Abstract
Background: There is a limited literature on the survival rates of male and female patients in surviving pulmonary embolism in Intensive Care Units (ICU). As such, this calls for study of its disparities. Our study aims to compare gender disparities in outcomes following pulmonary embolism treatment in the ICU.
Methods: A multicenter retrospective cohort study was conducted between January 2013 and December 2020 where we analyzed and compared gender disparities followed by outcomes. Data were retrieved from hospital databases, and patient medical records of P.E patients admitted to the intensive care unit (ICU) of six hospitals in China.
Results: The study included 100 confirmed pulmonary embolism patients with a mean age of 62 ± 14 years. Of them, 55 were female with co-morbidity chronic heart disease (20% vs. 4.4%), cigarette smokers (9.1% vs. 51.1%) all had (p<0.05) as compared to males. Also, females had APACHE II score of (17 ± 12 vs. 15 ± 10, p>0.05), 30-days and 6-months deaths, 4(7%) and 6(11%) with (p>0.05), respectively. Interestingly, sex-based outcomes on kidney injury revealed a statistically significant difference between females and males (p<0.05). In addition, there were also ssignificant differences in blood pressure between male and female non-survivors (p<0.05). Moreover, for non-survivors, factors attributed to mortality in descending order, were as follows; cardiopulmonary resuscitation (OR6.48, p<0.001) 95%CI, acute liver injury (OR6.23,p-0.02) 95%CI, vasopressors (OR5.46, p<0.001) 95%CI, acute kidney injury (OR5.27,p-0.02) 95%CI, mechanical ventilation (OR 4.90, p-0.02) 95%CI, acute respiratory failure (OR 4.00, p-0.02) 95%CI, APACHE II score (OR 1.07, p-0.03) 95%CI, Infections (OR 5.86, p-0.06) 95%CI, pH (OR 3.47, p-0.70) 95%CI, HCO3-(mmol/L) (OR 1.02, p-0.08) 95%CI, extracorporeal membrane oxygenation (OR 0.16, p-0.04) 95%CI.
Conclusion: Our study revealed no statistically significant sex-based differences in PE severity, outcomes or co-morbidities, with the exception of renal damage, which has been proven to have a negative impact. PE-related consequences and co-morbidities have been identified as statistically significant contributors to poor prognosis and mortality.
Keywords
Pulmonary embolism, Deep vein thrombosis, Acute physiology and chronic health evaluation II, Cardiopulmonary resuscitation
Introduction
Pulmonary embolism (PE) is a blockage of blood flow in the pulmonary artery bed that can result in a life-threatening and potentially reversible right ventricular failure [1]. PE remains one of the leading causes of poor prognosis and death, particularly when a shock or right ventricular failure occurs [2]. According to studies, PE is generally manifested in a nonspecific manner [3,4]. The difference in the presentation of symptoms and pathophysiology between males and females must be considered during management [5]. Recently, studies indicate that imaging modalities and cardiac biomarkers have been used in risk assessments and diagnosis [2,6].
Literature shows that PE affects roughly 60 to 70 people per 100,000 [7]. The literature also indicates that PE has a global incidence (0.3–30%) and death rate (16.9–31%) among individuals who have undergone surgery [8]. For instance, among the Chinese population with deep vein thrombosis (DVT), PE alone, and PE with DVT had annual occurrences of 30.0, 8.7 and 3.0 per 100,000 population, respectively, with a male-to-female ratio of 1:1.24 [9]. In addition to this, the 30- day mortality rate for DVT, PE alone, and PE with DVT were 9.0%, 17.4%, and 13.3%, respectively [9]. Studies have shown that a 30-day mortality rate is higher in males compared to females (9.8% vs.8.9%) [10, 11].
Furthermore, several studies have suggested that patients hospitalized in the ICU have at least one co-morbidity [12]. However, despite the severe therapeutic options in the ICU, the outcomes remain unpredictable [13]. It is believed that widening understanding and knowledge of PE in ICU settings is essential for management and risk identification. Similarly, understanding prognosis trends help to prevent late consequences in critically ill patients. Other scholars have indicated that better outcomes in acute PE have been achieved through institutional PE response teams offering timely and cost-reliable services for referred and discharged PE patients [14,15].
Early detection of high-risk PE in ICU patients has been considered critical for treatments and better outcomes. Many researchers have used the APACHE II scores at admission to predict 30-day mortality [2,11,16]. To explain this, PE patients with an APACHE II score >18 and the need for invasive ventilation (MV) are said to be susceptible to poor outcomes and/or death [11]. However, the latest data on sex-specific patterns and trends in therapy, outcomes, and complications after acute PE in the intensive care unit appears limited and contentious [17,18]. Given the significant prevalence of DVT and the high fatality rate of acute PE, whereby high-risk PE accounts for less than 5% of all PE [11], we anticipated PE outcomes to differ by gender due to differences in comorbidities in each group in ICU settings[12]. Therefore, it is critical and important to understand the impact of gender on outcomes among critically ill patients. We therefore conducted a study to compare gender disparities in outcomes following pulmonary embolism treatment in the intensive care unit.
Materials and Methods
Study population
The retrospective study was conducted in six ICU centers in Shandong province, Peoples Republic of China, between January 2013 and December 2020. This study protocols were approved by a review board of the medical research ethics committee.
Inclusion criteria: In our study we included all adult patients that were aged ≥18 years of age, and only included patients that had been confirmed to be diagnosed using computed tomography pulmonary angiography (CTPA) [1].
Exclusion criteria: In our study we excluded all the patients diagnosed with other modalities, such as a ventilation/perfusion (V/Q) scan, particularly those who were contraindicated for CTPA testing, such as those with severe renal failure, pregnant women, or those allergic to iodine contrast.
Data collection and definitions for patient characteristics
Based on the nature of our research design, no power analyses were performed. We obtained all data from hospital electronic medical records (EMR). All medical records were captured in the first 24 hours of admission of the patients. This includes age, gender, vital signs, arterial blood gases, and laboratory results, APACHE II. Thereafter, we screened the data for risk factors, and risks were defined as smoking habits, postpartum status, co-morbidities, and history of DVT. We also collected information on outcomes and treatment using low molecular weight heparin (LWMH), Urokinase (rtPa), vasopressor, embolectomy, number of patients who received CPR, and medical appliances used such as MV, extracorporeal membrane oxygenation (ECMO) and/or continuous renal replacement therapy (CRRT)[11]
Definition of APACHE II and blood tests
The APACHE II severity score is commonly used in the ICU to predict mortality and is measured during the first 24 hours of admission; its variables include age points and chronic illness points, having a maximum score of 71. Based on the results, the APACHE II scores of 25 and >35 indicate 50% and 80% chances of death, respectively [19]. The results of each patient’s APACHE II score test was obtained. Furthermore, laboratory cardiac biomarkers test results were recorded such as, creatine kinase-MB (CK-MB) 0.3-4ng/ml, cardiac troponin I (CTNI) 30.0 ng/l, myoglobin (0-70ng/ml), and high N-terminal pro-brain natriuretic peptide (NT-proBNP) levels ≥ 600 pg/ml; as well as D-dimer levels (<0.5 microgr/ml) and blood platelets (125-350) *10^9/L.
