Abstract
Babesia is a single celled protozoan parasite which may be transmitted through the bite of an infected tick, blood transfusion and/ or maternal-fetal transmission. We describe the case of a woman previously treated for Lyme disease and babesiosis who relapsed with severe malaria-like symptoms during the 3rd trimester of two consecutive pregnancies. Testing for active babesiosis was positive in both pregnancies despite prior conventional therapies, and she was subsequently treated with high dose atovaquone (1500 mg PO BID) and Zithromax during each of her 3rd trimesters, along with clindamycin during her 3rd pregnancy. At term, neonates were healthy with no clinical evidence of active babesiosis (no fever, acute respiratory distress syndrome [ARDS], hepatosplenomegaly or jaundice) and no laboratory evidence of babesiosis (no positive blood smears, hemolytic anemia, neutropenia or thrombocytopenia). Intramuscular benzathine penicillin was simultaneously used throughout her four pregnancies to prevent transmission of Borrelia burgdorferi, the agent of Lyme disease, with no evidence of transmission of the spirochete to the fetuses. Although this novel Lyme and Babesia therapy was effective in preventing congenital transmission of Borrelia burgdorferi and Babesia microti in consecutive pregnancies, future studies are needed to define the optimal management strategy for pregnant patients with Lyme disease and babesiosis due to the possible relapsing, remitting nature of the disease and the potential for associated morbidity and mortality.
Introduction
The genus Babesia comprises over 100 species of tick-transmitted protozoal intraerythrocytic pathogens (piroplasms) [1], causing malarial-type illness. The most common human pathogens in the United States are B. microti [2] and Babesia duncani (WA- 1) [3]; Less common species include Babesia MO-1 [4] and KO-1 [5], as well as Babesia divergens and Babesia venatorum (EU-1) in Europe [6]. Although transmission of Babesia spp. is primarily through the bite of an infected tick [7], transmission through blood transfusion [8-10], solid organ transplantation [11], and maternal-fetal transmission [12,13] have all been well documented. Congenital transmission of babesiosis has been reported in 8 prior case studies [13-20], where most infants presented with fever, anemia and thrombocytopenia and required blood transfusions [13].
We present the case of a woman with a well-documented history of infection with B. burgdorferi, the agent of Lyme disease, who was co-infected with B. microti before and during her pregnancy. Despite traditional antibiotic and antimalarial therapy for Lyme disease and babesiosis she remained ill. At the beginning of her third trimester, and during two consecutive pregnancies, she complained both times of the sudden onset of drenching sweats, flushing, and chills along with worsening fatigue and headaches. Retesting for babesia showed evidence of an active infection in both pregnancies despite prior treatments with clindamycin [21], and atovaquone and azithromycin [22], both established regimens for the treatment of babesiosis [23]. She was subsequently rotated to a combination regimen of higher dose atovaquone (1500 mg PO BID) and azithromycin, along with a 10-day course of clindamycin during her third pregnancy. At birth, all neonates were asymptomatic with healthy Apgar scores and no clinical or laboratory signs of active babesiosis. Published medical literature on treatment for babesiosis during pregnancy recommends either clindamycin and quinine [24], or atovaquone and azithromycin [25], with variable outcomes in neonates, oftentimes resulting in thrombocytopenia and hemolytic anemia requiring a blood transfusion [17]. It is therefore imperative to find novel therapies that are safe and effective. Our case report is to our knowledge the first to describe the use of a novel combination of Lyme disease and babesiosis therapies during pregnancy which were well tolerated and beneficial in the mother, without clinical or laboratory signs of active infections of B. burgdorferi or B. microti in the newborns.
Case History
A 27-year-old white female with a past medical history significant for Lyme disease, babesiosis, Helicobacter pylori, hypothyroidism, neurocardiogenic syncope, celiac disease, food allergies, migraines, irritable bowel syndrome and multiple chemical sensitivity came to see us with the chief complaints of symptomatic fatigue, headaches, and multiple joint pains. She had a history of chronic joint pain for several years of unknown etiology, followed by getting a tick bite and erythema migrans rash while living in Europe. Her ELISA and IgM Western blot became CDC positive after her tick bite (positive 23 KDA [OspC], 39 KDA, 41 KDA), along with a positive B.microti IgM titer (1:160+), and despite prompt treatment she remained ill and symptomatic for years. She then moved to the West Coast of the United States and underwent treatment with rotations of antibiotics for a chronic relapsing H. pylori infection, which included several courses of amoxicillin, metronidazole, and clarithromycin. Her G.I. symptoms eventually improved. She subsequently received treatment for her Lyme disease and babesiosis, which included rotations of antibiotics including doxycycline + mefloquine; atovaquone + telithromycin; as well as metronidazole + clarithromycin. Despite these antibiotic rotations, there was no clinical improvement in her resistant fatigue, headaches or joint pain.
The patient and her family then moved to the East Coast of the United States and came to our medical center for a diagnostic evaluation. At the time of her initial visit, her chief complaints included: daily day and night sweats which were drenching; constant hot flashes and chills; chronic fatigue; sore throat; hair loss; the sensation of “air hunger” and shortness of breath, both at rest and exertion; chest pain with tenderness to palpation of the chest wall; palpitations, which improved with the use of metoprolol; migratory joint pain which was worse in her shoulders, neck, ankles, knees, and bilateral great toes; soreness of the soles of her feet; myalgias in her upper and lower extremities; constant tingling and numbness of her fingers and toes, which increased with the use of atovaquone and clarithromycin, but not metronidazole; occasional paresthesias of the face; blurry vision and photosensitivity; sound sensitivity and increased sensitivity to smells; dizziness with episodes of vertigo; tinnitus; hearing loss (prior to antibiotic use); memory and concentration problems with word finding problems and emotional instability. She felt that she was functioning at 30 to 40 percent of her normal level due to the severity of these symptoms.
Social history and family history were unremarkable. One brother was alive and well with a history of syncopal episodes. She grew up around cats as pets. Allergies included dermatological reactions to nickel (rash) and intolerance to azithromycin and metronidazole (nausea), mefloquine (nausea, weakness, visual hallucinations), and cortisone injections (anaphylactic shock). Medications at the time of her initial visit included clarithromycin 500 milligrams PO BID and metronidazole 500 milligrams BID along with metoprolol 25 milligrams QD; Armour Thyroid 15 milligrams QD; a baby aspirin, meloxicam 15 milligrams QD for pain and a birth control pill (microgestin 1/20 QD).
Review of systems was negative except for previously described symptoms as well as occasional edema of the lower extremities; intermittent dysphagia, gas and stomach pain; rare yeast infections with urinary frequency; muscle weakness; eczema; anxiety and multiple syncopal episodes.
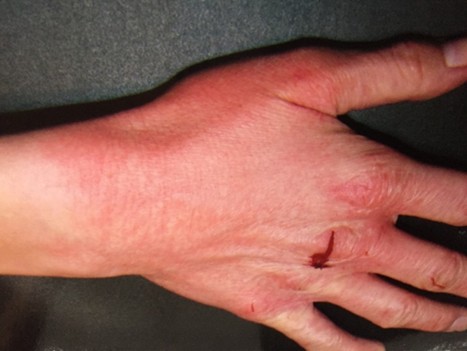
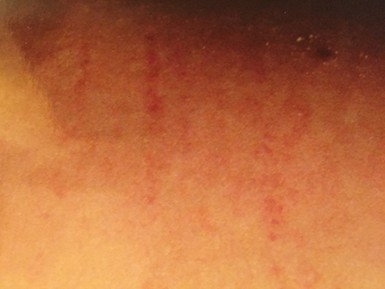
Physical examination revealed a well-developed, wellnourished white female in no apparent distress. She was afebrile. Her blood pressure was 118/74 with a pulse of 74 beats per minute (regular), and respiratory rate of 16 per minute. Physical exam was unremarkable except for cool, mottled extremities consistent with probable dysautonomia; dry skin of her hands with a bilateral erythematous, violaceous rash, consistent with acrodermatitis chronicum atrophicans. (Figure 1) and reddish streaks and striae on various parts of her body which were perpendicular to skin planes (Figure 2), suggestive of possible bartonellosis.
Figure 1. Acrodermatitis chronica atrophicans rash, June 2007.
Figure 2. Striae, June 2007.
Laboratory evaluation was significant for a Centers for Disease Control and Prevention (CDC) positive IgM Western blot and B.microti IgM titer which was positive at 1:160, positive Babesia fluorescent in situ hybridization test (FISH+, RNA testing) despite 6 months of taking atovaquone and telithromycin before our initial visit, prior exposure to Mycoplasma pneumonia (1.45 ISR, normal less than 0.90), low C3 serum complement levels (81 micrograms/deciliter; normal range 90 to 180 mg/dl), low C4 serum complement levels (11 micrograms/deciliter; normal range 16 to 47 mg/dl), elevated C4a complement levels (25,425 nanograms/milliliter; normal range from 0 to 2830 ng/ml), low serum glucose (60 milligrams/ deciliter, normal range 65 to 99 mg/dl, consistent with reactive hypoglycemia), low cortisol levels at noon, and a positive tissue transglutaminase (TTG), confirming the history of celiac disease. Measurement of omega-3 fatty acids showed elevated levels of omega-3 fatty acids with low polyunsaturated Omega 6 fatty acid levels, including low levels of arachidonic acid (317, normal range 330-633). A dimercaptosuccinic Acid (DMSA) urine toxic metal test was performed for her resistant symptoms and was positive for exposure to mercury and nickel. A comprehensive digestive stool analysis (CDSA) was done for her irritable bowel syndrome (IBS) symptoms which revealed low levels of Lactobacillus and Bifidobacterium, moderate levels of candida krusei and trichosporin species, as well as high levels of gram-negative bacteria, including alpha hemolytic streptococcus, gamma hemolytic streptococcus and Klebsiella pneumonia.
She was negative for antibodies to Babesia duncani, Anaplasma phagocytophilum (human granulocytic anaplasmosis, HGA), Ehrlichia chaffeensis (human monocytic ehrlichiosis, HME), Bartonella henselae, Bartonella quintana, Rickettsia rickettsii (Rocky mountain spotted fever), Coxiella burnetti (Q fever), chlamydia pneumonia, H. pylori, M. fermentans (PCR negative), active viral infections (negative PCRs for HSV-1, HSV-2, HHV6, CMV, EBV), toxoplasmosis, gastrointestinal parasites (negative ova and parasites on stool examination), or active fungal infections (negative PCR’s for Aspergillus fumigatus, cryptococcus neoformans or trichosporin).
