1, max 4), patients answered the standardized General Symptom Questionnaire-30 (GSQ-30) [5] and donated urine for proteomic analysis. Mass spectrometry proteomic analysis revealed that Borrelia-specific urinary peptide hits correlated with standardized and clinically validated GSQ-30 symptom scores. The only significant predictor of the GSQ-30 pain score was Borrelia peptide hits (coefficient 0.31, p < 0.001), whereas the other predictors considered (Lyme serology, Borrelia miyamotoi serology, and age) did not show significant effects (coefficient -5.0, p = 0.174; coefficient -4.1, p = 0.412; coefficient 0.01, p = 0.928, respectively). While the linear regression model demonstrated a statistically significant relationship between the peptide spectrum matches (PSMs) and the GSQ-30 score (p < 0.001), the adjusted r-squared value was 0.18, which suggests that the model explained 18% of the variability in the GSQ-30 scores. This phenomenon may be attributed to potential GSQ-30 and mass spectrometry data variability, and the fact that other independent variables might need to be investigated. GSQ-30 scores capture patient-reported measures of multisystem symptom burden. As these scores are reported by patients, they may be subjective and exhibit interpatient variability. The variability of mass spectrometry data can range from 10-25% [16,17]. When considered together, these factors might contribute to a relatively low r-squared value. We emphasize, however, that the GSQ-30 questionnaire was validated in a cohort of well-characterized early-stage Lyme disease individuals, demonstrated strong internal consistency, and was considered an appropriate clinical measure for future correlative studies with novel disease-relevant biomarkers [5]. In previous publications, we applied the urinary Borrelia peptide mass spectrometry assay to a clinical cohort of tick-borne disease patients, and we validate the technology for studying tick-borne pathogen infections. Analytical challenges include the large dynamic range of urinary proteins and the presence of Borrelia proteins at exceedingly low concentrations (e.g., 1 pg/mL), several orders of magnitude less than human proteins, which further exacerbates the analytical complexity. We demonstrated that pre-analytical sample processing steps and stringent bioinformatic analysis can effectively increase the signal-to-noise ratio and improve the sensitivity of the analysis [4]. In the absence of established clinical biomarkers to quantitatively characterize the study population, we compared a rigorously validated self-reported symptom score system with the results of an analytical platform that has shown promise in clinical studies.
In this study, the percentages of Lyme disease serology and B. miyamotoi serology positivity were 65% and 33%, respectively, and there was an overlap of positive serology in 20% of patients. This data should be contextualized with the analytical and diagnostic merits of the serological tests, the shared mechanisms of the two Borrelia species, and the available data on B. miyamotoi seroprevalence. Cross-reactivity between B. burgdorferi and B. miyamotoi serology has been previously reported [6], the extent of which depends on the antigen used for the assay. The enzyme glycerophosphoryl diester phosphodiesterase (GlpQ) has been used to effectively distinguish between B. miyamotoi and B. burgdorferi, while demonstrating opportunities for diagnostic sensitivity improvements. GlpQ-based serology assays have been reported to reach B. miyamotoi infection detection sensitivity of 54-97% and 38-87% for IgM and IgG, and specificity of 97-100% and 92-100% for IgM and IgG, respectively [18-20]. The inclusion of additional B. miyamotoi antigens, such as variable major proteins (Vmps), increased the sensitivity to 79-100% for IgM and 87-98% for IgG while maintaining high specificity [21,22]. While the incorporation of Vmps in B. miyamotoi serology assays has demonstrated efficacy in enhancing accuracy [23], the selection of specific Vmp markers is of paramount importance due to the homology between B. miyamotoi variable large proteins Vlps and the B. burgdorferi s.l. outer membrane Vmp-like sequence expressed (VlsE) protein [24,25]. In fact, C6 ELISA cross-reactivity was observed in both B. miyamotoi infected mice and humans. In order to overcome these issues, novel epitopes are currently being investigated, and the surface lipoprotein BmaA exhibited favorable IgG serology performance in both murine models and PCR-confirmed B. miyamotoi patients, with minimal cross-reactivity in LD patients [24].