Radiologic evaluation
Following initial resuscitation and clinical stabilization, all patients underwent CTPA. Bilateral PE was defined as any thrombus in the main pulmonary artery (PA) in both the right and left PA, whereas left or right sided PE was defined as any thrombus in the left or right branches of the pulmonary arteries.
Follow-up and outcomes
Lastly, the duration of stay in the hospital(LOS), ICUs, and the number of patients who died in the hospital during the first 30 days were both documented. In our observation we targeted that the 30-day mortality rate to be the primary objective, whereas, a 6-month death was the secondary objective. On the other hand, we contacted survivors who had already been discharged to do a six-month follow-up. Lastly, we used a sixmonth point as our reference point.
Therapies for PE
In this study all patients had treatment for PE. The low molecular weight heparin (1 mg/kg subcutaneous) was used to start the therapy, and the activated partial thromboplastin time was measured every 5-6 hours. In the absence of contraindications, thrombolytic therapy provided in individuals with hypotensive (systolic blood pressure<90 mmHg). A multidisciplinary team was set to decide on thrombolytic therapy to reduce the risk of sequelae such as thrombocytopenia, cerebral haemorrhage, and hematomas. Patients with respiratory distress (respiratory rate>30 breaths/min, abnormal breathing pattern, O2 saturation 90% for >5 minutes) and blood pressure instability were clinically stabilized using various methods, including vasopressors, CPR, and invasive MV. In contrast, other patients received more advanced therapies such as embolectomy, ECMO, and/ or CRRT.
Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) Version 26; IBM Corporation, Armonk, NY, USA software. Continuous variables were expressed as the median and interquartile range (IQR), and categorical variables were expressed as numbers and percentages. Continuous variables were compared with Student’s t-test or Mann–Whitney U test, whereas categorical variables were compared with chi-square or Fisher’s exact tests. An adjusted odds ratio (OR) and a 95% (CI) were reported for each independent factor. Multivariate logistic regression analysis was used to identify the possible risk factors for ICU mortality. Variables with a probability (p) value less than 0.1 in univariate models were introduced into the multivariate logistic models. A two-tailed p value<0.05 was considered statistically significant.
Results
Identification of patients
Table 1 shows patients’ characteristics and differences between males and females. A total of 100 confirmed PE patients aged ≥ 18 years were admitted to ICUs between 2013 and 2020, of which, 55 were female. The mean age of male vs. female (62 ± 15 vs 62 ± 14, p>0.05) not significant. The differences in cigarette smoking were (9.1% vs. 51.1%) had (p<0.05) as compared to males. As expected, disease severity APACHE II score, co-morbidities, arterial blood test results and outcomes were prevalent, although not statistically significant (all p>0.05). There was one significant co-morbidity, chronic heart disease, which was at (4.4% vs. 20%, p<0.05). In addition, CTPA data showed no significant differences in PE location, effusion, or atelectasis between male and female all comprised (p>0.05).
| variables | All patients n=100 | male=45 | female=55 | P-value |
|---|---|---|---|---|
| Age (year) | 62 ± 14 | 62 ± 15 | 62 ± 14 | 0.84 |
| Smoking (n, %) | 28 (28%) | 23 (51.1%) | 5 (9.1%) | <0.001 |
| surgery<1month (n, %) | 13 (13%) | 5 (11.1%) | 8 (14.5%) | 0.61 |
| Postpartum (n, %) | 7 (7%) | 0 (0.00%) | 7 (13.0%) | 0.01 |
| APACHE II | 16 ± 11 | 15 ± 10 | 17 ± 12 | 0.38 |
| Co-morbidities (n, %) | ||||
| Cancer | 23 (23%) | 11 (24.4%) | 12 (21.8%) | 0.76 |
| Hypertension | 23 (23%) | 11 (24.4%) | 12 (21.8%) | 0.76 |
| Diabetes mellitus | 15 (15%) | 7 (15.6%) | 8 (14.5%) | 0.88 |
| Chronic heart disease | 13 (13%) | 2 (4.4%) | 11 (20%) | 0.02 |
| Vital signs and arterial blood gases on admission | ||||
| Heart rate (beats/min) | 99 ± 24 | 100 ± 27 | 98 ± 21 | 0.54 |
| Systolic Bp (mmhg) | 119 ± 28 | 121 ± 26 | 118 ± 29 | 0.67 |
| Diastolic Bp (mmhg) | 73 ± 19 | 74 ± 18 | 72 ± 21 | 0.70 |
| RR (b/min) | 23 ± 8 | 24 ± 8 | 22 ± 8 | 0.56 |
| Ph | 7.41 ± 0.12 | 7.41 ± 0.105 | 7.40 ± 0.134 | 0.86 |
| PaCO2 (mmhg) | 38 ± 11 | 38 ± 11 | 37 ± 11 | 0.88 |
| PaO2 (mmhg) | 95 ± 55 | 86 ± 46 | 103 ± 61 | 0.11 |
| PaHCO3 (mmol/L) | 24 ± 6 | 25 ± 6 | 24 ± 7 | 0.69 |
| SaO2 (%) | 92 ± 12 | 91 ± 13 | 93 ± 12 | 0.39 |
| Lactate (mmol/L) | 3.11 ± 4 | 3.10 ± 4 | 3.12 ± 4.23 | 0.84 |
| Related complications (n, %) | ||||
| Acute respiratory failure | 48 (48%) | 19 (42.2%) | 29 (52.7%) | 0.30 |
| Deep vein Thrombosis | 27 (27%) | 14 (31.1%) | 13 (23.6%) | 0.89 |
| CPR | 16 (16%) | 5 (11.1%) | 11 (20%) | 0.23 |
| Acute kidney injury | 9 (9%) | 4 (8.9%) | 5 (9.1%) | 0.97 |
| Obstructive shock | 7 (7%) | 2 (8.9%) | 5 (9.1%) | 0.37 |
| HIE | 7 (7%) | 1 (2.2%) | 6 (11%) | 0.90 |
| Acute liver injury | 9 (9%) | 4 (8.9%) | 5 (9.1%) | 0.97 |
| Laboratory findings | ||||