Blood tests revealed a normal complete blood count (CBC), comprehensive metabolic profile (CMP), thyroid functions, Dehydroepiandrosterone (DHEA) sulfate level, growth hormone (GH) level (normal insulin like growth factor 1 [IGF-1], which varied over time), B12, methylmalonic acid (MMA), homocysteine and folic acid levels, immunoglobulin levels and subclasses, mineral levels (copper, zinc, magnesium, iron), C-reactive protein (CRP), sedimentation rates, creatinine phosphokinase (CPK) and rheumatoid factors, anti-myelin associated glycoproteins (anti- MAG antibodies), along with a normal IgE level and IgE food allergy panel. IgG 4 food antibody levels however were positive for mild allergies to rye, peanuts, almonds, sesame, sunflower, avocado, garlic, mustard and green peppers with moderate reactions to wheat, soy, and eggs. These results were consistent with the diagnosis of leaky gut. Over time, Epstein-Barr virus and herpesvirus 6 titers became low positive, but without a fourfold increase in titers.
Review of prior outpatient testing revealed a possible episode of ventricular tachycardia while having an IV placed for a tilt table test, to rule out Postural Orthostatic Tachycardia Syndrome (POTS) so she was sent for a cardiology evaluation and cardiac stress test, which showed normal myocardial perfusion and no significant cardiac abnormalities. A 24-hour Holter monitor revealed extra beats without significant arrhythmias. She did intermittently have evidence of hypotension, dropping her blood pressure from 100/64 to 85/55 with a 14 point increase in heart rate and lightheadedness, consistent with the diagnosis of moderate POTS. A brain SPECT scan showed severe hypoperfusion. A fivehour glucose tolerance test was also performed for the history of fatigue, lightheadedness, palpitations and syncope with the history of a low glucose level one time, which revealed a hypoglycemic response during the 4th hour when the blood sugar dropped to 53 milligrams/ deciliter (normal range 65-99 mg/dl) and the patient became symptomatic. She was subsequently placed on a low carbohydrate diet with small frequent meals and adequate levels of protein, which helped stabilize postprandial fatigue. Her endocrinologist also placed her on a small dose of hydrocortisone (Cortef, 5 mg/day) for her low adrenal function, with mild improvement in fatigue and stamina.
Since the patient had never used a cell wall antibiotic (penicillins, cephalosporins) for Lyme disease and complained of significant neurocognitive symptoms, she was placed on IV ceftriaxone (Rocephin) 2 grams per day, ursodiol (Actigall) 300 milligrams PO BID, atovaquone (Mepron) 750 milligrams, 2 teaspoons PO BID with a high-fat meal, clarithromycin XL 500 milligrams BID, hydroxychloroquine (Plaquenil) 200 milligrams PO BID and nystatin 500,000 units tablets, 2 PO BID. Despite this protocol, she complained of daily drenching sweats, severe fatigue, increased joint and muscle pain, headaches, and worsening cognitive impairment. Bactrim double strength (sulfamethoxazole/trimethoprim) one PO BID was therefore added to the regimen, along with extra detoxification support, including IV glutathione 3 days per week. She reported improvements days that she was on IV glutathione and her sweats and chills became less frequent with the addition of Bactrim to the regimen. Herxheimer reactions however continued the days on IV ceftriaxone, despite trying to pulse the IV medication 4 days in a row per week, so she was subsequently taken off this protocol, and switched to IV doxycycline 200 milligrams per day, infused over a 2 hour time period along with Plaquenil, nystatin, and rifampin 300 milligrams PO BID. Day and night sweats, chills, and peripheral neuropathy symptoms increased off her atovaquone and Rocephin, so she was rotated to clindamycin 300 milligrams, 2 capsules 3 × per day, atovaquone 2 teaspoons PO BID along with clarithromycin 500 milligrams BID for her babesiosis. High dose B12 injections were also started for the resistant neuropathy along with alpha lipoic acid 600 milligrams QD with B6 50 milligrams TID.
After one month, the only improvement she experienced was a decrease in sweats for one to two days, which subsequently increased in intensity with severe fatigue, severe cognitive dysfunction, headaches, leg pain, myalgias and peripheral neuropathy. She was therefore rotated to doxycycline, ciprofloxacin 500 milligrams PO BID and atovaquone/proguanil to 50/100 milligrams, 4 tablets per day, with low dose mefloquine (250 milligrams Q5 days) for the possibility of resistant bartonellosis along with Lyme disease and babesiosis, but unfortunately this regimen was ineffective in significantly relieving symptomatology, so all antibiotics were stopped, and the patient was placed on a yeast-free, sugar-free diet with an herbal protocol for her tickborne diseases. Herbals included Samento (cat’s claw), Cumunda, Enula, Burbur, and parsley. Within days of being off her antibiotics, all symptoms worsened including severe depression where she was crying for hours at a time, so she was placed back on a Babesia protocol, including atovaquone, clarithromycin and Bactrim DS twice a day. This immediately helped resolve the mood swings and decreased associated fevers, sweats, chills, and palpitations.
At the end of 2008, after several months on this protocol, the patient decided to try getting pregnant. Options were discussed for her pregnancy including oral penicillin’s, cephalosporins or intramuscular benzathine penicillin (IM Bicillin) with azithromycin which have all been shown to be safe for the fetus [26]. She chose intramuscular Bicillin 1.2 million units IM twice a week with EMLA cream one hour prior to the injection. She was to stay on atovaquone, clarithromycin and Bactrim, which were controlling her Babesia symptoms until she got pregnant. Over the next few months, the Bicillin injections helped her to feel better with improvement in energy and cognitive functioning, and eventually the Bactrim was stopped. One month later, the patient became pregnant and tapered off clarithromycin and nystatin but stayed on low-dose hydrocortisone (2.5 milligram BID) and Armour thyroid (dosage of 90 mg/day) as per her endocrinologist, along with Bicillin and azithromycin (Zithromax). She followed the pregnancy with a highrisk OB/GYN at Yale who placed her on a prenatal multivitamin with folic acid and extra vitamin D (7000 IU/d) for subtherapeutic vitamin D levels, and her Armour thyroid was increased to 120 mg/day in the third trimester for borderline elevated levels of TSH (3.04 microIU/ml, optimum range below 2.5 microIU/ml). IgF1 levels were intermittently low (121 ng/ml, normal range 138-410 ng/ml), suggestive of growth hormone (GH) deficiency, but due to normal fetal growth, levels were followed. A beta blocker, labetalol, proven to be safe in pregnancy [27], was also prescribed at 100 mg PO BID and was effective in controlling palpitations. She carried the fetus to term and gave birth to a healthy baby girl by vaginal delivery, with no signs or symptoms of active Lyme or babesiosis. Real time Polymerase chain reaction (PCR) testing of the placenta and cord blood was negative for B. burgdorferi or B. microti. The patient began breast feeding, and breast milk was also checked for any evidence of B. burgdorferi, which was negative. A subsequent IgF1 level became normal (147, normal range 114-492) and a glucagon stimulation test indicated normal GH production (13.2 ng/ml, peak GH of 3 ng/ml or higher being normal). 25-OH Vitamin D levels also became normal (60 ng/ml, range 20-100 ng/ ml) over time but 1,25 OH Vit D levels were elevated at 153 pg/ml (normal range 19-67 pg/ml). Vitamin D dosing was decreased accordingly.
Shortly after giving birth and during the period of breast feeding for approximately one year, the patient noticed increased symptoms of resistant fatigue and sweats. She had remained on Bicillin injections during this time, which were increased post-partum to 2.4 million units twice a week (total of 4.8 million units) for resistant fatigue, which improved with the higher dose of intramuscular penicillin. Bactrim DS was added to Zithromax along with higher dose atovaquone (3 tsp PO BID) for resistant Babesia symptoms, which also improved symptomatology. Her perceived level of functioning increased from 30% to 65% of normal during that time, a significant improvement from prior baseline levels of functioning.
Approximately one year into breast feeding (10/2010) the patient became pregnant for the second time and all medications were stopped except for Bicillin injections and Armour thyroid. She was able to taper off low dose hydrocortisone without difficulty, since subsequent cortisol measurements were within normal limits. Labetalol, an alpha and beta blocker, was also stopped because of urinary urge incontinence which developed post-partum, which improved off the medication. The patient remained at 70% normal functioning during the second pregnancy without any yeast or diarrhea (she was continuously on three probiotics twice a day with Saccharomyces boulardii during the course of treatment) [28]. Resistant joint pain bilaterally in the wrists, knees and shoulders persisted, as did fatigue, dizziness, headaches, light and sound sensitivity and memory/concentration problems, which varied between mild to severe in intensity. Although palpitations were initially controlled off Labetalol, the medication needed to be re-added at half dose (50 mg BID) once extrasystoles returned later in the pregnancy. The labetalol was better tolerated at lower dosing without any associated incontinence, and no orthostatic intolerance was seen with the addition of the low dose beta blocker.
At term on 7/2011, the patient gave birth vaginally to a healthy baby boy. Again, B. burgdorferi Real-Time PCR of the placenta and B. burgdorferi Real-Time PCR and B. microti Real-Time PCR of the cord blood were negative without signs or symptoms of active babesiosis in the neonate. The patient also denied significant day or night sweats or air hunger during her second pregnancy or several months into the post-partum period (symptoms of active babesiosis), although these symptoms relapsed by 1/2012 when day sweats and night sweats returned several times a week. She felt that Bicillin injections remained her best Lyme disease treatment to date, despite resistant symptoms of fatigue, joint pain, and headaches as these symptoms often improved immediately post injection. Zithromax and rifampin were added post-partum (rifampin may be used while pregnant and breastfeeding, as there is no known risk of infant harm) [29], and the patient felt an improvement in her moods, fatigue and headaches with the addition of the 2nd intracellular medication. Dosing of rifampin higher than 150 milligrams PO BID caused significant Herxheimer reactions however, suggestive of an active intracellular infection, and any attempt to lower the Bicillin injections to 1.2 million units twice a week resulted in an increase in headaches and mood swings.
The patient remained stable on this regimen until 10/2012 when she had a syncopal episode post venipuncture. It was subsequently determined that she was pregnant for the third time. An ultrasound at 16 weeks was normal and she was again followed by her high-risk OB-GYN.