The two Borrelia species demonstrate shared ecological niche and infection mechanisms, such as tick transmission, antigenic variation, immune evasion, systemic dissemination, induction of inflammation, and persistence over time [26]. When characterizing the tick ecology, scientists have determined that B. burgdorferi is more prevalent than B. miyamotoi in the same geographical area (up to a ratio of 10:1), while infected ticks tend to have a higher amount of B. miyamotoi organisms in their tissues [26]. One hypothesis to explain this behavior is that B. burgdorferi is capable of persisting in the skin of its vertebrate host for a longer period, thus possibly extending the transmission window to feeding ticks [26]. Although the two species share the same ecological niche and have overlapping nutritional requirements, they appear to have no antagonistic or synergistic relationship but rather a neutral one. A coexistence characterized by indifference, where presence and activities do not significantly affect one another’s survival might be supported by the following evidence. Studies showed that their seasonal peaks in rodents (spring for B. burgdorferi and summer for B. miyamotoi), tick transovarian transmission capability (prevalent in B. miyamotoi, very rare in B. burgdorferi), and tick tissue colonization (salivary gland for B. miyamotoi and midgut for B. burgdorferi) differ [26].
Available data on B. miyamotoi serology prevalence are reviewed in a recent meta-analysis of published data encompassing more than 45,000 unique individuals and 504 well-described cases of B. miyamotoi infection. The study revealed that the aggregated B. miyamotoi seroprevalence rate in the general population consisting of ~90% of participants at risk and 10% healthy controls was 4.4% (min = 0–max =25.6) [30]. The seroprevalence rates in symptomatic individuals suspected of having a tick-borne disease and in asymptomatic individuals with no active B. miyamotoi infection (controls) were 11.9% (min = 2.0 – max = 25.6) and 1.3% (min = 0–max = 6.9), respectively [30]. In the symptomatic individuals suspected of having a tick-borne disease analyzed in this study, B. miyamotoi seropositivity was 33%, which was greater than that determined in the meta-analysis of similar populations (one-sample Z-test for proportions Z=5.29, p < 1.2e-07) and healthy controls (one-sample Z-test for proportions Z=22, p < 1e-07).
A seropositivity rate of 33% is also in line with a recently published paper by Delaney et al., which analyzed 82 patients seeking consultation for long-term sequelae after suspected tick-borne illness and found a seropositivity rate of 26% [6]. There, patients with positive B. miyamotoi serology exhibited significantly greater sleepiness and pain than patients who were only positive for Lyme disease serology. In our study, serological positivity for either Lyme disease or B. miyamotoi was not associated with higher GSQ-30 symptom severity scores (coefficient -5.0, p = 0.174; coefficient -4.1, p = 0.412, respectively). In contrast, urinary Borrelia peptides showed a significant positive correlation with GSQ-30 symptom scores (Figure 4, coefficient 0.31, p<0.001). The high incidence of B. miyamotoi seropositivity in this study and in [6] merits attention. B. miyamotoi infection is a serious medical issue, particularly due to its potential for severe complications like meningoencephalitis [27-29]. B. miyamotoi infection’s non-specific symptoms and the need for laboratory confirmation make it a challenging diagnosis [29]. Although effective treatment options are available [26,30,31], the fact that clinicians do not routinely recommend B. miyamotoi serology to their patients might lead to missed diagnoses and delays in care [6].
Extracellular vesicles have been recognized for their potential as biomarker sources in cancer and other infectious diseases [32,33] because their molecular composition reflects the physiological status of the producing cell [32], and they contain cell-specific biomolecules. Borrelia BEVs have been reported to contain bacterium-specific proteins such as outer surface proteins [34]. Significant technical challenges, such as BEV small size and limited quantities in bodily fluids, have hindered the routine measurement of BEV-associated molecules in urine and other bodily fluids for research and diagnostic purposes [33]. In this clinical study, however, we demonstrated that enhanced proteomic and immunoassay workflows afford analytical sensitivity sufficient to detect BEV-associated molecules in urine.