| CKMB (ng/ml) | 10 ± 48 | 15 ± 10 | 17 ± 12 | 0.38 |
| CTNI (ng/L) | 419 ± 2979 | 174 ± 915 | 623 ± 3953 | 0.46 |
| Myoglobin (ng/mL) | 98 ± 203 | 90 ± 168 | 104 ± 229 | 0.74 |
| NT-Pro BNP (pg/mL) | 2844 ± 5521 | 2859 ± 5865 | 2831 ± 5273 | 0.98 |
| Platelets (x109/L) | 193 ± 102 | 180 ± 100 | 203 ± 102 | 0.27 |
| D-dimers | 359 ± 1632 | 673 ± 2346 | 102 ± 498 | 0.12 |
| Treatments& interventions (n, %) | ||||
| LMWH | 86 (86%) | 38 (84.4%) | 48 (87.3%) | 0.69 |
| MV | 37 (37%) | 19 (42.2%) | 18 (32.7%) | 0.33 |
| rtPA | 9 (9%) | 4 (8.9%) | 5 (9.1%) | 0.97 |
| ECMO | 6 (6%) | 2 (4.4%) | 4 (7.3%) | 0.55 |
| CRRT | 5 (5%) | 3 (6.7%) | 2 (3.6%) | 0.49 |
| CTPA findings (n, %) | ||||
| Bilateral sided PE | 47 (47%) | 23 (51%) | 24 (44%) | 0.51 |
| Right sided PE | 24 (24%) | 8 (18%) | 16 (29%) | 0.88 |
| Left sided PE | 9 (9%) | 5 (11%) | 4 (7%) | 0.17 |
| Bilateral multiple PE | 17 (17%) | 8 (18%) | 9 (16%) | 0.52 |
| Other findings (n, %) | ||||
| Pleural effusion | 59 (59%) | 29 (64%) | 30 (55%) | 0.32 |
| Atelectasis | 23 (23%) | 9 (20%) | 14 (26%) | 0.41 |
| Outcomes | ||||
| LOS (days,IQR) | 18 ± 15 | 18 ± 12 | 18 ± 18 | 0.98 |
| ICUs (days,IQR) | 9 ± 8 | 7 ± 6 | 10 ± 9 | 0.20 |
| 30-days mortality (n,%) | 6 (6%) | 2 (4.4%) | 4 (7%) | 0.58 |
| 6-months mortality (n,%) | 10 (10%) | 4 (9%) | 6 (11%) | 0.45 |
P<0.05 significance: CPR: Cardiopulmonary Resuscitation; APACHE II- Acute Physiology and Chronic Health Evaluation II; CTNI-Cardiac Troponin I; CK-MB-Creatine Kinase-MB; NT Pro BNP: N-Terminal Pro Brain-type Natriuretic Peptide; HIE: Hypoxic Ischemic Encephalopathy; LOS: Length of Hospital Stays; LICU: Length of ICU stay; MV: Mechanical Ventilation; ECMO: Extracorporeal Membrane Oxygenation; CRRT: Continuous Renal Replacement Therapy; LMWH: Low Molecular Weight Heparin; rtPA: Urokinase
Table 1: Patient characteristics.
Outcome data
Table 2 shows that all patients had severe co-morbidities and abnormal physiological indicators in the first 24 hours following their admission in the hospital (ICU). As anticipated, non-survivors had more severe sickness severity (APACHE II score), co-morbidities such as chronic heart disease and cancer, as well as PE-related complications comprised of acute respiratory failure, acute kidney damage, acute liver failure, infection and, physiological derangements (pH, HR & HCO3-), and cardiac troponin I (ctn I)) (p <0.05 for all). There were also two more typical problems in PE patients undergoing therapy subarachnoid hemorrhage (SAH) and thrombocytopenia which showed (p<0.05). Treatments used for severe PE patients, such as MV, ECMO, and pulmonary embolectomy, all had (p<0.05). In addition to this, the majority of non-survivors the mortality rate and CPR measures were both found to be statistically significant (p<0.05).
| Variables | All patients=100 | survivors=84 | non-survivors=16 | p-value |
|---|---|---|---|---|
| Age (years) | 62 ± 14 | 61 ± 15 | 68 ± 11 | 0.09 |
| Male | 45 | 39 (46%) | 6 (40%) | 0.56 |
| Immobility | 37 (37%) | 27 (32%) | 10 (63%) | 0.02 |
| APACHE II | 16 ± 11 | 14 ± 10 | 24 ± 12 | <0.001 |
| Co-morbidity (%) | ||||
| Cancer | 23 (23%) | 15 (18%) | 8 (50%) | 0.01 |
| Arterial blood gases on admission | ||||
| Ph | 7.41 ± 0.12 | 7.42 ± 0.11 | 7.31 ± 0.162 | 0.03 |
| Heart rate(beats/min) | 98 ± 24 | 96 ± 24 | 108 ± 22 | 0.05 |
| Lactate (mmol/L) | 3.05 ± 3.91 | 6.64 ± 7.14 | 2.5 ± 2.85 | 0.06 |
| HCO3 (mmol/L) | 24 ± 7 | 20 ± 7.14 | 25 ± 6.34 | <0.001 |
| Related complications (n,%) | ||||
| ARF | 9 (9%) | 5 (6%) | 4 (25%) | 0.02 |
| CPR | 16 (16%) | 9 (11%) | 7 (44%) | <0.001 |
| AKI | 9 (9%) | 5 (6%) | 4 (25%) | 0.02 |
| ALI | 6 (6%) | 3 (4%) | 3 (19%) | 0.02 |
| SAH | 1 (1%) | 0 (0.0%) | 1 (7.7%) | 0.01 |
| Other illness (n,%) | ||||
| Infection | 4 (4%) | 2 (2%) | 2 (13%) | 0.05 |
| Thrombocytopenia | 4 (4%) | 1 (1.1%) | 3 (13%) | 0.02 |
| Laboratory results | ||||
| CTNI (ng/L) | 419 ± 2979 | 139 ± 700 | 1873 ± 7254 | 0.03 |
| NT-Pro BNP (pg/mL) | 2844 ± 5521 | 2585 ± 4687 | 4186 ± 8762 | 0.49 |
| D-dimers (mcg/mL) | 359 ± 1632 | 360 ± 1683 | 356 ± 1381 | 0.99 |
| Platelets(x109/L) | 193 ± 102 | 194 ± 103 | 185 ± 95 | 0.75 |
| CTPA findings (n, %) | ||||
| Bilateral sided PE | 47 (47%) | 40 (48%) | 7 (44%) | 0.75 |
| Bilateral multiple PE | 17 (17%) | 12 (15%) | 5 (31%) | 0.10 |
| Right sided PE | 24 (24%) | 21 (25%) | 3 (19%) | 0.58 |
| Left sided PE | 9 (9%) | 9 (11%) | 0 (0.0%) | 0.17 |
| LMWH | 86 (86%) | 73 (87%) | 13 (81%) | 0.55 |
| MV | 37 (37%) | 26 (31%) | 11 (69%) | <0.001 |
| Vasopressors | 25 (25%) | 16 (19%) | 9 (56%) | <0.001 |
| rtPA | 9 (9%) | 7 (8.3%) | 2 (13%) | 0.59 |
| ECMO | 6 (6%) | 3 (4%) | 3 (19%) | 0.02 |
| Embolectomy | 4 (4%) | 1 (1.2%) | 3 (13%) | 0.02 |
| Outcomes | ||||
| LOS(IQR) | 18 ± 15 | 18 ± 16 | 15 ± 13 | 0.40 |
| ICUs(IQR) | 9 ± 8 | 9 ± 8 | 9 ± 11 | 1.00 |
| 30-days mortality(n,%) | 6 (6%) | 0 (0.0%) | 6 (53%) | <0.001 |
| 6-months mortality(n,%) | 10 (10%) | 0 (0.0%) | 10 (63%) | <0.001 |
ARF: Acute Respiratory Failure; AKI: Acute Kidney Injury; ALI: Acute Lver Injury; SAH: Sub-Arachnoid Hemorrhage
Table 2: Comparison of outcomes.