At the end of her second trimester her night sweats began to return with increased fatigue and headaches. Repeat testing for tick-borne diseases was performed and revealed an ongoing positive B.microti IgM antibody at 1:160 + (IgeneX laboratories), new positive Babesia duncani (WA-1) IgG immunofluorescent antibody at 1:256 + (Bioreference laboratory, normal reference range below 1:256). Testing for Coxiella burnetti (Q-fever), Brucella, Rickettsia rickettsii (Rocky mountain spotted fever), Bartonella henselae, and toxoplasma gondii were all negative. ANA, rheumatoid factor and sedimentation rates along with thyroid functions were also within normal limits. No other explanation for the sweats could be found, so a confirmatory blood test was sent to Mayo Clinic which showed a low positive IgM B. microti at 1:40+ with a negative PCR. A blood smear was sent to visualize parasites, but the specimen was deemed too old to be useful. A repeat Babesia FISH test was therefore sent to IgeneX laboratories as it provides a significant increase in sensitivity and specificity over standard Giemsa stained smears for the presence of intraerythrocytic parasites (piroplasms) in RBC’s [23,30]. The Babesia FISH test returned positive during the beginning of her third trimester indicating active babesiosis despite prior anti-Babesia therapies.
Laboratory blood tests during that time intermittently showed toxic vacuolation and granulations present in the white cells with myelocytes present, a mild anemia with a hemoglobin of 11.5 g/dl (normal range 12-16 g/dL), hemoglobin of 34.9 (normal range 36-46%), slightly low platelet counts ranging between 116,000/ UL and 130,000/UL (normal range 140-400,000/ UL) with normal liver and kidney functions. She was placed back on clindamycin 300 mg 2 PO BID for 10 days along with atovaquone 750 mg, two tsp PO BID with a high fat meal and azithromycin 250 mg PO BID throughout the third trimester. After one week on atovaquone the sweats significantly decreased and she felt an improvement in her overall symptomatology, although muscle and joint pains and neuropathy continued to be moderate to severe in intensity. She remained on Bicillin injections during this time and a long acting oral amoxicillin (Moxatag 775 mg) was added during the 3rd trimester as higher dose Bicillin injections helped decrease symptomatology in the past. This resulted in improvement in headaches and fatigue.
Her high-risk OB/GYN and neonatal infectious disease specialist were consulted, who agreed with the plan, and the patient gave birth to her 3rd child, a healthy girl, in the summer of 2013. Apgar scores were normal. Testing of the cord blood for B. burgdorferi (Lyme disease), B. microti, B. henselae, Bartonella quintana, and Mycoplasma species were all negative by qualitative and Real-Time PCR through Medical Diagnostic Laboratories (MDL). Placental biopsies were also negative by Real-Time PCR for Lyme disease, B. henselae and B. quintana. There was no clinical or laboratory evidence of infection in the neonate (lack of leucopenia, thrombocytopenia, hemolytic anemia, transaminitis) and the patient breastfed during the following year without any complications in the newborn.
During 2014, the patient continued to breastfeed and clinically improved as long as she stayed on Bicillin injections, atovaquone, azithromycin, and Bactrim DS with a low carbohydrate, Paleo diet which also helped stabilize her energy and headaches. By August 2014, she was functioning at 85% of normal functioning, a significant improvement from her baseline. However, each time she tried to lower the dose of Bicillin injections or Moxatag/probenecid for her Lyme disease, or discontinue atovaquone and Bactrim for Babesia, her fatigue, headaches, migratory joint pain and sweats significantly increased. A high salt diet and fluids helped decrease orthostatic changes.
The patient decided to try for her 4th child in the fall of 2014 after breastfeeding for 17 months and became pregnant by the end of December 2014. Cefixime (Suprax) 400 mg QD was added during the first trimester due to significant joint pain, which helped decrease the intensity. During her visit of 6/2015, at the end of her second trimester, all prenatal checkups were fine and higher dose Bicillin and cefixime were helping to decrease her joint pain. Although she complained of resistant fatigue and dizziness, there were no orthostatic changes or increases in heart rate during her physical examination, ruling out significant dysautonomia as a potential etiology.
The patient initially denied significant fever, sweats or chills, but did complain of ongoing “air hunger” and a cough, atypical symptoms of babesiosis. Based on these symptoms and her prior history of relapses with proof of active infection, repeat Babesia titers and a FISH test were performed, as well as repeat titers for other tickborne infections. Almost immediately upon returning to her home in the southeastern United States, and beginning her 3rd trimester, her sweats began to return, occasionally drenching in nature. Although she denied any new tick bites, her B. microti IgM titer had now increased to 1:320+, her IgG B.microti titer turned positive at 1:40+ while her Babesia FISH test remained positive. Repeat Western blots for Lyme disease revealed an increase in the 31 KDA band (outer surface protein A) and decrease of her 23 KDA band (outer surface protein C) on her IgM blot from baseline measurements.
All other tickborne titers were negative, including antibodies for Borrelia burgdorferi (negative C6 ELISA), relapsing fever borreliosis (Borrelia hermsii), Anaplasma phagocytophilum (HGA), Ehrlichia chaffeensis (HME), Rickettsia rickettsii (Rocky mountain spotted fever), Coxiella burnetti (Q-fever), Bartonella henselae, Bartonella quintana, and B. Abortus (Brucellosis). Thyroid functions also remained within normal limits and a vascular endothelial growth factor (VEGF) was negative (an indirect marker of Bartonella) [31]. CBC as well as kidney and liver functions also remained within normal limits, with no evidence of anemia, thrombocytopenia, or transaminitis.
The patient therefore was placed back on high dose Mepron (atovaquone) 2 teaspoons PO BID with azithromycin during her 3rd trimester for active relapsing babesiosis. Clindamycin, which had been added during her 3rd pregnancy, was held due to ongoing nausea. Symptoms significantly improved once high dose atovaquone was re-added to the protocol, with a significant decrease in both the frequency and intensity of headaches and decrease in sweats (which were primarily at night, 2 to 3 times per week).
She gave birth at term by vaginal delivery to a healthy boy in the fall of 2015, with normal Apgar scores and no signs or symptoms of babesiosis (lack of anemia, thrombocytopenia, transaminitis, hepatosplenomegaly or jaundice). Repeat real-time PCR’s of the cord blood for B. burgdorferi, B. microti, B. henselae, B. quintana, and qualitative PCRs for Mycoplasma species from MDL laboratories were all negative. Clindamycin and Bactrim needed to be added several months into breastfeeding however (the pediatrician concurred) when drenching night sweats returned. Despite a temporary increase in emotional lability with Bactrim, symptoms subsequently resolved.
Follow-up titers for babesiosis months later showed a further increase in B. microti IgM titers to 1:640+ (a fourfold increase from her initial titers) with the IgG B. microti titer turning negative, yet the patient remained stable on her regimen and baby continued to do well. To date, years later, all four children have remained healthy. This case report is to our knowledge the first one to describe the safe and effective use of high dose benzathine penicillin injections along with high dose atovaquone, azithromycin and clindamycin to prevent the perinatal transmission of Lyme disease and babesiosis in multiple consecutive pregnancies.
Discussion
Tick-borne diseases can pose significant risks to both the mother and fetus during pregnancy. Maternalfetal transmission has been reported for babesiosis [12-16], as well as multiple tick-borne bacterial infections, including B. burgdorferi (Lyme disease) [32-39], relapsing fever borrelia (B. hermsii) [40-41], A.phagocytophilum (human granulocytic anaplasmosis) [42], Ehrlichia chaffeensis (human monocytic ehrlichiosis) [43,44], and Rickettsia rickettsii (Rocky mountain spotted fever) [45,46]. Bartonella species have also been implicated in adverse fetal outcomes [47,48], whether vectored by a tick [49], or other modes of transmission [50].
Tick bites within 3 years preceding conception have been shown to be significantly associated with congenital malformations [51] and our patient had a history of chronic joint pain for several years after getting a tick bite and erythema migrans rash while living in Europe. She also had a bilateral erythematous, violaceous rash on her hands, consistent with acrodermatitis chronicum atrophicans (Figure 1). This is a chronic dermatological manifestation seen more often in women and associated with Borrelia afzelii in Europe [52]. In North America, although Lyme disease is primarily caused by B.burgdorferi sensu stricto and/or eight other sensu lato species (B. mayonii, B. americana, B. andersonii, B. bissettii, B. californiensis, B. carolinensis, B. garinii, and B. kurtenbachii) [53-58], in Europe the majority of cases of Lyme borreliosis are caused by Borrelia afzelii and Borrelia garinii leading to a broad range of clinical manifestations [59]. As our patient had a history of both classic dermatological skin lesions seen in Lyme disease, an EM rash and ACA, several borrelia species may have been involved in the chronicity of her illness.
Successes and failures of treating ACA and EM rashes with traditional antibiotics have been reported. In one study of ACA, B. burgdorferi DNA excretion was still positive after 12 months [60], and atypical forms of B. burgdorferi were visualized under the microscope with positive staining for borrelia genusspecific monoclonal flagellar antibody [61]. These may have represented in vivo morphologic variants of this bacterium [61], which have been shown to form round body forms [62,63], stationary phase cells and persister cells [64-66], as well as aggregates in biofilms [67-69], depending on environmental conditions and stressors [65,70]. Successful cultivation of spirochetes from skin lesions of patients with erythema migrans (EM) and Acrodermatitis chronica atrophicans with a disease duration of greater than 10 years also suggests that the spirochetes may survive in the human body for a considerable time [71]. This was confirmed in a study of recurrent erythema migrans despite extended antibiotic treatment [72]. In that study, persistent B. burgdorferi infection was found in the blood by polymerase chain reaction (PCR) while skin biopsy specimen contained Borrelia-compatible structures [72]. These findings are consistent with other published scientific literature showing persistence of B. burgdorferi despite seemingly adequate courses of antibiotics [73,74], where the bacteria can persist in various parts of the body where antibiotics may not easily penetrate. These include the ligamentous tissue [75], intracellular compartment and synovium [76,77], joints [78], joint fluid [79], spleen [80], eyes [81,82], neuronal and glial cells [83], endothelial cells and macrophages [84], and central nervous system [85]. Persistence in skin lesions in EM and ACA may be due to immune evasion [86], biofilms protecting the organism [68,87], as well as invasion of human skin fibroblasts by B. burgdorferi [88], where fibroblasts can protect the Lyme disease spirochete from the effect of antibiotics, including ceftriaxone [89], which the patient received early on in the course of her treatment.
