Abstract
A 69-year-old woman with hypertension, hyperlipidemia, sleep apnea, gastroesophageal reflux disease, and recent knee replacement was brought to the emergency room (ER) for syncope. She had her physiotherapy session earlier in the day and became symptomatic with dizziness, shortness of breath and had loss of consciousness. In the ER, systolic blood pressure (SBP) was noted to be 90 mmHg and an oxygen saturation (O2 sat) of 80% on room air. Patient received fluid bolus with improvement of SBP to 110 mmHg. O2 sat improved to 99% with 10 L of oxygen. A bedside echocardiogram showed right ventricular (RV) distension. A Computerized Tomographic Angiogram (CTA) of the chest showed bilateral main stem pulmonary emboli (PE) with signs of RV strain. Initially EKG showed sinus tachycardia, right bundle branch block, and a S1Q3T3 pattern which resolved rapidly the next day. Patient was admitted, remained hemodynamically stable, and was treated with full dose of Enoxaparin subcutaneously. A follow up EKG was performed the next day, which showed complete resolution of initial findings. Follow up echocardiogram also showed rapid resolution of RV strain and complete restoration of RV size and function. Patient was eventually discharged home on full dose apixaban.
Introduction
In the United States, approximately 900,000 people are affected by venous thromboembolism (VTE) yearly. About 100,000 people die of a pulmonary embolism (PE) annually [1]. Venous thrombi usually form in deep veins of the lower extremities. These thrombi then detach from their formation sites, travel through into the right atrium (RA) and right ventricle (RV) and eventually obstruct pulmonary vasculature. Deep vein thromboses (DVTs) have an incidence of 1.5 per 1,000 individuals. About 79% of patients with acute PE have evidence of DVT. About half of the patients with a proximal DVT go on to have an episode of acute PE [2].
Case Presentation
Investigation
69-year-old female with past medical history of hypertension, hyperlipidemia, gastroesophageal reflux disease, obstructive sleep apnea, and recent history of left knee replacement was brought to the emergency department after a syncopal episode while crossing the street after a physiotherapy session. In the emergency room, patient was fully alert and oriented. Patient denied any preceding chest pain or palpitations. Patient did have shortness of breath and was noted to be hypoxic at presentation with O2 Sat of 80% on room air.
Diagnosis
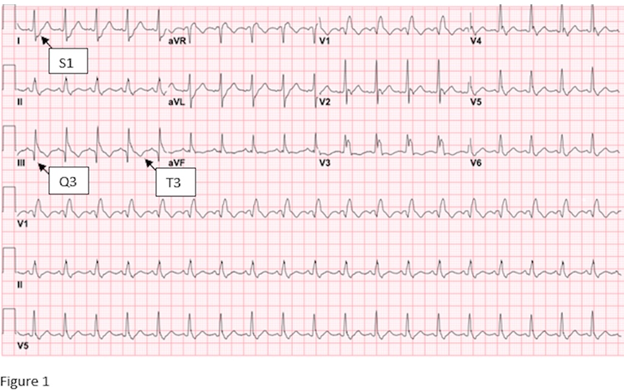
Initial chest x-ray showed cardiomegaly, prominent aorta, prominent pulmonary arteries which suggested pulmonary arterial hypertension (PAH). Initial EKG showed sinus tachycardia with right bundle branch block and a S1Q3T3 pattern (Figure 1). Bilateral lower extremity doppler was negative for DVT. CT Angiography (CTA) showed filling defect seen within the bilateral main stem pulmonary arteries which extend into the lower segmental arteries bilaterally. Right heart strain was noted. The pulmonary artery measured 3.2 cm, which was nonspecific but suggestive of pulmonary arterial hypertension (PAH). Mild reflux of contrast in the IVC and hepatic veins was suggestive of right heart strain.
Figure 1. Initial EKG showing sinus tachycardia with right bundle branch block and a S1Q3T3 pattern.
Treatment
In the ER, patient's systolic blood pressure (SBP) was initially 90 mmHg. After receiving one liter of fluid bolus SBP improved to 110 mmHg. She was placed on 5 L of oxygen via nasal cannula with O2 sat improving to 90% and later upgraded to 10 L Oxy-mask with saturation improving to 99%. Patient was started full dose of enoxaparin subcutaneously.