Table 3 shows the results of the data and differences according to sex. As anticipated, non-survivors exhibited blood pressure (systolic and diastolic) derangements all had (p<0.05). Furthermore, the complication of renal injury was included with (p<0.05) for both gender outcome results. Figure 1 illustrates the Kaplan–Meier survival plot of survivors based on gender at 6-month period. The median 6-month survival for both gender groups could not be ascertained, however at 88 days, roughly 91% of females and 97% of men consecutively were still alive following PE treatments.
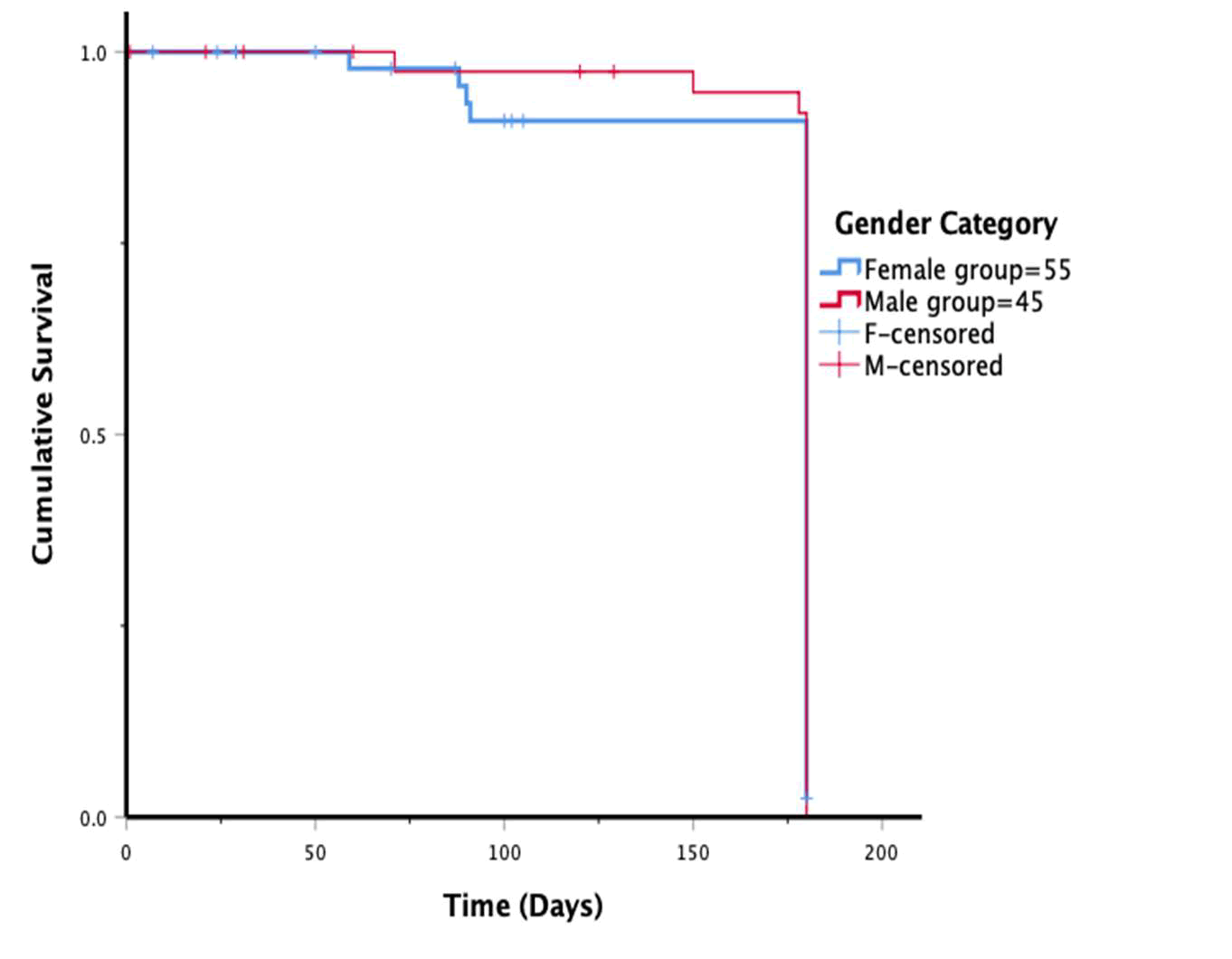
Figure 1: Kaplan–Meier survival graphs for PE patients based on gender +, censored (logrank, P>0.05); female; male.