A 2018 systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn reported significantly fewer adverse birth outcomes in women reported to have been treated for gestational Lyme disease (LD) (11%, 95% confidence interval [CI]) compared to those who were not treated during pregnancy (50%, 95% CI) providing indirect evidence of an association between gestational LD and adverse birth outcomes [90]. Among those fetal abnormalities noted, adverse outcomes with a possible association included syndactyly, cortical blindness, hydrocephalus, intrauterine fetal death [38], prematurity, rash in the newborn [91], as well as stillbirth [92] neonatal distress syndrome, intrauterine growth retardation, maternal toxemia of pregnancy and sudden infant death syndrome [38].
Despite these findings, prior reviews of the literature concerning the effects of gestational LD from case reports and series, epidemiologic studies, and experimental animal models have yielded conflicting results [93,94] and inconsistent evidence for adverse birth outcomes of gestational LD in the epidemiological research [90]. Also, no comparative studies on the effectiveness of different therapeutic approaches for LD are available regarding preventing vertical transmission in pregnancy [95], apart from a large series of pregnant women who had a favorable course and outcome using intravenous ceftriaxone [96]. What is clear however, is that gestational LD appears to be associated with a low risk of adverse pregnancy outcome, particularly with appropriate antibiotic therapy [93]. In one case study reported by Horowitz [97], a healthy woman with a history of Lyme disease who was previously treated with several months of antibiotics miscarried at week eighteen when she was no longer on antibiotics, and both the placenta and fetus were positive for PCR evidence of B. burgdorferi. As per the CDC, “Lyme disease acquired during pregnancy may lead to infection of the placenta and possible stillbirth; however, no negative effects on the fetus have been found when the mother receives appropriate antibiotic treatment” [98].
According to US Preventative Services Task Force, all women should be screened serologically early on in pregnancy for syphilis, another spirochetal infection [99], due to the possibility of stillbirth, neonatal death and neurologic impairment [99]. These are some of the same symptoms reported in congenital Lyme disease, another spirochetal infection [38,92]. Treatment of syphilis involves intramuscular injection of long acting Benzathine penicillin G (2.4 million units administered intramuscularly) for primary, secondary or early latent syphilis and has been found to be effective [100]. Since B. burgdorferi has been found in a newborn despite the use of oral penicillin for Lyme borreliosis during pregnancy [34], and as Bicillin injections have been found effective in the treatment of both Lyme arthritis [101,102] and other resistant symptoms of LD (chronic fatigue, myalgias, arthralgias, headaches, paresthesias and cognitive difficulties) unresponsive to oral therapy [103], we decided to institute IM benzathine penicillin after an informed discussion of the risks and benefits.
Our patient was on intramuscular benzathine penicillin (Bicillin) throughout her four pregnancies, ranging from 2.4 to 4.8 million U IM/week with no adverse outcomes in the fetus, and the route of administration of antibiotic therapy has been shown to play a role in pregnancy outcomes. In a study of 95 women with Lyme borreliosis during pregnancy, adverse outcomes were seen in 12.1% of parentally treated women, 31.6% of orally treated women, and 60% untreated women [104]. In comparison to patients treated with antibiotics, untreated women had a significantly higher risk of adverse pregnancy outcome (odds ratio [OR] 7.61, p=0.004). The advantage of benzathine penicillin intramuscularly is that parenteral penicillin therapy has been shown to be effective in the treatment of Lyme disease and Lyme arthritis [101,102], and as opposed to oral regimens with varying peak and trough levels, sustained therapeutic levels of benzathine penicillin may be effective by inhibition of germ replication or by lysis of the spirochetes leaving their sanctuaries [102]. This may have contributed to healthy outcomes in all four newborns.
Despite ongoing treatment, our patient had frequent complaints of symptoms of fatigue, migratory joint pain, neuralgia, severe headaches, and cognitive difficulties. These are all part of the classic constellation of symptoms seen with Lyme disease [105,106], although it is possible that she also suffered from occult bartonellosis with her history of unexplained striae (Figure 2), Herxheimer flares with rifampin, high 1-25 OH/25 OH Vitamin D ratios (consistent with an active intracellular infection) [107,108], and resistant symptoms. The type of striae seen on her physical examination have been found to be associated with bartonella [109,110], and laboratory testing in New York only allowed evaluation of two species, B. henselae and B. quintana, although 36 different Bartonella species have now been discovered [50], and at least seventeen of them have been associated with an expanding spectrum of human diseases [111]. Bartonella may complicate and worsen clinical presentations. Bartonella species were recently found in 46.6% of Lyme patients with rheumatological symptoms in one case series [112], and 46.5% of neurologically impaired Lyme patients in a recent 2019 retrospective chart review [113].
Our patient also frequently complained of drenching day and night sweats, chills, flushing, an unexplained cough and shortness of breath described as ‘air hunger’. These are commonly experienced symptoms in patients infected with babesiosis [105]. Laboratory diagnosis of babesiosis is typically made by examination of peripheral thick and thin blood smears using Giemsa staining, visualizing ring forms and Maltese cross forms (smears can be negative with low levels of parasitemia), as well as serology (IFA, ELISA), culture and nucleic acid amplification (PCR, RNA) and sequencing [114]. FISH staining of blood smears has also proven effective [115]. Our patient had evidence of exposure to B. microti with a fourfold rise in titer over time and multiple positive FISH tests, proving persistence [116]. She also had a positive antibody titer to B. duncani, a species primarily found on the west coast. The patient did live in California for several years before moving to the eastern seaboard but did not report a known tick bite during that time. It is possible that she was exposed to several different Babesia species, as two recent reports have indicated the probable spread of B. duncani across the US as well as Canada [113,117,118]. It is also possible that her B. duncani test represented cross reactivity with B. microti, as only 13 confirmed cases have been reported to date by the CDC [113,119], although antibody responses among the three dominant human pathogens of humans, B. microti, B. duncani, and B. divergens appear to be species specific [120].
Epidemiological studies have demonstrated that babesiosis is spreading in the United States [121,122] and Europe [123-125], and that simultaneous infection with B. microti and B. burgdorferi are now the most common tick-transmitted coinfections in the U.S. [122,126,127]. B. microti-B. burgdorferi coinfected patients suffer from significantly more diverse and intense symptoms, which persist longer than those in patients infected with each pathogen individually [128]. Several reasons may exist for the increased severity of illness. It has been suggested that coinfection not only increases the severity of disease, but also may impair host defense mechanisms [129]. In support of this hypothesis, patients with coinfections report a longer duration of illness and exacerbated symptoms including fatigue, myalgia, sweats and erythema migrans [128,130]. Our patient complained of resistant fatigue, myalgias, arthralgias, sweats and an ACA rash. Co-infection has also been reported to result in a death from pancarditis [131].
Impaired host defense mechanisms have been described in detail for babesiosis [126]. The adaptive immune system and a strong TH1 response increasing production of IFN-γ is needed to combat intracellular pathogens and Babesia, while a strong Th2 response with an increase in IL-4 production and B cell production of antibodies is essential for host defense against extracellular pathogens and Lyme disease [132]. A robust B cell response has also been shown to be necessary for rapid resolution of Lyme disease [133], and in the acute phase of co-infection in the mouse model, marginal zone disruption of the spleen and B cell atrophy were seen where B. microti infection ultimately resulted in reduction in splenic B cells and pathogen specific antibody production [126,134]. B. burgdorferi infection has also been shown to attenuate parasitemia in mice while B. microti subverts the splenic immune response, leading to a marked decrease in splenic B and T cells, reduction in antibody levels and diminished functional humoral immunity [135]. Clearance of both pathogens could therefore be impaired by imbalances in the immune system.
Infection with B. burgdorferi has previously been shown in the mouse model to cause a T cell independent response in the lymph nodes where there is a lack of strongly induced, protective borrelia-specific IgG antibodies [136]. Suppression of a long-lived humoral immunity following an infection with B. burgdorferi ultimately results in a temporary immunosuppression of the host where early germinal centers systemically fail to produce antigen-specific memory B cells [137]. This can result in elevations of IgM antibodies and a decrease in IgG protective antibodies. The same type of immune deficiencies has been reported in humans infected with B. burgdorferi and/or co-infected with babesiosis [113]. This could potentially explain our patient’s four-fold rise in IgM titers to B. microti over time, with evidence of persistent Babesia by RNA analysis (FISH), without significant production of protective IgG antibodies.
Numerous studies highlight immune suppression in pregnancy, where progesterone can act as a natural immune suppressor [138]. Advanced age, splenectomy and immune suppression all increase the risk of critical outcomes with Babesia [119]. Pregnant women have also been shown to be more severely affected by infections with certain organisms vs. non-pregnant women, including the influenza virus, hepatitis E virus (HEV), herpes simplex virus (HSV) and the malaria parasite [139]. Babesiosis and malaria are both intraerythrocytic parasitic infections and share certain similar clinical characteristics, including febrile hemolytic anemia [140]. Considering the risk of immunosuppression in pregnancy with the possibility of transplacental infection and congenital babesiosis, certain authors have therefore advocated for the treatment of all pregnant women diagnosed with babesiosis, even if asymptomatic [25].
Babesiosis in pregnancy can also imitate HELLP syndrome [25] which is an important differential diagnosis, since both diseases can result in hemolysis, elevated liver functions and low platelet counts. Our patient only had evidence of very mild anemia later in her pregnancies (normally seen in pregnancy with hemodilution) with very mild thrombocytopenia (platelet counts ranging between 116,000/UL and 130,000/UL [normal range 140-400,000/UL)] and normal liver and kidney functions. There was no evidence of hemolytic anemia, even though her initial exposure to Babesia was in Europe, where the European species B. divergens has been shown to be more highly associated with hemolytic anemia [141]. Babesiosis can also result in symptoms ranging from mild anemia, to severe pancytopenia, with complications including splenic rupture, disseminated intravascular coagulopathy (DIC), hemophagocytic lymphohistiocytosis (HLH) [142], and acute respiratory distress syndrome (ARDS) [143], as well as warm autoimmune hemolytic anemia (WAHA) [144]. Congenital babesiosis in the fetus can result in anemia, neutropenia, and thrombocytopenia [13], with fevers, jaundice, and hepatosplenomegaly [16]. Apart from following the pregnancy with a highrisk OB/GYN, the pediatric team should be alerted to a history of maternal babesiosis, so appropriate blood work and close surveillance are performed postdelivery.
In our patient, all four children were healthy at delivery with no signs or symptoms of borreliosis or babesiosis. Clindamycin with quinine is one of the most common treatments for babesiosis in pregnancy, due to good placental penetration and evidence of decreased vertical transmission to the fetus [12]. Transplacental passage of azithromycin was initially postulated to occur at low rates [145], but further pharmacokinetic studies have proven adequate penetration into the amniotic fluid with adequate doses, dosing intervals and duration of therapy [146]. Azithromycin in higher doses as monotherapy have also been shown to be efficacious in treating B. microti in the animal model [147].