Follow-up and outcomes
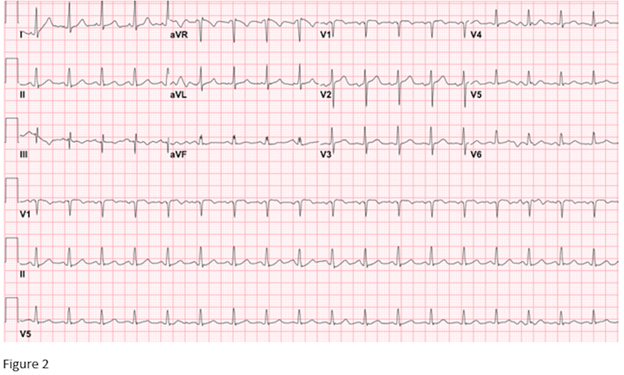
A repeat EKG performed the next day showed sinus tachycardia at 110 BPM with resolution of RBBB and S1Q3T3 pattern (Figure 2). An echocardiogram done the next day showed normal left ventricular ejection fraction at 60% with pulmonary artery systolic pressure (PASP) of 32.16 mmHg, normal IVC size, and normal RV systolic function with complete resolution of RV strain. Patient was eventually discharged home with oral anticoagulant.
Figure 2. A repeat EKG (performed the next day) showing sinus tachycardia at 110 BPM with resolution of RBBB and S1Q3T3 pattern.
Discussion
Patients with acute pulmonary embolism can present with a variety of clinical symptoms. These include chest pain, palpitations, dyspnea, dizziness, syncope, lower extremity pain, and/or swelling. Physical examination findings may include tachypnea, tachycardia, jugular venous distension, parasternal heave, loud S2 sound, right-sided S3 sound, tricuspid regurgitation murmur, pulsatile liver, and peripheral edema. Since most clinical presentations are nonspecific, frequent misdiagnosis can occur [3].
PE compromises both gas exchange and circulation. There is a sudden rise in pulmonary artery pressure and pulmonary vascular resistance, which abruptly increases RV afterload. This rise in RV afterload is due to mechanical obstruction and vasoconstriction (through increased release of thromboxane A2 and serotonin) [4]. Increased right ventricular afterload causes RV dysfunction due to dilation and increased wall tension. As the RV dilates, the interventricular septum shifts toward the LV, thereby compressing the LV. This results in decreased filling of the LV and diastolic dysfunction, which leads to decreased cardiac output and blood pressure. Increased RV wall tension itself reduces right coronary artery flow, increases oxygen demand, and leads to RV ischemia. At the cellular level, RV ischemia occurs is from myocyte necrosis and myocardial inflammation. The myocardial inflammation occurs due to macrophage, T cell, and neutrophil infiltration and is distinct from the epicardial vessel occlusion pattern normally seen in a myocardial infarction [2].
To determine the pretest probability of PE, the Geneva score and the Wells score are used. The pretest probability of PE can be categorized as low, intermediate or high. D-dimer testing is highly sensitive and can rule out PE in patients with low or intermediate pretest probability [3].
Chest x-ray findings can include Hampton hump or Westermark sign. On EKG, sinus tachycardia or atrial fibrillation is the most common finding [3]. Other EKG signs of RV strain can include T wave inversions in V1-V4, QR pattern in V1, the S1Q3T3 pattern, and incomplete or complete right bundle-branch block. RBBB is thought to be caused by acute RV overload and dilatation, accompanied by subendocardial ischemia in the right bundle. The presence of RV strain on EKG correlates to the extent of pulmonary vascular obstruction and outcomes related to acute PE. At least 25% of patients with acute PE have signs of RV impairment on 2D transthoracic echocardiography (TTE) [2].