The presence of BEVs in urine has been supported by repeated experimental evidence over the last 10 years [35]. The heterogeneous urinary vesicle population originates from different urogenital tract cell types and resident immune cells, bacteria, and yeasts. Additionally, BEVs from non-urogenital organs have been reported to enter urine from blood circulation [35]. Microbial-specific markers have been measured in the urinary vesicles of human subjects affected by pulmonary tuberculosis and allergic disease of the airways [8,36-38] either originating from pathogens or microbiota. In this study, we detected urinary Borrelia-specific molecules that have been previously associated with Borrelia BEVs: glycerophosphodiester phosphodiesterase, BAN67553, variable outer membrane protein, WP_012538010, variable small protein, WP_015633341, variable large protein, WP_025444237, Mlbp family lipoprotein, WP_119024480, Bdr protein, ACH93789, and p66 (bb0603) [14,15]. The functional significance of these proteins resides in their roles in the spirochete’s ability to evade the immune system and persist in the host, with emphasis on energy metabolism, membrane remodeling, and tissue invasion. Glycerophosphodiester phosphodiesterase (GlpQ, BAN67553) is involved in glycerol metabolism, mainly in the conversion of glycerophosphodiesters into sn-glycerol 3-phosphate and alcohol. This reaction helps the bacteria utilize phospholipids from its host environment for energy production [39,40]. Variable major proteins (Vmps, WP_015633341, WP_025444237) including variable large proteins (Vlps) and variable small proteins (Vsps) contribute to antigenic variability and membrane integrity [41]. Variable outer membrane proteins (WP_012538010) functions as adhesins, thus mediating the first step in tissue invasion. These adhesins can bind to specific host cell receptors, components of the extracellular matrix, or plasma proteins. Tissue adhesion protects the spirochete from physical defenses of the host [42]. The multicopy lipoprotein (Mlp) Family Lipoprotein (WP_119024480) belongs to a group of membrane-associated proteins that support the structural integrity of the bacterial membrane and mediate interactions with the host immune system, thus mediating immune evasion and pathogenesis. The Borrelia direct repeat (Bdr) protein (ACH93789) belongs to a family of proteins that are thought to be involved in the regulation of gene expression and possibly in the adaptation to different environmental conditions. Borrelia p66 (bb0603) functions both as an integrin-binding adhesin and as a porin, is produced during mammalian infection [43], and has a role in dissemination and vascular transmigration, tissue colonization and tissue persistence [44,45]. P66 is a β-barrel protein that forms a unique oligomeric transmembrane structure of 7-8 monomeric subunits versus the more common 3 monomeric units in the vast majority of Gram-negative bacteria [44]. These proteins may contribute to the ability of the pathogen to persist in the host by modulating the expression of virulence factors and other important proteins. All these proteins are immunogenic, and in some instances, are used for clinical diagnosis. GlpQ [19] and Vmps [19] are utilized for the serological diagnosis of Borrelia miyamotoi disease. Variable outer membrane proteins such as OspA, OspC, VlsE, and p66 are used as antigens for Lyme disease serology [44,46,47]. The Borrelia burgdorferi Mlp paralogous family is also immunogenic in the vertebrate host and it is divided into two antigenic classes: class I (masses of 18 to 23 kDa), and class II Mlps (13 to 15 kDa) [48]. Finally, the B. burgdorferi Bdr proteins, while not commonly used as primary targets in serological tests for Lyme disease, can elicit an antibody response during human infection. In a study, 51% of Lyme disease patient sera had detectable antibodies to one or more Bdr proteins. For these proteins, cross-reactivity was observed across various Borrelia species, including B. burgdorferi, B. garinii, and B. afzelii [49]. Taken together, this evidence supports the systemic presence of Borrelia-derived BEVs in the body, their shedding into the urine, and their utility as diagnostic biomarkers.
Extracellular vesicles are recognized as key facilitators of intercellular communication, allowing the transfer of functionally active molecules between cells [32,34]. The BEV systemic circulation and the presence of immunogenic macromolecules derived from the cell wall in the Borrelia BEVs support a novel hypothesis on the etiology of PTLDS, the Borrelia BEVs might be the vehicle for the persistent antigenic debris that has been postulated to play a role in disease pathogenesis [50,51]. One of the farthest-reaching unanswered questions in the field of Lyme disease is the etiology of symptom persistence, months to years after patients suffer from acute Lyme disease. Two mechanisms have been proposed to explain persistent symptoms: 1) persistence of infection or antigenic debris and 2) persistence of improper immune activation and inflammation, or a combination thereof [50]. The mechanisms of Borrelia BEV production, their role in bacterial physiology, and host-pathogen interactions are largely unknown and a promising field of investigation. Macromolecules enclosed by or associated with lipid-bound vesicles are protected from degradation and persist in host tissues longer than soluble molecules [52]. This would contribute to explaining the unusually long persistence of antigenic debris in patients with PTLDS. If confirmed, the hypothesis that BEVs serve as vehicles for maintaining antigenic pressure in the human host would have significant implications for identifying future therapeutic targets. This could open new avenues for developing strategies to block BEV production or its interaction with human cells.