We chose atovaquone and azithromycin as it has been shown to be as effective as clindamycin and quinine for both mild and severe infections [22,148], with less potential side effects. For example, quinine may induce nausea, vomiting, rashes, and QT prolongation with torsade de point [149]. Both regimens however may be inadequate to completely clear the parasitic infection [150]. A 2019 study by Abittan et al., found clindamycin and quinine as well as atovaquone and azithromycin to be ineffective in treating babesiosis during pregnancy [24]. That was our experience as well. Our patient continuously relapsed with signs and symptoms of active babesiosis with multiple positive FISH (RNA) tests before and during her pregnancy despite long term use of atovaquone and azithromycin. Clindamycin was also used for 10 days during the third trimester of her third pregnancy along with atovaquone and azithromycin for resistant symptoms. This was helpful in relieving her symptomatology and preventing vertical transmission but was not curative.
Treatment regimens for babesiosis are limited by the emergence of drug-resistant parasites, potential toxicity and failure of drugs [151,152]. Lemieux et al. found mutations in the cytb and rpl4 genes of B. microti from patients with resistant infections [153], and there are now difficulties and failures treating B. microti and B. duncani in both those with normal functioning immune systems as well as in those who are immunosuppressed [154,155]. Those at highest risk are patients with immunodeficiencies resulting from B-cell lymphomas, organ transplants, HIV/AIDS, or treatment with immunosuppressive drugs such as Rituximab [156-159] and severe disease can also be seen in hospitalized patients with concurrent chronic illnesses, including diabetes, congestive heart failure (CHF) and renal failure [160]. Yet babesiosis may be fatal even in those with no clear underlying medical problems, especially when transmitted by blood transfusion. [4,156,161].
Lyme disease patients co-infected with Babesia spp. oftentimes are sicker and more resistant to therapy [157,162], and this is especially the case in pregnancy when underlying immunosuppression is present [128,151,154,163]. Recently a pregnant patient with babesiosis who failed clindamycin and quinine as well as atovaquone and azithromycin required an exchange transfusion [24]. Other treatments that have been shown to be helpful in certain cases of resistant babesiosis, including the use of higher dose Bactrim [164], mefloquine [165], atovaquone/proguanil, artemisinin derivatives and dapsone [119,166-168] are contraindicated during pregnancy. Similarly, disulfiram [169] and tafenoquine [170] which have the potential to treat resistant babesiosis are unable to be used due to potential toxicity in the fetus. Novel and more effective treatments for resistant babesiosis are urgently needed.
In our patient, we used higher doses of atovaquone (two teaspoons BID, i.e., 1500 mg BID) combined with azithromycin during all four pregnancies to try and overcome resistance to standard drug regimens. Clindamycin was also used in combination with atovaquone and azithromycin during the third pregnancy. Combination therapies have previously been shown to be superior to monotherapy [147], and our use of higher doses of atovaquone with azithromycin and clindamycin proved to be effective in preventing congenital transmission, even if ineffective in clearing Babesia in the mother. Atovaquone used alone can cause drug resistance but evidence that clindamycin and atovaquone with azithromycin can effectively treat Babesia has previously been reported in both animals [171], and humans [172], although there is now greater recognition of resistance to standard Babesia regimens [153,163], especially in immunocompromised patients with babesiosis when 6 weeks of therapy or longer may be necessary [172].
At birth, none of the neonates of our patient demonstrated signs of active infection with Lyme or babesiosis during pediatric follow-up. Follow-up ranged from 1-10 years. In prior case series of congenitally transmitted babesiosis among 9 children [13] most mothers were unaware of being infected and therefore were not treated, leading to increased morbidity in the neonates who presented with fever, hemolytic anemia, thrombocytopenia and neutropenia [13].
Conclusion
Considering the significant increase in exposure to Lyme disease in the United States in the past decade [173-175] as well as the increase in cases of babesiosis [127,176,177] it is important for all women of childbearing age to be aware of the risks of congenital transmission of tick-borne diseases and to take appropriate preventative measures. A screening questionnaire to establish the pre-test possibility of tick-borne infection before and during pregnancy could be useful [105], as well as determining optimal testing and management approaches [24,178]. Penicillins, cephalosporins, macrolides, clindamycin, quinine and atovaquone may all be used in pregnancy [91,179-183], although high dose quinine should be avoided in the first trimester secondary to the possibility of spontaneous abortion [12].
Our case report is the first one to our knowledge to describe both the safe and effective use of IM benzathine penicillin and azithromycin in four consecutive pregnancies to prevent vertical transmission of LD, along with high dose atovaquone and azithromycin (with or without clindamycin) to prevent vertical transmission of babesiosis. Future research into effective treatments of Lyme disease and babesiosis in both the pregnant and non-pregnant patient is urgently needed due to the possible relapsing, remitting nature of both diseases and potential for associated morbidity and mortality.
Disclaimer
The views expressed are those of Dr. Richard Horowitz, and do not represent the views of the Tick-Borne Disease Working Group, HHS or the United States.
Acknowledgement
The authors thank the MSIDS Research Foundation for their support of research reported in this paper.
References
2. Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. Babesiosis. Clinical Microbiology Reviews. 2000 Jul 1;13(3):451-69.
3. Thomford JW, Conrad PA, Telford III SR, Mathiesen D, Bowman BH, Spielman A, Eberhard ML, Herwaldt BL, Quick RE, Persing DH. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. Journal of Infectious Diseases. 1994 May 1;169(5):1050-6.
4. Herwaldt BL, Persing DH, Precigout EA, Goff WL, Mathiesen DA, Taylor PW, Eberhard ML, Gorenflot AF. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Annals of Internal Medicine. 1996 Apr 1;124(7):643-50.
5. Vannier EG, Krause PJ. Human babesiosis. 2008; 22(3):469G488.
6. Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga P, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, Pampiglione S. Molecular characterization of a non–Babesia divergens organism causing zoonotic babesiosis in Europe. Emerging Infectious Diseases. 2003 Aug;9(8):943.
7. Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. International Journal for Parasitology. 2000 Nov 1;30(12-13):1323-37.
8. Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, Reed W, Hunter R, Ryals R, Hagar W, Xayavong MV. The third described case of transfusiontransmitted Babesia duncani. Transfusion. 2012 Jul;52(7):1517-22.
9. Cangelosi JJ, Sarvat B, Sarria JC, Herwaldt BL, Indrikovs AJ. Transmission of Babesia microti by blood transfusion in Texas. Vox Sanguinis. 2008 Nov;95(4):331- 4.
10. Leiby DA. Babesiosis and blood transfusion: flying under the radar. Vox Sanguinis. 2006 Apr;90(3):157-65.
11. Brennan MB, Herwaldt BL, Kazmierczak JJ, Weiss JW, Klein CL, Leith CP, He R, Oberley MJ, Tonnetti L, Wilkins PP, Gauthier GM. Transmission of Babesia microti parasites by solid organ transplantation. Emerging Infectious Diseases. 2016 Nov;22(11):1869.
12. Feder Jr HM, Lawlor M, Krause PJ. Babesiosis in pregnancy. New England Journal of Medicine. 2003 Jul 10;349(2):195-6.
13. Saetre K, Godhwani N, Maria M, Patel D, Wang G, Li KI, Wormser GP, Nolan SM. Congenital babesiosis after maternal infection with Borrelia burgdorferi and Babesia microti. Journal of the Pediatric Infectious Diseases Society. 2017 Sep 16;7(1):e1-5.
14. Esernio-Jenssen D, Scimeca PG, Benach JI, Tenenbaum MJ. Transplacental/perinatal babesiosis. Obstetrical & Gynecological Survey. 1987 Nov 1;42(11):691- 2.
15. New DL, Quinn JB, Qureshi MZ, Sigler SJ. Vertically transmitted babesiosis. The Journal of Pediatrics. 1997 Jul 1;131(1):163-4.
16. Sethi S, Alcid D, Kesarwala H, Tolan Jr RW. Probable congenital babesiosis in infant, New Jersey, USA. Emerging Infectious Diseases. 2009 May;15(5):788.
17. Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, Horowitz HW, Aguero-Rosenfeld ME, Moore JM, Abramowsky C, Wormser GP. Vertical transmission of Babesia microti, United States. Emerging Infectious Diseases. 2012 Aug;18(8):1318.
18. Aderinboye O, Syed SS. Congenital babesiosis in a four-week-old female infant. The Pediatric Infectious Disease Journal. 2010 Feb 1;29(2):188.
19. Yager PH, Luginbuhl LM, Dekker JP. Case 6-2014: a 35-day-old boy with fever, vomiting, mottled skin, and severe anemia. New England Journal of Medicine. 2014 Feb 20;370(8):753-62.
20. Surra ND, Jesus JE. The anemic and thrombocytopenic febrile neonate. The Journal of Emergency Medicine. 2015 Jun 1;48(6):675-8.
21. Dorman SE, Cannon ME, Telford III SR, Frank KM, Churchill WH. Fulminant babesiosis treated with clindamycin, quinine, and whole-blood exchange transfusion. Transfusion. 2000 Mar;40(3):375-80.
22. Krause PJ, Lepore T, Sikand VK, Gadbaw Jr J, Burke G, Telford SR, et al. Atovaquone and azithromycin for the treatment of babesiosis. New England Journal of Medicine. 2000;343(20):1454-1458.
23. (PDF) Human babesiosis and ehrlichiosis - current status. Retrieved from https://www.researchgate.net/publication/285992807_Human_babesiosis_and_ehrlichiosis_-_current_status
24. Abittan B, Nizam A, Oey M, Callan F, Simmonds L, Pachtman SL. A Case of Babesiosis in a Pregnant Patient Treated with Red Blood Cell Exchange Transfusion. Case Reports in Obstetrics and Gynecology. 2019;2019.
25. Khangura RK, Williams N, Cooper S, Prabulos AM. Babesiosis in Pregnancy: An Imitator of HELLP Syndrome. American Journal of Perinatology Reports. 2019 Apr;9(02):e147-52.
26. Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2015 Nov;35(11):1052-62.
27. Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014 Mar 1;74(3):283- 96.
28. Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infection and Immunity. 1999 Jan 1;67(1):302-7.