CT Angiography (CTA) has high sensitivity and specificity and is used as the gold standard for diagnosis. Ventilation/perfusion scan (V/Q scan) can be used for pregnant women, patients with iodine contrast allergy or renal disease. TTE should also be done in patients who have a high pretest probability of PE, are hemodynamic unstable and/or cannot be safely transported to CTA. TTE can show right heart dilation, compression of interventricular septum, increased tricuspid regurgitation, or RV hypokinesis. Lower extremity doppler ultrasound can also be performed to look for a venous thromboembolism when PE is suspected and when imaging cannot be performed [3].
Anticoagulation should be started as soon as PE is diagnosed (or sometimes even prior to diagnosis especially when there is high clinical suspicion and low bleeding risk) [3]. Initial therapy with heparin followed by long-term anticoagulation with vitamin K antagonists was considered the main treatment of PE [5]. The disadvantages of these therapies included multiple interactions with foods/drugs and frequent INR monitoring with dose adjustments [6]. However, direct oral anticoagulants (DOACs) can now be safely used for both acute and long-term treatment of PE (without the need for initial heparin therapy) [5]. DOACs can be given in fixed oral doses without routine monitoring, have rapid onset of action, have minimal food and drug interaction, and can be safely switched to after Vitamin K antagonist therapy. For provoked PE, anticoagulation is recommended for 3 months. For unprovoked PE, extended duration of DOAC anticoagulation is recommended. Few contraindications to DOAC use includes: CrCl <30 ml/min, moderate or severe hepatic impairment), concomitant use of P-glycoprotein inhibitors (amiodarone, quinidine, verapamil), strong CYP3A4 inhibitors or inducers, and pregnancy or breastfeeding [6].
Systemic thrombolysis can be used in critically ill patients (with massive clot burden and low bleeding risk) to increase pulmonary perfusion and to reduce RV afterload. Surgical pulmonary embolectomy (SPE) is another option for patients who have contraindications to thrombolytics use [7]. IVC filter placement (for prevention of PE) should also be considered in patients who have an absolute contraindication to anticoagulation, have had complications, or had failure of prior anticoagulation therapy [4].
Conclusion
In our case, the patient presented with new RBBB due to RV strain from acute PE as shown by the CT-angiogram and bedside echocardiogram. The initial 12-lead electrocardiogram (EKG) showed sinus tachycardia (ST) at 117 beats per minute (BPM), right bundle branch block (RBBB) and S1Q3T3 pattern (Figure 1). A repeat EKG done the following day showed ST at 110 BPM with resolution of RBBB and resolution of S1Q3T3 pattern (Figure 2). The S1Q3T3 pattern is considered as a “classic” EKG pattern associated with acute PE, even though it is neither sensitive nor specific for acute PE. The rapid resolution of the EKG findings, complete improvement in RV hemodynamics and overall clinical improvement of the patient is worth noting. Our case gives further insight into the diagnosis of PE. In addition to performing CT angiography, our case adds to the literature to reiterate the importance of unique findings (such as S1Q3T3 pattern associated only with PE) and other supportive findings (such as on echocardiography).
References
2. Gerges C, Skoro-Sajer N, Lang IM. Right ventricle in acute and chronic pulmonary embolism (2013 Grover Conference series). Pulmonary circulation. 2014 Sep;4(3):378-86.
3. Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT Consortium. Clinical and Applied Thrombosis/Hemostasis. 2019 Jun 10;25:1076029619853037.
4. DeYoung E, Minocha J. Inferior vena cava filters: guidelines, best practice, and expanding indications. Seminars in Interventional Radiology. 2016 Jun;33(02):65-70.
5. Eldredge JB, Spyropoulos AC. Direct oral anticoagulants in the treatment of pulmonary embolism. Current Medical Research and Opinion. 2018 Jan 2;34(1):131-40.
6. Tromeur C, van der Pol LM, Mairuhu AT, Leroyer C, Couturaud F, Huisman MV, et al. Novel anticoagulant treatment for pulmonary embolism with direct oral anticoagulants phase 3 trials and clinical practice. Seminars in Interventional Radiology. 2018 Jun; 35(2):83-91.
7. Iaccarino A, Frati G, Schirone L, Saade W, Iovine E, D’Abramo M, et al. Surgical embolectomy for acute massive pulmonary embolism: state of the art. Journal of Thoracic Disease. 2018 Aug;10(8):5154.