| Variables | survivors=84 | p-value | non-survivors=16 | p-value | ||
|---|---|---|---|---|---|---|
| Male=39 | Female=46 | Male=6 | Female-10 | |||
| Age (year) | 60 ± 15 | 62 ± 14 | 0.58 | 71 ± 7 | 60 ± 16 | 0.12 |
| APACHE II | 14 ± 10 | 15 ± 11 | 0.57 | 22. ± 9 | 25 ± 14 | 0.66 |
| Vital signs & Arterial blood gases on admission | ||||||
| Heart rate (beat/min) | 94 ± 23 | 97 ± 25 | 0.48 | 117 ± 21 | 103 ± 22 | 0.25 |
| Systolic Bp (mmhg) | 118 ± 24 | 121 ± 28 | 0.54 | 131 ± 22 | 102 ± 22 | 0.03 |
| Diastolic Bp (mmhg) | 72 ± 16 | 75 ± 19 | 0.54 | 86 ± 16 | 61 ± 10 | <0.001 |
| RR (b/min) | 23 ± 7.5 | 25 ± 99 | 0.52 | 25 ± 4 | 18 ± 8 | 0.10 |
| pH | 7.41 ± 0.11 | 7.43 ± 0.11 | 0.28 | 7.42 ± 0.07 | 7.30 ± 0.07 | 0.08 |
| PaCO2 (mmhg) | 38 ± 8 | 37 ± 10 | 0.77 | 37 ± 7 | 35 ± 13 | 0.72 |
| PaO2 (mmhg) | 88 ± 40 | 91 ± 49 | 0.38 | 94 ± 73 | 128 ± 95 | 0.46 |
| SaO2 (%) | 90 ± 19 | 94 ± 10 | 0.56 | 87 ± 16 | 83 ± 10 | 0.29 |
| Lactate (mmol/L) | 2.76 ± 3.48 | 2.53 ± 2.28 | 0.72 | 2.72 ± 2.21 | 6.85 ± 7.87 | 0.15 |
| HCO3 (mmol/L) | 25 ± 6 | 26 ± 6 | 0.57 | 24 ± 9 | 19 ± 8 | 0.19 |
| Co-morbidities (n,%) | ||||||
| Cancer | 11 (28%) | 8 (17%) | 0.29 | 2 (40%) | 4 (40%) | 0.73 |
| CHD | 2 (5%) | 8 (17%) | 0.68 | 1 (20%) | 4 (40%) | 0.28 |
| DM | 6 (15%) | 7 (15%) | 0.94 | 2 (40%) | 3 (30%) | 0.93 |
| Related complications (n,%) | ||||||
| ARF | 16 (41%) | 21 (46%) | 0.53 | 4 (80%) | 7 (70%) | 0.72 |
| DVT | 11 (28%) | 10 (10%) | 0.90 | 3 (50%) | 3 (30%) | 0.42 |
| AKI | 5 (15%) | 1 (2%) | 0.03 | 0 (0.0%) | 5 (50%) | 0.02 |
| CPR | 4 (10%) | 7 (15%) | 0.44 | 1 (20%) | 5 (50%) | 0.14 |
| ALI | 1 (3%) | 2 (4%) | 0.63 | 1 (20%) | 3 (30%) | 0.51 |
| Obstructive shock | 4 (10%) | 2 (4%) | 0.32 | 2 (40%) | 1 (10%) | 0.25 |
| Treatment and interventions (n,%) | ||||||
| MV | 16 (41%) | 13 (28%) | 0.29 | 4 (80%) | 7 (70%) | 0.72 |
| ECMO | 2 (5%) | 1 (2%) | 0.49 | 0 (0.0%) | 3 (30%) | 0.12 |
| LMWH | 33 (85%) | 41 (89%) | 0.26 | 5 (100%) | 5 (50%) | 0.12 |
| rtPA | 3 (8%) | 4 (9%) | 0.81 | 1 (20%) | 0 (0.0%) | 0.19 |
| Vasopressors | 7 (18%) | 8 (17%) | 0.97 | 3 (60%) | 7 (70%) | 0.25 |
| Embolectomy | - | - | - | 2 (40%) | 0 (0.0%) | 0.63 |
| CTPA findings(n, %) | ||||||
| Bilateral sided PE | 20 (51%) | 20 (44%) | 0.58 | 3 (60%) | 4 (40%) | 0.83 |
| Bilateral multiple PE | 5 (13%) | 7 (15%) | 0.72 | 3 (30%) | 2 (20%) | 0.26 |
| Right sided PE | 8 (21%) | 18 (39%) | 0.27 | 0 (0.0%) | 2 (20%) | 0.22 |
| Pleural effusion | 24 (61%) | 27 (59%) | 0.79 | 5 (100%) | 3 (30%) | 0.06 |
| Atelectasis | 9 (23%) | 12 (26%) | 0.48 | 0 (0.0%) | 2 (20%) | 0.23 |
| Outcomes | ||||||
| LOS (days,IQR) | 18 ± 12 | 19 ± 18 | 0.85 | 16 ± 11 | 14 ± 14 | 0.79 |
| ICUs (days,IQR) | 7 ± 7 | 10 ± 8 | 0.19 | 8 ± 7 | 9 ± 13 | 0.76 |
Table 3: Comparison of outcomes according to sub-gender groups.
Table 4 shows a multivariate logistic regression analysis. The risk factors that contributed positively to the poor outcomes for non-survivors in descending order were as follows; CPR (OR6.48, p<0.001) 95%CI, acute liver injury (OR6.23,p-0.02) 95%CI, vasopressors (OR5.46, p<0.001) 95%CI, acute kidney injury (OR 5.27, p-0.02) 95%CI, mechanical ventilation (OR 4.90, p-0.02) 95%CI, acute respiratory failure (OR 4.00, p-0.02) 95%CI, APACHE II score (OR 1.07, p-0.03) 95%CI, Infections (OR 5.86, p-0.06) 95%CI, pH (OR 3.47, p-0.70) 95%CI, HCO3- (mmol/L) (OR 1.02, p-0.08) 95%CI, ECMO (OR 0.16, p-0.04) 95%CI.
| Variables | Odds ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Clinical score | |||
| HCO3 (mmol/L) - |
1.02 | 0.76–1.02 | 0.08 |
| pH | 3.47 | 0.01–2089 | 0.70 |
| APACHE II | 1.07 | 1.00–1.14 | 0.03 |
| Co-morbidity (n,%) | |||
| Chronic heart disease | 1.71 | 0.41–7.05 | 0.46 |
| Related complications (n, %) | |||
| Acute respiratory failure | 4.00 | 1.19–13.43 | 0.02 |
| Acute kidney injury | 5.27 | 1.24–22.41 | 0.02 |
| Acute liver injury | 6.23 | 1.13–34.25 | 0.02 |
| CPR | 6.48 | 1.94–21.64 | <0.001 |
| Other illness (n, %) | |||
| Infections | 5.86 | 0.76–45.06 | 0.06 |
| Drugs & laboratory findings (n,%) | |||
| ECMO | 0.16 | 0.03–0.88 | 0.04 |
| MV | 4.90 | 1.55–15.56 | <0.001 |
| Vasopressors | 5.46 | 1.77–16.88 | <0.001 |
Table 4: Independent predictors of non-survivor according to multivariate logistic regression analysis.
Discussion
Our study was aimed to asses any gender differences in the outcomes of patients who were admitted in the ICU with a condition of acute PE. The findings of this study revealed three significant conclusions. Firstly, the differences in severity based on the APACHE II score, length of hospital stay (LOS and ICUs), and thirty-days and six-month mortality between males and females were statistically insignificant. Secondly, PE-related complications and co-morbidities have been identified as contributing factors to poor prognosis and mortality. Finally, our findings revealed high APACHE II values >18 upon admission, and invasive MV was a significant predictor of poor outcomes in the non-survivor group as also found by other scholars [11].