29. Bothamley G. Drug treatment for tuberculosis during pregnancy. Drug Safety. 2001 Jun 1;24(7):553-65.
30. Shah J, Mark O, Weltman H, Barcelo N, Lo W, Wronska D, Kakkilaya S, Rao A, Bhat ST, Sinha R, Omar S. Fluorescence in situ hybridization (FISH) assays for diagnosing malaria in endemic areas. PLoS One. 2015 Sep 2;10(9):e0136726.
31. Kempf VA, Volkmann B, Schaller M, Sander CA, Alitalo K, Rieß T, Autenrieth IB. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cellular Microbiology. 2001 Sep;3(9):623- 32.
32. Bale Jr JF. Murph Jr. Congenital infections and the nervous. Pediatric Clinics of North America. 1996;39:669- 90.
33. Schlesinger PA, Duray PH, Burke BA, Steere AC, Stillman MT. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Annals of Internal Medicine. 1985 Jul 1;103(1):67-8.
34. Weber K, Bratzke HJ, Neubert U, Wilske B, Duray PH. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. The Pediatric Infectious Disease Journal. 1988;7(4):286-289.
35. Alexander JM, Cox SM. Lyme disease and pregnancy. Infectious Diseases in Obstetrics and Gynecology. 1995;3(6):256-61.
36. Carlomagno G, Luksa V, Candussi G, Rizzi GM, Trevisan G. Lyme borrelia positive serology associated with spontaneous abortion in an endemic Italian area. Acta Europaea Fertilitatis. 1988;19(5):279-81.
37. Macdonald AB. Human fetal borreliosis, toxemia of pregnancy, and fetal death. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene. Series A: Medical Microbiology, Infectious Diseases, Virology, Parasitology. 1986 Dec 1;263(1-2):189-200.
38. MacDonald AB. Gestational Lyme borreliosis. Implications for the fetus. Rheumatic Disease Clinics of North America. 1989;15(4):657-677.
39. MacDonald AB, Benach JL, Burgdorfer W. Stillbirth following maternal Lyme disease. New York State Journal of Medicine. 1987 Nov;87(11):615-6.
40. Steenbarger JR. Congenital tick-borne relapsing fever: report of a case with first documentation of transplacental transmission. Birth Defects Original Article Series. 1982;18(3 Pt A):39.
41. Mahram M, Ghavami MB. Congenital tick-borne relapsing fever: report of a case with transplacental transmission in the Islamic Republic of Iran.
42. Brzostek T. Human granulocytic ehrlichiosis coincident with Lyme borreliosis in pregnant woman--a case study. Przeglad epidemiologiczny. 2004;58(2):289-94.
43. Muffly T, McCormick TC, Cook C, Wall J. Human granulocytic ehrlichiosis complicating early pregnancy. Infectious Diseases in Obstetrics and Gynecology. 2008;2008.
44. Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clinical Infectious Diseases. 2007 Sep 1;45(5):589- 93.
45. Licona-Enriquez JD, Delgado-De La Mora J, Paddock CD, Ramirez-Rodriguez CA, del Carmen Candia-Plata M, Hernández GÁ. Rocky Mountain spotted fever and pregnancy: four cases from Sonora, Mexico. The American Journal of Tropical Medicine and Hygiene. 2017 Sep 7;97(3):795-8.
46. Stallings SP. Rocky Mountain spotted fever and pregnancy: a case report and review of the literature. Obstetrical & Gynecological Survey. 2001 Jan 1;56(1):37- 42.
47. Balakrishnan N, Ericson M, Maggi R, Breitschwerdt EB. Vasculitis, cerebral infarction and persistent Bartonella henselae infection in a child. Parasites & Vectors. 2016 Dec;9(1):254.
48. Breitschwerdt EB, Maggi RG, Farmer P, Mascarelli PE. Molecular evidence of perinatal transmission of Bartonella vinsonii subsp. berkhoffii and Bartonella henselae to a child. Journal of Clinical Microbiology. 2010 Jun 1;48(6):2289-93.
49. Reis C, Cote M, Le Rhun D, Lecuelle B, Levin ML, Vayssier-Taussat M, et al. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLOS Neglected Tropical Diseases. 2011;5(5):e1186.
50. Breitschwerdt EB. Bartonellosis, One Health and all creatures great and small. Advances in Veterinary Dermatology. 2017 Jun 12;8:111-21.
51. Strobino BA, Williams CL, Abid S, Ghalson R, Spierling P. Lyme disease and pregnancy outcome: A prospective study of two thousand prenatal patients. American Journal of Obstetrics and Gynecology. 1993 Aug 1;169(2):367-74.
52. Strle F, Wormser GP, Mead P, Dhaduvai K, Longo MV, Adenikinju O, Soman S, Tefera Y, Maraspin V, Lotric- Furlan S, Ogrinc K. Gender disparity between cutaneous and non-cutaneous manifestations of Lyme borreliosis. PloS one. 2013 May 30;8(5):e64110.
53. Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti JC, Assous M, Grimont PA. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. International Journal of Systematic and Evolutionary Microbiology. 1992 Jul 1;42(3):378-83.
54. Postic D, Ras NM, Lane RS, Hendson M, Baranton G. Expanded Diversity among Californian Borrelia Isolates and Description of Borrelia bissettii sp. nov.(Formerly Borrelia Group DN127). Journal of Clinical Microbiology. 1998 Dec 1;36(12):3497-504.
55. Muzaffar SB, Smith Jr RP, Jones IL, Lavers J, Lacombe EH, Cahill BK, Lubelczyk CB, Rand PW. The Trans-Atlantic Movement ofthe Spirochete Borrelia garinii. Emerging Avian Disease: Published for the Cooper Ornithological Society. 2012 May 2;42:23.
56. Rudenko N, Golovchenko M, Grubhoffer L, Oliver Jr JH. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks and tick-borne diseases. 2011 Sep 1;2(3):123-8.
57. Margos G, Hojgaard A, Lane RS, Cornet M, Fingerle V, Rudenko N, Ogden N, Aanensen DM, Fish D, Piesman J. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks and Tick-borne Diseases. 2010 Dec 1;1(4):151-8.
58. Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio- Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. The Lancet Infectious Diseases. 2016 May 1;16(5):556-64.
59. Gerold S, Gary PW, Jeremy G. Lyme borreliosis. Lancet. 2012;379:461-73.
60. Hofmann H, Fingerle V, Hunfeld KP, Huppertz HI, Krause A, Rauer S, Ruf B. Cutaneous lyme borreliosis: guideline of the German dermatology society. GMS German Medical Science. 2017;15.
61. Aberer E, Kersten A, Klade H, Poitschek C, Jurecka W. Heterogeneity of Borrelia burgdorferi in the skin. The American Journal of Dermatopathology. 1996 Dec 1;18(6):571-9.
62. Brorson O, Brorson SH. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection. 1997;25(4):240-246.
63. MacDonald AB. Spirochetal cyst forms in neurodegenerative disorders, hiding in plain sight. Medical Hypotheses. 2006;67(4):819-832.
64. Rudenko N, Golovchenko M, Kybicova K, Vancova M. Metamorphoses of Lyme disease spirochetes: phenomenon of Borrelia persisters. Parasites & Vectors. 2019;12(1):237.
65. Vancová M, Rudenko N, Vanecek J, Golovchenko M, Strnad M, Rego RO, Tichá L, Grubhoffer L, Nebesárová J. Pleomorphism and viability of the Lyme disease pathogen Borrelia burgdorferi exposed to physiological stress conditions: A correlative cryo-fluorescence and cryo-scanning electron microscopy study. Frontiers in Microbiology. 2017 Apr 11;8:596.
66. Feng J, Shi W, Zhang S, Zhang Y. Persister mechanisms in Borrelia burgdorferi: implications for improved intervention. Emerging Microbes & Infections. 2015 Aug;4(8):e51.
67. Sapi E, Bastian SL, Mpoy CM, et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS ONE. 2012;7(10):e48277.
68. Sapi E, Balasubramanian K, Poruri A, Maghsoudlou JS, Socarras KM, Timmaraju AV, Filush KR, Gupta K, Shaikh S, Theophilus PA, Luecke DF. Evidence of in vivo existence of Borrelia biofilm in borrelial lymphocytomas. European Journal of Microbiology and Immunology. 2016 Mar;6(1):9-24.
69. Di Domenico EG, Cavallo I, Bordignon V, D’Agosto G, Pontone M, Trento E, Gallo MT, Prignano G, Pimpinelli F, Toma L, Ensoli F. The Emerging Role of Microbial Biofilm in Lyme Neuroborreliosis. Frontiers in Neurology. 2018;9:1048.
70. Alban PS, Johnson PW, Nelson DR. Serum-starvationinduced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology (Reading, Engl).2000;146 ( Pt 1):119-127.
71. Åsbrink E, Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathologica Microbiologica Scandinavica Series B: Microbiology. 1985 Sep;93(1-6):161-3.
72. Liegner KB, Shapiro JR, Ramsay D, Halperin AJ, Hogrefe W, Kong L. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting Borrelia burgdorferi infection. Journal of the American Academy of Dermatology. 1993;28(2):312- 314.
73. Middelveen MJ, Sapi E, Burke J, et al. Persistent Borrelia Infection in Patients with Ongoing Symptoms of Lyme Disease. Healthcare (Basel). 2018;6(2):33.
74. Embers ME, Barthold SW, Borda JT, et al. Persistence of Borrelia burgdorferi in Rhesus Macaques following Antibiotic Treatment of Disseminated Infection. PLOS ONE. 2012;7(1):e29914.
75. Häupl T, Hahn G, Rittig M, et al. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheumatology. 1993;36(11):1621-1626.
76. Ma Y, Sturrock A, Weis JJ. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infection and Immunity. 1991 Feb 1;59(2):671-8.
77. Girschick HJ, Huppertz HI, Rüssman H, Krenn V, Karch H. Intracellular persistence ofBorrelia burgdorferi in human synovial cells. Rheumatology International. 1996 May 1;16(3):125-32.
78. Battafarano DF, Combs JA, Enzenauer RJ, Fitzpatrick JE. Chronic septic arthritis caused by Borrelia burgdorferi. Clinical Orthopaedics and Related Research. 1993 Dec(297):238-41.
79. Schmidli J, Hunziker T, Moesli P, Schaad UB. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. Journal of Infectious Diseases. 1988;158(4):905-906.
80. Cimmino MA, Azzolini A, Tobia F, Pesce CM. Spirochetes in the spleen of a patient with chronic Lyme disease. American Journal of Clinical Pathology. 1989;91(1):95-97.