The practical implications of urinary peptide detection as a diagnostic tool and for disease monitoring purposes are profound. If validated, a direct assay targeting urinary peptides offers many advantages, including ease of biospecimen collection, specificity, sensitivity, and the ability to guide therapy. Direct tests can be used to monitor disease progression. By targeting a substance that is specific to the pathogenic microorganism, an infection can be identified at an early stage without waiting for a person to produce antibodies in response to the microorganism. In addition, a direct test can be used in individuals whose immune systems are unable to produce sufficient antibodies. We have published anectodical data supporting the ability of urinary peptides to detect B. burgdorferi infection at a very early stage prior to the development of erythema migrans and seroconversion [4]. By nature, a mass spectrometry assay targeting Borrelia-unique peptides affords high specificity because the analysis is precise at the amino acid level and the sequences are filtered to exclude peptides belonging to the human microbiome, Homo sapiens, and all organisms for which we have a database, which as of January 2025 are over 162,000 organisms (NCBI non-redundant database). The urinary peptide assay was calibrated to achieve 100% specificity in previous clinical studies, including 250 negative controls [4]. We have shown that proper preanalytical sample processing yields an analytical sensitivity of 1 pg/mL and a diagnostic sensitivity of 90% in a small cohort of acute Lyme disease patients [4]. The emphasis on urine as a diagnostic biospecimen opens up the possibility of point-of-need sample collection or testing [53]. We have developed a novel biomaterial that, when incorporated into a urine collection cup for room-temperature shipment and storage, maintains the precision of the downstream analytical platform [54]. This technology might enable a telehealth model including home sample collection coupled with room-temperature shipping and laboratory testing that simultaneously increases patient access and ensures highly accurate testing results. In the future, a highly sensitive urine assay can be deployed for point-of-care testing for rapid disease detection [55].
Conclusion
The B. miyamotoi seroprevalence (33%) in a symptomatic population with clinical suspicion of a tick-borne illness was higher than the estimated seroprevalence in the general population. As it provides an objective measurement correlated with a standardized symptom burden score, urinary Borrelia peptide detection offers a promising avenue for future assay development. This proteomic analysis supports the presence of Borrelia BEVs in the urine of symptomatic patients with suspected tick-borne illnesses.
References
2. Raffetin A, Saunier A, Bouiller K, Caraux-Paz P, Eldin C, Gallien S, et al. Unconventional diagnostic tests for Lyme borreliosis: a systematic review. Clin Microbiol Infect. 2020;26:51-9.
3. Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JWR, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090.
4. Magni R, Almofee R, Yusuf S, Mueller C, Vuong N, Almosuli M, et al. Evaluation of pathogen specific urinary peptides in tick-borne illnesses. Sci Rep. 2020;10:19340.
5. Fallon BA, Zubcevik N, Bennett C, Doshi S, Rebman AW, Kishon R, et al. The General Symptom Questionnaire-30 (GSQ-30): A Brief Measure of Multi-System Symptom Burden in Lyme Disease. Front Med. 2019;6:283.
6. Delaney SL, Murray LA, Aasen CE, Bennett CE, Brown E, Fallon BA. Borrelia miyamotoi Serology in a Clinical Population With Persistent Symptoms and Suspected Tick-Borne Illness. Front Med (Lausanne). 2020;7:567350.
7. Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, et al. Human Borrelia miyamotoi infection in the United States. N Engl J Med. 2013;368:291-3.
8. Su K-Y, Koh Kok J-Y, Chua Y-W, Ong S-D, Ser HL, Pusparajah P, et al. Bacterial extracellular vesicles in biofluids as potential diagnostic biomarkers. Expert Review of Molecular Diagnostics. 2022;22:1057-62.
9. Liang A, Korani L, Yeung CLS, Tey SK, Yam JWP. The emerging role of bacterial extracellular vesicles in human cancers. J Extracell Vesicles. 2024;13:e12521.
10. Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res. 2013;22:75-84.
11. Tamburro D, Fredolini C, Espina V, Douglas TA, Ranganathan A, Ilag L, et al. Multifunctional core-shell nanoparticles: discovery of previously invisible biomarkers. J Am Chem Soc. 2011;133:19178-88.
12. Douglas TA, Tamburro D, Fredolini C, Espina BH, Lepene BS, Ilag L, et al. The use of hydrogel microparticles to sequester and concentrate bacterial antigens in a urine test for Lyme disease. Biomaterials. 2011;32:1157-66.
13. Hejazian SM, Pirmoradi S, Zununi Vahed S, Kumar Roy R, Hosseiniyan Khatibi SM. An update on Glycerophosphodiester Phosphodiesterases; From Bacteria to Human. Protein J. 2024;43:187-99.