In continuation, our study found no differences in severity APACHE II scores based on gender outcomes; overall, females registered more deaths than men but not statistically significant (Table 3). In comparison to males, most females had severe symptoms, even cardiac markers (NT-pro BNP and Ctn1), as well as D-dimers, were found to be higher in the blood, indicated the severity of PE [20] (Tables 1 and 2). We postulated PE related complications and other metabolic defects such as low pH, lactic acidosis, ventilation-related problems, and therapeutic complications. All these could have instigated poor outcomes. Similarly, Agarwal et al., studied more than 200,000 people discharged with PE. This study revealed that women had more severe symptoms such as low blood pressure (1.19 % vs. 1.04 %), more episodes of blood transfusions (6.49% vs. 4.47%), and were treated more with thrombolytics (27% vs. 23%) compared to men [17]. Also, a review study by Angela et al., showed that when given anticoagulants, women were 46% vs 21% more likely to suffer from bleeding problems than men [21] In addition, from our findings, the APACHE II severity scores were 24 and (OR; 1.07, 95%CI, p-0.03) which was found to be a predictor of death in non-survivors (Table 4). Corresponding to our findings, Ergan et al., retrospectively studied 56 patients, with 13% mortality also found APACHE II score >18 cut-offs to be a predictor of poor outcome and increased mortality risk, however, more deaths were recorded in males (32.1% vs. 69.2%; p=0.943) inversely co-related to our findings [11]. Also, from our findings we postulated that disparities in outcomes could be due to the disease severity scores resemblances across gender groups in ICU. Moreover, similar to our findings Keller et al., prospectively studied 569 PE patients, the overall 14.8% adverse 30-day outcomes were seen but no differences in death outcomes between male and female were observed [22]. Contrasting our findings, the above study focused on normotensive patients with few symptoms and did not require intrusive treatment methods. Contradictory to our findings, Borrero et al., retrospectively studied 15,531 patients, found out that males had a higher crude 30-day mortality (9.8% vs. 8.9%) and poor outcomes which were caused by high rates of recurrent VTE and suboptimal anticoagulation intake due to poor compliances in men [10]. However, the above study used diagnostic codes, which could have resulted in patient selection bias and/or it lacked specificity because their study did not focus on ICU patients or confirmed diagnosis by CTPA compared to what we used in our study.
In addition, studies have suggested that patients who undergo CPR have a 57% mortality rate, as compared to 12% of those who did not [23]. The overall mortality rate associated with large PE remains around 30% [24]. Research reveals that when CPR is needed, mortality rates rise. Even today, the operative mortality rate for patients with extensive PE who require CPR can be as high as 75% [23,24]. Our findings suggested that CPR had a statistically significant and positive impact on cause mortality in non-survivors (Table 4), and we anticipated that refractory low blood pressure, respiratory failure, hypoxia ischemic encephalopathy and related comorbidities could be the causes of death in resuscitated patients [25]. As a continuation, for gender-based outcomes, females had received more CPR than males but not significant (Table 3), yet the prognosis remained ambiguous due to post- CPR effects and the impacts of vasopressors used during CPR. Our findings suggested that the use of vasopressors during CPR could have positively contributed to the probability of death in PE patients (Tables 3 and 4). In this study we further hypothesized that increased vasopressor such as norepinephrine administration was linked to adverse effects that could lead to a poor prognosis; however, co-morbidities also played a significant role. Similarly, other scholars like Yamamura et al., and Martin et al., have conducted studies that revealed that multiple doses of norepinephrine >1 mcg/min promote immunosuppression, ischemia and pulmonary edema that might contribute to poor prognosis and/or mortality [26,27]. Regarding CPR the findings of Konstantinov et al, of 2007, corresponded to our findings; studied retrospectively 8 patients with massive PE required CPR. The overall 30-day mortality rate was 28.5% and 1 patient died after 8-months due to long-term consequences of brain injury sustained during resuscitation [23]. The study differs from ours in that it was conducted in a single center and had a smaller sample size. Similarly, several studies have postulated that cardiac arrest after PE has a poor prognosis. Nevertheless, surgical interventions, on the other hand, are found to enhance early survival rates [23,28]. Furthermore, retrospectively Dutta et al., studied 996 cardiac arrest patients, 87 (8.7%) of whom had acute PE. They found no difference in outcomes between cardiac arrest survivors who had PE-related cardiac arrest (68.3%) and those who had other types of cardiac arrest (64%) [29], whereas, older age, female sex, and co-morbidities were all predictors of mortality in PE patients in 24 hours after CPR resuscitation.
Furthermore, in our study, six patients were kept on ECMO and had a statistical significance (Table 2). ECMO was found to have protective effects as it offered hemodynamic and respiratory support in massive PE cases (Table 4). Although ECMO therapy had no statistical significance in genderrelated outcomes in both groups, it had benefits in minimizing multiple organ damage in patients with major PE and shock (Table 3). Corresponding with our findings, Hobohm et al, retrospectively analyzed 1,172,354 individuals hospitalized with PE, of whom 2,197 (0.2%) received ECMO assistance. Cardiac arrest occurred in 77,196 people (6.5%), necessitating CPR indicating that patients who received embolectomy or thrombolysis together with VA-ECMO or VA-ECMO alone had a lower risk of death compared to thrombolysis alone [30]. In addition, a meta-analysis study by Karami et al., has suggested patients ≤ 60 years who received VA-ECMO plus surgical embolectomy seemed to have better outcomes but no survival benefits were observed for acute PE therapies[31]. Despite its profound benefits, a meta-analysis study by Zangrillo, A. et al,2013, when employed for acute PE therapy, ECMO therapy has been linked to cause poor results and mortality[32]. According to our findings, we postulated ECMO should not be used as a stand-alone therapy for PE patients, but rather as a temporary therapy to enhance surgical or pharmacological therapy in order to achieve better outcomes.
Additionally, PE related complications and co-morbidity were statistically significant and negatively impacted patients’ prognosis (Tables 2 and 4), most organs can be harmed as a result of the low blood pressure caused by massive PE, resulting in the production of inflammatory mediators, tissue necrosis, and subsequent multiple organ damage [33]. In addition, kidney injury showed statistically significant findings for gender subgroup outcomes (Table 3). Henceforth we hypothesized that mechanisms of kidney and liver injury could have caused endothelial injuries, activated procoagulants [34], and impaired the coagulation system could have led to thrombus formation, exacerbate PE recurrences and poor prognosis [35,36]. A case report by Tuchscherer et al, has suggested in PE, hypoxemic respiratory distress increases breathing drive which produces peripheral vasoconstriction and increase in venous return, which in turn raise pulmonary blood flow causing edemas and lung failure [37]. Similarly, retrospective study of Mameli et al, comprised of 971 PE cases showed chronic heart failure played a significant role in the hospital mortality in PE patients [36]. Contrast to ours, the study above used diagnostic codes, which could have resulted in selection bias, not included ICU patients and some of patients were diagnosed by V/Q scan. Also, Gupta et al., examined retrospectively 183 subjects for 4.1 years have discovered that co-morbidities such as cancer (19%), chronic heart disease (20%), myocardial infarction (17%) and obstructive pulmonary diseases (24%), diabetes mellitus (19%) all played a role in causing mortality in PE [38]. However, within the above-mentioned research, interventions such as MV, ECMO, or anticoagulants, were not thoroughly examined, all of which could have led to problems and affect prognosis [11,30,32].