81. Preac-Mursic V, Pfister HW, Spiegel H, Burk R, Wilske B, Reinhardt S, Böhmer R. First isolation of Borrelia burgdorferi from an iris biopsy. Journal of Clinical Neuro- Ophthalmology. 1993 Sep;13(3):155-61.
82. Meier P, Blatz R, Gau M, Spencker FB, Wiedemann P. Pars-plana-Vitrektomie bei Borrelia burgdorferi- Endophthalmitis. Klinische Monatsblätter für Augenheilkunde. 1998 Dec;213(12):351-4.
83. Livengood JA, Gilmore Jr RD. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes and Infection. 2006 Nov 1;8(14- 15):2832-40.
84. Montgomery RR, Nathanson MH, Malawista SE. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery. The Journal of Immunology. 1993;150(3):909-915.
85. Lawrence C, Lipton RB, Lowy FD, Coyle PK. Seronegative chronic relapsing neuroborreliosis. European Neurology. 1995;35(2):113-7.
86. Berndtson K. Review of evidence for immune evasion and persistent infection in Lyme disease. International Journal of General Medicine. 2013;6:291-306.
87. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318-1322.
88. Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. Journal of Infectious Diseases. 1993 May 1;167(5):1074-81.
89. Georgilis K, Peacocke M, Klempner MS. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro Journal of Infectious Diseases. 1992;166(2):440-444.
90. Waddell LA, Greig J, Lindsay LR, Hinckley AF, Ogden NH. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLOS ONE. 2018;13(11):e0207067.
91. Markowitz LE, Steere AC, Benach JL, Slade JD, Broome CV. Lyme disease during pregnancy. JAMA. 1986;255(24):3394-3396.
92. Goldenberg RL, Thompson C. The infectious origins of stillbirth. American Journal of Obstetrics and Gynecology. 2003 Sep 1;189(3):861-73.
93. Elliott DJ, Eppes SC, Klein JD. Teratogen update: Lyme disease. Teratology. 2001;64(5):276-281.
94. Mylonas I. Borreliosis during pregnancy: a risk for the unborn child?. Vector-Borne and Zoonotic Diseases. 2011 Jul 1;11(7):891-8.
95. Esposito S, Bosis S, Sabatini C, Tagliaferri L, Principi N. Borrelia burgdorferi infection and Lyme disease in children. International Journal of Infectious Diseases. 2013;17(3):e153-e158.
96. Maraspin V, Ružic-Sabljic E, Pleterski-Rigler D, Strle F. Pregnant women with erythema migrans and isolation of borreliae from blood: course and outcome after treatment with ceftriaxone. Diagnostic Microbiology and Infectious Disease. 2011 Dec 1;71(4):446-8.
97. Horowitz RI. Lyme disease and pregnancy: Implications of chronic infection, PCR testing, and prenatal treatment. In Proceedings of the 16th International Scientific Conference on Lyme Disease & Other Tick– Borne Disorders, Hartford, CT, USA 2003 Jun (pp. 7-8).
98. Transmission | Lyme Disease | CDC. Retrieved From https://www.cdc.gov/lyme/transmission/index.html
99. Screening for Syphilis Infection in Pregnancy: U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. Annals of Internal Medicine. 2009;150(10):705
100. CDC – Syphilis Treatment. Retrieved From https:// www.cdc.gov/std/syphilis/treatment.htm
101. Steere AC, Green J, Schoen RT, Taylor E, Hutchinson GJ, Rahn DW, Malawista SE. Successful parenteral penicillin therapy of established Lyme arthritis. New England Journal of Medicine. 1985 Apr 4;312(14):869-74.
102. Cimmino MA, Accardo S. Long term treatment of chronic Lyme arthritis with benzathine penicillin. Annals of the Rheumatic Diseases. 1992 Aug 1;51(8):1007-8.
103. Horowitz R. Bicillin Therapy And Lyme Disease: A Retrospective Study Of The Safety And Efficacy Of High Dose Intramuscular Bicillin In The Treatment Of Chronic Resistant Lyme Disease: Abstract, 12th International Scientific Conference On Lyme Disease And Other Spirochetal & Tick- Borne Disorders, LDA, April 1999. In: ; 1999.
104. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcome. International Journal of Infectious Diseases. 2010;14(6):e494-e498.
105. Citera M, Freeman PR, Horowitz RI. Empirical validation of the Horowitz multiple systemic infectious disease syndrome questionnaire for suspected Lyme disease. International Journal of General Medicine. 2017;10:249.
106. Shadick NA. The Long-Term Clinical Outcomes of Lyme Disease: A Population-Based Retrospective Cohort Study. Annals of Internal Medicine. 1994;121(8):560.
107. Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflammation Research. 2014 Oct 1;63(10):803-19.
108. Verma RK, Kaur J, Kumar K, Yadav AB, Misra A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrobial Agents and Chemotherapy. 2008 Sep 1;52(9):3195-201.
109. Maggi RG, Ericson M, Mascarelli PE, Bradley JM, Breitschwerdt EB. Bartonella henselae bacteremia in a mother and son potentially associated with tick exposure. Parasites & Vectors. 2013 Dec;6(1):101.
110. Ericson M, Balakrishnan N, Mozayeni BR, Woods CW, Dencklau J, Kelly S, Breitschwerdt EB. Culture, PCR, DNA sequencing, and second harmonic generation (SHG) visualization of Bartonella henselae from a surgically excised human femoral head. Clinical Rheumatology. 2017 Jul 1;36(7):1669-75.
111. Breitschwerdt EB. Bartonellosis: one health perspectives for an emerging infectious disease. ILAR Journal. 2014 Jan 1;55(1):46-58.
112. Maggi RG, Mozayeni BR, Pultorak EL, Hegarty BC, Bradley JM, Correa M, Breitschwerdt EB. Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease–endemic region. Emerging Infectious Diseases. 2012 May;18(5):783.
113. Horowitz RI, Freeman PR. Precision medicine: retrospective chart review and data analysis of 200 patients on dapsone combination therapy for chronic Lyme disease/post-treatment Lyme disease syndrome: part 1. International Journal of General Medicine. 2019;12:101.
114. Parija SC, Dinoop KP, Venugopal H. Diagnosis and management of human babesiosis. Tropical Parasitology. 2015 Jul;5(2):88.
115. Office of HIV/AIDS and Infectious Disease Policy AS for H (ASH). Report of the Other TBDs and Co-Infections Subcommittee. HHS.gov. Retrieved from https://www.hhs.gov/ash/advisory-committees/tickbornedisease/reports/other-tbds-2018-5-9/index.html
116. Akoolo L, Schlachter S, Khan R, Alter L, Rojtman AD, Gedroic K, Bhanot P, Parveen N. A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiology. 2017 Dec;17(1):16.
117. Scott JD. First record of locally acquired human babesiosis in Canada caused by Babesia duncani: A case report. SAGE Open Medical Case Reports. 2017 Aug 26;5:2050313X17725645.
118. Scott JD, Scott CM. Human Babesiosis Caused by Babesia duncani Has Widespread Distribution across Canada. Healthcare (Basel). 2018;6(2).
119. Office of HIV/AIDS and Infectious Disease Policy AS for H (ASH). Report of Other TBDS and Co-Infections Subcommittee. HHS.gov. https://www.hhs.gov/ash/ advisory-committees/tickbornedisease/reports/othertbds- 2018-5-9/index.html. Published May 9, 2018. Accessed May 21, 2018.
120. Priest JW, Moss DM, Won K, et al. Multiplex Assay Detection of Immunoglobulin G Antibodies That Recognize Babesia microti Antigens. Clinical and Vaccine Immunology : CVI. 2012;19(9):1539.
121. Westblade LF, Simon MS, Mathison BA, Kirkman LA. Babesia microti: from mice to ticks to an increasing number of highly susceptible humans. Journal of Clinical Microbiology. 2017 Oct 1;55(10):2903-12.
122. Hersh MH, Ostfeld RS, McHenry DJ, et al. Co- Infection of Blacklegged Ticks with Babesia microti and Borrelia burgdorferi Is Higher than Expected and Acquired from Small Mammal Hosts. PLOS ONE. 2014;9(6):e99348.
123. Moutailler S, Moro CV, Vaumourin E, Michelet L, Tran FH, Devillers E, Cosson JF, Gasqui P, Mavingui P, Vourc’h G, Vayssier-Taussat M. Co-infection of ticks: the rule rather than the exception. PLoS Neglected Tropical Diseases. 2016 Mar 17;10(3):e0004539.
124. Lempereur L, Shiels B, Heyman P, Moreau E, Saegerman C, Losson B, Malandrin L. A retrospective serological survey on human babesiosis in Belgium. Clinical Microbiology and Infection. 2015 Jan 1;21(1):96- e1.
125. Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, Jouglin M, Chauvin A, De Briel D. Babesiosis in immunocompetent patients, Europe. Emerging Infectious Diseases. 2011 Jan;17(1):114.
126. Djokic V, Primus S, Akoolo L, Chakraborti M, Parveen N. Age-related differential stimulation of immune response by Babesia microti and Borrelia burgdorferi during acute phase of infection affect diseases severity. Frontiers in immunology. 2018;9:2891.
127. Dunn JM, Krause PJ, Davis S, Vannier EG, Fitzpatrick MC, Rollend L, Belperron AA, Stacey A, Bockenstedt LK, Fish D, Diuk-Wasser MA. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One. 2014 Dec 29;9(12):e115494.
128. Krause PJ, Telford SR, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. JAMA. 1996 Jun 5;275(21):1657-60.
129. Goldstein EJ, Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clinical Infectious Diseases. 2001 Sep 1;33(5):676-85.
130. Dos Santos CC, Kain KC. Two tick-borne diseases in one: a case report of concurrent babesiosis and Lyme disease in Ontario. CMAJ: Canadian Medical Association Journal. 1999 Jun 29;160(13):1851.
131. Marcus LC, Steere AC, Duray PH, Anderson AE, Mahoney EB. Fatal pancarditis in a patient with coexistent Lyme disease and Babesiosis. Annals of Internal Medicine. 1985;103:374-76.
132. Palmer MV, Thacker TC, Waters W, Gortázar C, Corner LA. Mycobacterium bovis: a model pathogen at the interface of livestock, wildlife, and humans. Veterinary Medicine International. 2012;2012.
133. Blum LK, Adamska JZ, Martin DS, Rebman AW, Elliott SE, Cao RR, Embers ME, Aucott JN, Soloski MJ, Robinson WH. Robust B cell responses predict rapid resolution of Lyme disease. Frontiers in Immunology. 2018;9:1634.