14. Templeton TJ. Borrelia outer membrane surface proteins and transmission through the tick. J Exp Med. 2004;199:603-6.
15. Shang ES, Skare JT, Exner MM, Blanco DR, Kagan BL, Miller JN, et al. Isolation and characterization of the outer membrane of Borrelia hermsii. Infect Immun. 1998;66:1082-91.
16. Piehowski PD, Petyuk VA, Orton DJ, Xie F, Moore RJ, Ramirez-Restrepo M, et al. Sources of technical variability in quantitative LC-MS proteomics: human brain tissue sample analysis. J Proteome Res. 2013;12:2128-37.
17. Anderle M, Roy S, Lin H, Becker C, Joho K. Quantifying reproducibility for differential proteomics: noise analysis for protein liquid chromatography-mass spectrometry of human serum. Bioinformatics. 2004;20:3575-82.
18. Molloy PJ, Telford SR, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, et al. Borrelia miyamotoi Disease in the Northeastern United States: A Case Series. Ann Intern Med. 2015;163:91-8.
19. Koetsveld J, Kolyasnikova NM, Wagemakers A, Stukolova OA, Hoornstra D, Sarksyan DS, et al. Serodiagnosis of Borrelia miyamotoi disease by measuring antibodies against GlpQ and variable major proteins. Clin Microbiol Infect. 2018;24:1338.e1-1338.e7.
20. Jahfari S, Sarksyan DS, Kolyasnikova NM, Hovius JW, Sprong H, Platonov AE. Evaluation of a serological test for the diagnosis of Borrelia miyamotoi disease in Europe. J Microbiol Methods. 2017;136:11-6.
21. Wagemakers A, Koetsveld J, Narasimhan S, Wickel M, Deponte K, Bleijlevens B, et al. Variable Major Proteins as Targets for Specific Antibodies against Borrelia miyamotoi. J Immunol. 2016;196:4185-95.
22. Stukolova O, Koetsveld J, Kolyasnikova N, Sarksyan D, Toporkova M, Karan L, et al. Antibody response in Borrelia miyamotoi infection studied by protein microarray. International Journal of Infectious Diseases. 2019;79:18.
23. Harris EK, Harton MR, de Mello Marques MA, Belisle JT, Molins CR, Breuner N, et al. Immunoproteomic analysis of Borrelia miyamotoi for the identification of serodiagnostic antigens. Sci Rep. 2019;9:16808.
24. Koetsveld J, Platonov AE, Kuleshov K, Wagemakers A, Hoornstra D, Ang W, et al. Borrelia miyamotoi infection leads to cross-reactive antibodies to the C6 peptide in mice and men. Clin Microbiol Infect. 2020;26:513.e1-513.e6.
25. Molloy PJ, Weeks KE, Todd B, Wormser GP. Seroreactivity to the C6 Peptide in Borrelia miyamotoi Infections Occurring in the Northeastern United States. Clin Infect Dis. 2018;66:1407-10.
26. Cutler S, Vayssier-Taussat M, Estrada-Peña A, Potkonjak A, Mihalca AD, Zeller H. A new Borrelia on the block: Borrelia miyamotoi - a human health risk? Euro Surveill. 2019;24:1800170.
27. Wagemakers A, Staarink PJ, Sprong H, Hovius JWR. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31:260-9.
28. Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631-9.
29. Jiang B-G, Jia N, Jiang J-F, Zheng Y-C, Chu Y-L, Jiang R-R, et al. Borrelia miyamotoi Infections in Humans and Ticks, Northeastern China. Emerg Infect Dis. 2018;24:236-41.
30. Hoornstra D, Azagi T, van Eck JA, Wagemakers A, Koetsveld J, Spijker R, et al. Prevalence and clinical manifestation of Borrelia miyamotoi in Ixodes ticks and humans in the northern hemisphere: a systematic review and meta-analysis. Lancet Microbe. 2022;3:e772-86.
31. Ahmad S, Kasper D, Rubio L, Marinez JE. 853. Rapid, non-invasive detection of Borrelia miyamotoi infection using a plasma-based microbial cell-free DNA sequencing test. Open Forum Infectious Diseases. 2023;10:ofad500.898.
32. Morales R-TT, Ko J. Future of Digital Assays to Resolve Clinical Heterogeneity of Single Extracellular Vesicles. ACS Nano. 2022;16:11619-45.
33. Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM, et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881-906.
34. Shoberg RJ, Thomas DD. Specific adherence of Borrelia burgdorferi extracellular vesicles to human endothelial cells in culture. Infect Immun. 1993;61:3892-900.