Likewise, by comparing survivors and non-survivors, invasive MV had statistical significance and has shown to increase the probability of death in PE patients (Table 4). However, Invasive MV for gender-based outcomes had no statistical significance (Table 3). We postulated that MV-induced high intrathoracic pressure exacerbates right ventricular heart failure, which can further limit cardiac output, reducing tissue oxygen delivery, and causing a variety of organ damage that could have hampered patient recovery [39,40]. Also, hypotension, hypoxemia (as indicated by a changes in blood pH), and abrupt respiratory failure may have all played a role in the poor outcome [37] (Tables 2 and 4). Correspondingly, Winterton et al., did a retrospective study comprised of 2797 patients, and compared ventilated with non-ventilated outcomes. Their study found that MV patients had a poor prognosis and an overall mortality rate of 14.1% (41.0 % p-0.0001) of patients were kept on MV [6]. These findings differ to ours, some of the suggested reasons being that their study included a larger sample size but lacked precision in patient selection because not all patients were confirmed by CTPA. Similarly, Ergan et al., 2016, retrospectively compared high-risk patients and intermediate patients, of whom 29% required MV. This study found that the use of invasive MV (OR 30.10 95% CI 1.96–463.31) was discovered to be an independent predictor of death [11]. Conversely to our findings, in their study other therapeutic procedures that aid PE patients in the ICU, such as embolectomy and ECMO, as well as NT-Pro BNP readings which determine the severity of PE, were not included.
An interesting finding of this study is that no gender-based outcome difference is seen. This phenomenon has been also reported in previous studies which found no sex-based differences in PE severity, need for advanced therapies, or short-term outcomes [41]. Consistent with prior studies, our multivariate regression analysis revealed that co-morbidities have a substantial role in ICU patients’ poor prognosis and mortality. The reason why females have a higher risk of death is multifactorial. Consequently, the impact of gender on PErelated mortality requires further research.
Limitation
The study was based on a retrospective design. From six ICU centers in city of Shandong province in People’s Republic of China. Based on our study we have found that reporting PE in ICU with a relatively small research group is difficult. For instances the patients who underwent CTA were purposively selected for the study, so there was a considerable selection bias. Furthermore, a multi-center study implies that management is not homogeneous. Therefore, the results cannot be generalized and are limited by the small sample size, and more research with more ICU centers is required.
The strengths of our research findings. Firstly, It provides crucial insight into the most severe clinical presentation of PE and the overall outcomes to expect in ICU settings. Secondly, we used CTPA a gold standard diagnostic tool for PE, which is now widely accepted as the primary approach for detecting PE. As a result, we were able to prevent patient selection biases, misdiagnosis, and the inappropriate demography for our study [6,11]. Lastly, our study findings identified clinical features such as APACHE II, Ctn1, NT-Pro BNP, D-dimers, and platelets that can be used to predict the severity of PE and the risk variables that affect its prognosis, as well as guide clinical treatment [11,20].
Conclusion
Our study revealed no significant sex-based differences in PE severity, outcomes, or complications, with the exception of renal damage, which has been proven to have a negative impact. There were also substantial differences in blood pressure between male and female non-survivors. PE-related consequences and co-morbidities have been identified as significant contributors to poor prognosis and mortality. Further, we found that increased arterial blood gases indicators (pH and HCO3-) and the necessity for MV have negative prognostic indicators and contribute to poor outcomes. The reasons for these disparities are undoubtedly complex, but they should be a top priority for research to provide appropriate and evidence-based care to patients who have been hospitalized for a long time or who are critically ill.
Acknowledgments
We are thankful to the following Dr. Liu Han of Cheeloo ICU department, Philip Ndakama and Stanford Jeremiah both from Muhimbili national hospital for helping with data collection procedures. We’d also like to thank Natasha Robinson of the department of education at the University of Oxford for her help with language and writing skills.
Author Contributions
E. R Mkama designed the research and wrote the manuscript; J. Peng, Y. Zhou, T. Zhou, J. Du, G. Pang and M. Si: obtained the medical data; Likewise, Y. Li: assisted in the collection of medical data and coordination of the project. W. Qin: Participated in the study’s design and performed out the statistical analysis. X. Chen: proofread the manuscript. All authors read and approved the final manuscript.
Disclosure of Conflict of Interests
No competing financial interests exist.
Funding Information
This research didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
2. Bach AG, Taute BM, Baasai N, Wienke A, Meyer HJ, Schramm D, et al. 30-day mortality in acute pulmonary embolism: prognostic value of clinical scores and anamnestic features. PLoS One. 2016 Feb 11;11(2):e0148728.
3. Tapson VF. Acute pulmonary embolism. New England Journal of Medicine. 2008 Mar 6;358(10):1037-52.
4. Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. Derivation and validation of a prognostic model for pulmonary embolism. American Journal of Respiratory and Critical Care Medicine. 2005 Oct 15;172(8):1041-6.
5. Deng X, Li Y, Zhou L, Liu C, Liu M, Ding N, et al. Gender differences in the symptoms, signs, disease history, lesion position and pathophysiology in patients with pulmonary embolism. PLoS One. 2015 Jul 24;10(7):e0133993.
6. Winterton D, Bailey M, Pilcher D, Landoni G, Bellomo R. Characteristics, incidence and outcome of patients admitted to intensive care because of pulmonary embolism. Respirology. 2017 Feb;22(2):329-37.
7. Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Experimental & Clinical Cardiology. 2013;18(2):129.
8. Temgoua MN, Tochie JN, Noubiap JJ, Agbor VN, Danwang C, Endomba FT, et al. Global incidence and case fatality rate of pulmonary embolism following major surgery: a protocol for a systematic review and meta-analysis of cohort studies. Systematic reviews. 2017 Dec;6(1):1-6.
9. Law Y, Chan YC, Cheng SW. Epidemiological updates of venous thromboembolism in a Chinese population. Asian Journal of Surgery. 2018 Mar 1;41(2):176-82.
10. Borrero S, Aujesky D, Stone RA, Geng M, Fine MJ, Ibrahim SA. Gender differences in 30-day mortality for patients hospitalized with acute pulmonary embolism. Journal of Women’s Health. 2007 Oct 1;16(8):1165-70.