134. Djokic V, Akoolo L, Parveen N. Babesia microti infection changes host spleen architecture and is cleared by a Th1 immune response. Frontiers in Microbiology. 2018 Jan 31;9:85.
135. Djokic V, Akoolo L, Primus S, Schlachter S, Kelly K, Bhanot P, Parveen N. Protozoan parasite Babesia microti subverts adaptive immunity and enhances Lyme disease severity. Frontiers in Microbiology. 2019 Jul 10;10:1596.
136. Tunev SS, Hastey CJ, Hodzic E, Feng S, Barthold SW, Baumgarth N. Lymphoadenopathy during Lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS pathogens. 2011 May 26;7(5):e1002066.
137. Elsner RA, Hastey CJ, Olsen KJ, Baumgarth N. Suppression of long-lived humoral immunity following Borrelia burgdorferi infection. PLoS pathogens. 2015 Jul 2;11(7):e1004976.
138. Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. American Journal of Reproductive Immunology. 2010 Jun;63(6):425-33.
139. Kourtis AP, Read JS, Jamieson DJ. Pregnancy and Infection The New England Journal of Medicine.2014;370(23):2211-2218.
140. Krause PJ, Daily J, Telford SR, Vannier E, Lantos P, Spielman A. Shared features in the pathobiology of babesiosis and malaria. Trends in Parasitology. 2007 Dec 1;23(12):605-10.
141. Management of Babesia divergens Babesiosis Without a Complete Course of Quinine Treatment - ProQuest. Retrieved from https://search.proquest.com/ openview/77dbfc06f22d0d9278b92f0e017cb921/1?pqorigsite= gscholar&cbl=54107
142. Akel T, Mobarakai N. Hematologic manifestations of babesiosis. Annals of clinical microbiology and antimicrobials. 2017 Dec;16(1):6.
143. Alvarez De Leon S, Srivastava P, Revelo AE, Kadambi A, El Khoury MY, Wormser GP, Epelbaum O. Babesiosis as a cause of acute respiratory distress syndrome: a series of eight cases. Postgraduate Medicine. 2019 Feb 17;131(2):138-43.
144. Woolley AE, Montgomery MW, Savage WJ, Achebe MO, Dunford K, Villeda S, Maguire JH, Marty FM. Postbabesiosis warm autoimmune hemolytic anemia. New England Journal of Medicine. 2017 Mar 9;376(10):939- 46.
145. Heikkinen T, Laine K, Neuvonen PJ, Ekblad U. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. BJOG: An International Journal of Obstetrics & Gynaecology. 2000 Jun;107(6):770-5.
146. Acosta EP, Grigsby PL, Larson KB, James AM, Long MC, Duffy LB, Waites KB, Novy MJ. Transplacental transfer of azithromycin and its use for eradicating intraamniotic ureaplasma infection in a primate model. The Journal of Infectious Diseases. 2013 Oct 31;209(6):898- 904.
147. Weiss LM, Wittner M, Wasserman S, Oz HS, Retsema J, Tanowitz HB. Efficacy of Azithromycin for Treating Babesia microti Infection in the Hamster Model. The Journal of Infectious Diseases. 1993;168(5):1289- 1292.
148. Weiss LM. Babesiosis in humans: a treatment review. Expert Opinion on Pharmacotherapy. 2002 Aug 1;3(8):1109-15.
149. Sheehan ET, Frizzell JD, Gabaldon J, West MB. Quinine and the ABCs of long QT: a patient’s misfortune with arthritis, (alcoholic) beverages, and cramps. Journal of General Internal Medicine. 2016 Oct 1;31(10):1254-7.
150. Horowitz RI. Chronic Persistent Babesiosis after Clindamycin and Quinine/ Mepron and Zithromax. [Abstract]. 12th International Conference on Lyme Borreliosis. Presented at the: 12th International Conference on Lyme Borreliosis; April 1999; New York, NY.
151. Krause PJ, Spielman A, Telford SR, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH. Persistent parasitemia after acute babesiosis. New England Journal of Medicine. 1998 Jul 16;339(3):160-5.
152. Allred DR. Babesiosis: persistence in the face of adversity. Trends in Parasitology. 2003 Feb 1;19(2):51-5.
153. Lemieux JE, Tran AD, Freimark L, Schaffner SF, Goethert H, Andersen KG, Bazner S, Li A, McGrath G, Sloan L, Vannier E. Telford 3rd, S., Bailey, JA, Sabeti, PC. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nature Microbiology. 2016; 1, 16079.
154. Wormser GP, Prasad A, Neuhaus E, Joshi S, Nowakowski J, Nelson J, Mittleman A, Aguero-Rosenfeld M, Topal J, Krause PJ. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clinical Infectious Diseases. 2010 Feb 1;50(3):381-6.
155. Abraham A, Thekkiniath J, Kilian N, Lawres L, Gao R, DeBus K, He L, Yu X, Zhu G, Graham MM, Liu X. Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. Journal of Biological Chemistry. 2018 Dec 28;293(52):19974-81.
156. Krause PJ. Human babesiosis. International journal for parasitology. 2019 Jan 26.
157. Parveen N, Bhanot P. Babesia microti—Borrelia burgdorferi coinfection. Pathogens. 2019 Sep;8(3):117.
158. Raffalli J, Wormser GP. Persistence of babesiosis for> 2 years in a patient on rituximab for rheumatoid arthritis. Diagnostic Microbiology and Infectious Disease. 2016 Jun 1;85(2):231-2.
159. Falagas ME, Klempner MS. Babesiosis in patients with AIDS: a chronic infection presenting as fever of unknown origin. Clinical Infectious Diseases. 1996 May 1;22(5):809-12.
160. Fida M, Hamdi A, Saleh OA, O’Horo J. 421. Babesiosis: Retrospective Review of 38 Cases from Upper Midwest. In Open Forum Infectious Diseases 2018 Nov (Vol. 5, No. Suppl 1, p. S160). Oxford University Press.
161. Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Annals of Internal Medicine. 2011 Oct 18;155(8):509-19.
162. Grunwaldt E, Barbour AG, Benach JL. Simultaneous occurrence of babesiosis and Lyme disease. New England Journal of Medicine. 1983.
163. Krause PJ, McKay K, Gadbaw J, Christianson D, Closter L, Lepore T. Telford 3rd. SR, Sikand, V., Ryan, R., Persing, D., Radolf, JD, Spielman, A. 2003:431-6.
164. Horowitz R. High Dose Trimethoprim- Sulfamethoxazole Therapy: A Useful Adjunct To Combination Therapy In Treatment Resistant Babesiosis. Abstract, 12th International Scientific Conference On Lyme Disease and Other Spirochetal & Tick-Borne Disorders, April 1999, New York City, USA. In: NYC, NY; 1999.
165. Horowitz R. Mefloquine and artemesia: A prospective trial of combination therapy in chronic babesiosis. In 13th International Scientific Conference on Lyme Disease and Other Tick-Borne Disorders 2000 Mar 24. Hartford Marriott Farmington CT, USA.
166. Horowitz R. How Can I Get Better?: An Action Plan for Treating Resistant Lyme & Chronic Disease. St. Martin’s Griffin; 2017 Feb 14.
167. Vyas JM, Telford SR, Robbins GK. Treatment of refractory Babesia microti infection with atovaquoneproguanil in an HIV-infected patient: case report. Clinical infectious diseases. 2007 Dec 15;45(12):1588-60.
168. Goo YK, Terkawi MA, Jia H, Aboge GO, Ooka H, Nelson B, Kim S, Sunaga F, Namikawa K, Igarashi I, Nishikawa Y. Artesunate, a potential drug for treatment of Babesia infection. Parasitology International. 2010 Sep 1;59(3):481-6.
169. Liegner KB. Disulfiram (Tetraethylthiuram Disulfide) in the Treatment of Lyme Disease and Babesiosis: Report of Experience in Three Cases. Antibiotics. 2019 Jun;8(2):72.
170. Mordue DG, Wormser GP. Could the Drug Tafenoquine Revolutionize Treatment of Babesia microti Infection?. The Journal of Infectious Diseases. 2019 May 17.
171. Wittner M, Lederman J, Tanowitz HB, Rosenbaum GS, Weiss LM. Atovaquone in the treatment of Babesia microti infections in hamsters. The American Journal of Tropical Medicine and Hygiene. 1996 Aug 1;55(2):219-22.
172. Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: A Review. JAMA. 2016;315(16):1767-1777.
173. Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographic distribution and expansion of human Lyme Disease, United States. Emerging Infectious Diseases. 2015 Aug;21(8):1455-7.
174. How many people get Lyme disease? | Lyme Disease | CDC. Retrieved From https://www.cdc.gov/lyme/stats/ humancases.html
175. Record Number of Tickborne Diseases Reported in U.S. in 2017 | CDC Online Newsroom | CDC. Retrieved From https://www.cdc.gov/media/releases/2018/s1114- record-number-tickborne-diseases.html
176. Goethert HK, Molloy P, Berardi V, Weeks K, Telford III SR. Zoonotic Babesia microti in the northeastern US: Evidence for the expansion of a specific parasite lineage. PloS one. 2018 Mar 22;13(3):e0193837.
177. Gray EB. Babesiosis Surveillance — United States, 2011–2015. MMWR Surveill Summ. 2019;68. doi:10.15585/mmwr.ss6806a1
178. Horowitz RI. Why Can’t I Get Better?: Solving the Mystery of Lyme and Chronic Disease. Macmillan; 2013 Nov 12.
179. Czeizel AE, Rockenbauer M, Sørensen HT, Olsen J. Use of cephalosporins during pregnancy and in the presence of congenital abnormalities: a population-based, case-control study. American Journal of Obstetrics and Gynecology. 2001 May 1;184(6):1289-96.
180. Dinur AB, Koren G, Matok I, Wiznitzer A, Uziel E, Gorodischer R, Levy A. Fetal safety of macrolides. Antimicrobial Agents and Chemotherapy. 2013 Jul 1;57(7):3307-11.
181. Obonyo CO, Juma EA. Clindamycin plus quinine for treating uncomplicated falciparum malaria: a systematic review and meta-analysis. Malaria Journal. 2012 Dec;11(1):2.
182. Mcgready R, Thwai KL, Cho T, Looareesuwan S, White NJ, Nosten F. The effects of quinine and chloroquine antimalarial treatments in the first trimester of pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002 Apr 1;96(2):180-4.
183. Irvine MH, Einarson A, Bozzo P. Prophylactic use of antimalarials during pregnancy. Canadian Family Physician. 2011 Nov 1;57(11):1279-81.