35. Erdbrügger U, Blijdorp CJ, Bijnsdorp IV, Borràs FE, Burger D, Bussolati B, et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J Extracell Vesicles. 2021;10:e12093.
36. Dahiya B, Khan A, Mor P, Kamra E, Singh N, Gupta KB, et al. Detection of Mycobacterium tuberculosis lipoarabinomannan and CFP-10 (Rv3874) from urinary extracellular vesicles of tuberculosis patients by immuno-PCR. Pathogens and Disease. 2019;77:ftz049.
37. Samra MS, Lim DH, Han MY, Jee HM, Kim YK, Kim JH. Bacterial Microbiota-derived Extracellular Vesicles in Children With Allergic Airway Diseases: Compositional and Functional Features. Allergy Asthma Immunol Res. 2021;13:56.
38. Tulkens J, De Wever O, Hendrix A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat Protoc. 2020;15:40-67.
39. Skare JT, Mirzabekov TA, Shang ES, Blanco DR, Erdjument-Bromage H, Bunikis J, et al. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654-61.
40. Shang ES, Skare JT, Erdjument-Bromage H, Blanco DR, Tempst P, Miller JN, et al. Sequence analysis and characterization of a 40-kilodalton Borrelia hermsii glycerophosphodiester phosphodiesterase homolog. J Bacteriol. 1997;179:2238-46.
41. Brissette CA, Cooley AE, Burns LH, Riley SP, Verma A, Woodman ME, et al. Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int J Med Microbiol. 2008;298 Suppl 1:257-67.
42. Cullen PA, Haake DA, Adler B. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol Rev. 2004;28:291-318.
43. Lahey LJ, Panas MW, Mao R, Delanoy M, Flanagan JJ, Binder SR, et al. Development of a Multiantigen Panel for Improved Detection of Borrelia burgdorferi Infection in Early Lyme Disease. Munson E, editor. J Clin Microbiol. 2015;53:3834-41.
44. Curtis MW, Fierros CH, Hahn BL, Surdel MC, Kessler J, Anderson PN, et al. Identification of amino acid domains of Borrelia burgdorferi P66 that are surface exposed and important for localization, oligomerization, and porin function of the protein. Front Cell Infect Microbiol. 2022;12:991689.
45. Bernard Q, Thakur M, Smith AA, Kitsou C, Yang X, Pal U. Borrelia burgdorferi protein interactions critical for microbial persistence in mammals. Cellular Microbiology. 2019 Feb;21(2):e12885.
46. Liang FT, Alvarez AL, Gu Y, Nowling JM, Ramamoorthy R, Philipp MT. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol. 1999;163:5566–73.
47. Lone AG, Bankhead T. The Borrelia burgdorferi VlsE Lipoprotein Prevents Antibody Binding to an Arthritis-Related Surface Antigen. Cell Rep. 2020;30:3663-70.e5.
48. Yang XF, Hübner A, Popova TG, Hagman KE, Norgard MV. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect Immun. 2003;71:5012-20.
49. Zückert WR, Meyer J, Barbour AG. Comparative Analysis and Immunological Characterization of the Borrelia Bdr Protein Family. Orndorff PE, editor. Infect Immun. 1999;67:3257-66.
50. Bobe JR, Jutras BL, Horn EJ, Embers ME, Bailey A, Moritz RL, et al. Recent Progress in Lyme Disease and Remaining Challenges. Front Med (Lausanne). 2021;8:666554.
51. Sapi E, Kasliwala RS, Ismail H, Torres JP, Oldakowski M, Markland S, et al. The Long-Term Persistence of Borrelia burgdorferi Antigens and DNA in the Tissues of a Patient with Lyme Disease. Antibiotics (Basel). 2019;8:183.
52. O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585-606.
53. Lei R, Huo R, Mohan C. Current and emerging trends in point-of-care urinalysis tests. Expert Rev Mol Diagn. 2020;20:69-84.
54. Cornero R, Irfan SS, Cachaco S, Zhou W, Byne A, Howard M, et al. Identification of Unambiguous Borrelia Peptides in Human Urine Using Affinity Capture and Mass Spectrometry. Methods Mol Biol. 2024;2742:105-22.
55. Markowitz MA, Monti GK, Kim J-H, Haake DA. Rapid diagnostic testing in the management of urinary tract infection: Potentials and limitations. Diagn Microbiol Infect Dis. 2019;94:371-7.