11. Ergan B, Ergün R, Çalışkan T, Aydın K, Tokur ME, Savran Y, et al. Mortality related risk factors in high-risk pulmonary embolism in the ICU. Canadian Respiratory Journal. 2016 Jan 1;2016.
12. Simpson A, Puxty K, McLoone P, Quasim T, Sloan B, Morrison DS. Comorbidity and survival after admission to the intensive care unit: a population-based study of 41,230 patients. Journal of the Intensive Care Society. 2021 May;22(2):143-51.
13. Saad N. Aggressive management of pulmonary embolism. InSeminars in interventional radiology 2012 Mar (Vol. 29, No. 01, pp. 052-056). Thieme Medical Publishers.
14. Yamamoto T. Management of patients with high-risk pulmonary embolism: a narrative review. Journal of Intensive Care. 2018 Dec;6(1):1-9.
15. Rali PM, Criner GJ. Submassive pulmonary embolism. American Journal of Respiratory and Critical Care Medicine. 2018 Sep 1;198(5):588-98.
16. VijayGanapathy S, Karthikeyan VS, Sreenivas J, Mallya A, Keshavamurthy R. Validation of APACHE II scoring system at 24 hours after admission as a prognostic tool in urosepsis: A prospective observational study. Investigative and Clinical Urology. 2017 Nov 1;58(6):453-9.
17. Agarwal S, Clark III D, Sud K, Jaber WA, Cho L, Menon V. Gender disparities in outcomes and resource utilization for acute pulmonary embolism hospitalizations in the United States. The American Journal of Cardiology. 2015 Oct 15;116(8):1270-6.
18. Mosca L, Barrett-Connor E, Kass Wenger N. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011 Nov 8;124(19):2145-54.
19. Akavipat P, Thinkhamrop J, Thinkhamrop B, Sriraj W. Acute physiology and chronic health evaluation (APACHE) II score–the clinical predictor in neurosurgical intensive care unit. Acta Clinica Croatica. 2019 Mar;58(1):50.
20. Fu C, Chen YY, Zhu F, Liu J. Analysis of the Occurrence of Acute Pulmonary Embolism in the ICU Ward and Related Risk Factors Predicting Its Severity. The Heart Surgery Forum. 2022;25(2):E190-E195.
21. Jarman AF, Mumma BE, Singh KS, Nowadly CD, Maughan BC. Crucial considerations: Sex differences in the epidemiology, diagnosis, treatment, and outcomes of acute pulmonary embolism in non‐pregnant adult patients. Journal of the American College of Emergency Physicians Open. 2021 Feb;2(1):e12378.
22. Keller K, Rappold L, Gerhold-Ay A, Hobohm L, Hasenfuß G, Konstantinides SV, et al. Sex-specific differences in pulmonary embolism. Thrombosis Research. 2019 Jun 1;178:173-81.
23. Konstantinov IE, Saxena P, Koniuszko MD, Alvarez J, Newman MA. Acute massive pulmonary embolism with cardiopulmonary resuscitation: management and results. Texas Heart Institute Journal. 2007;34(1):41.
24. Kürkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, et al. Pulmonary embolism as cause of cardiac arrest: presentation and outcome. Archives of Internal Medicine. 2000 May 22;160(10):1529- 35.
25. Witten L, Gardner R, Holmberg MJ, Wiberg S, Moskowitz A, Mehta S, et al . Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation. 2019 Mar 1;136:93-9.
26. Yamamura H, Kawazoe Y, Miyamoto K, Yamamoto T, Ohta Y, Morimoto T. Effect of norepinephrine dosage on mortality in patients with septic shock. Journal of Intensive Care. 2018 Dec;6(1):1-7.
27. Martin C, Medam S, Antonini F, Alingrin J, Haddam M, Hammad E, et al. Norepinephrine: not too much, too long. Shock. 2015 Oct 1;44(4):305-9.
28. Laher AE, Moolla M, Motara F, Paruk F, Richards G. Survival after cardiac arrest secondary to massive pulmonary embolism. Case Reports in Emergency Medicine. 2018 Jan 31;2018.
29. Dutta A, Tayal B, Kragholm KH, Masmoudi Y, Azizian J, Mcdonald L, et al. Characteristics and outcomes of cardiac arrest survivors with acute pulmonary embolism. Resuscitation. 2020 Oct 1;155:6-12.
30. Hobohm L, Sagoschen I, Habertheuer A, Barco S, Valerio L, Wild J, et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation. 2022 Jan 1;170:285-92.
31. Karami M, Mandigers L, Miranda DD, Rietdijk WJ, Binnekade JM, Knijn DC, et al. Survival of patients with acute pulmonary embolism treated with venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. Journal of Critical Care. 2021 Aug 1;64:245-54.
32. Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Critical Care and Resuscitation. 2013 Sep;15(3):172-8.
33. Liu X, Chang S, Fu C, Huo Z, Zhou J, Liu C, et al. Predictors of midterm prognosis and adverse factors in acute pulmonary embolism. Therapeutic Advances in Respiratory Disease. 2017 Aug;11(8):293- 300.
34. Huang MJ, Wei RB, Wang Y, Su TY, Di P, Li QP, et al. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open. 2017 May 1;7(5):e014294.
35. Søgaard KK, Horváth-Puhó E, Montomoli J, Vilstrup H, Sørensen HT. Cirrhosis is associated with an increased 30-day mortality after venous thromboembolism. Clinical and Translational Gastroenterology. 2015 Jul;6(7):e97.
36. Mameli A, Palmas MA, Antonelli A, Contu P, Prandoni P, Marongiu F. A retrospective cohort study of patients with pulmonary embolism: the impact of comorbidities on patient’s outcome. European Respiratory Journal. 2016 Aug 1;48(2):555-7.
37. Tuchscherer D, Hollinger A, Bremerich J, Siegemund M. Prolonged respiratory failure due to pulmonary embolism in a young woman: a case report. Journal of Medical Case Reports. 2019 Dec;13(1):1-4.
38. Gupta R, Ammari Z, Dasa O, Ruzieh M, Burlen JJ, Shunnar KM, et al. Long-term mortality after massive, submassive, and low-risk pulmonary Embolism. Vascular Medicine. 2020 Apr;25(2):141-9.
39. Tanabe Y, Yamamoto T, Murata T, Mabuchi K, Hara N, Mizuno A, et al. Gender differences among patients with acute pulmonary embolism. The American Journal of Cardiology. 2018 Sep 15;122(6):1079-84.
40. Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. bmj. 2020 Aug 5;370.
41. Pribish A, Secemsky EA. Sex-Based Disparities Among Hospitalized Patients with Acute Pulmonary Embolism. American College of Cardiology. Apr 12, 2021.
