Abstract
Acute pulmonary embolism (PE) is when one or more thrombus travel to the lungs and obstruct the pulmonary artery or one of the branches of the pulmonary tree, producing signs and symptoms immediately after the obstruction. Saddle pulmonary embolism (SPE) is a rare type of acute PE that can lead to hemodynamic instability and death. The incidence of pulmonary embolism increases with age. In women, the risk of PE increases with pregnancy, hormonal contraceptives, and hormone replacement therapy. In this case, the patient was in her forties and presented with a sudden episode of continuous dyspnea that worsened over four hours. The dyspnea was associated with palpitations and diaphoresis. The clinical scoring tools had a low pre-test probability for PE. The patient had no risk factors for PE other than being obese. Significant laboratory workup showed troponin of 0.10, D-dimer of 8.10, and a B-type natriuretic peptide (BNP) of 1,160. Her computed tomography angiography (CTA) showed extensive pulmonary emboli in the bilateral main and segmental pulmonary arteries, with findings consistent with right heart strain. The patient was managed with an unfractionated heparin loading dose in the ED based on her weight, followed by a heparin drip. Then, the patient was transferred to a tertiary medical center for further interventions. Prompt recognition and treatment of a submassive PE are fundamental to improving patient mortality and morbidity.
Keywords
Saddle pulmonary embolism, Submassive pulmonary embolism, Acute pulmonary embolism, Interventional radiology guided embolization, Thrombus, Venous thromboembolism, Pulmonary embolism with cardiac strain, Pulmonary embolism with right ventricular strain
Introduction
Saddle pulmonary embolism (SPE) is a rare type of acute PE that can lead to hemodynamic instability and death [1]. Submassive PE is hemodynamically stable [2]. Pulmonary embolism is the third most common cause of cardiovascular death (after myocardial infarction and stroke) in the United States per year [3]. Patients with right ventricular strain have a mortality rate of up to 25% when hemodynamically stable and up to 65% when hemodynamically unstable [3]. The RV/LV ratio assesses RV compromise and the significance of PE burden. The RV/LV ratio is measured using the maximal RV and LV diameters from inner wall to inner wall on the axial slice that best approximates the four-chamber view [3] (Figure 2). PE presents a wide range of clinical presentations, from asymptomatic, making diagnosing PE challenging to death [4]. The most common presenting symptom is dyspnea. Other symptoms, such as chest pain, cough, and hemoptysis, might be present. With severe PE, patients can present with arrhythmias [2]. The provoking risk factors for PE are recent surgery, trauma, hormone therapy, immobilization, or active cancer. The non-provoking risk factors are obesity and heavy cigarette smoking [2]. This article describes a submassive PE with cardiac strain in a young woman with no PE risk factors or physical findings who was promptly diagnosed and managed with heparin.
Case Presentation
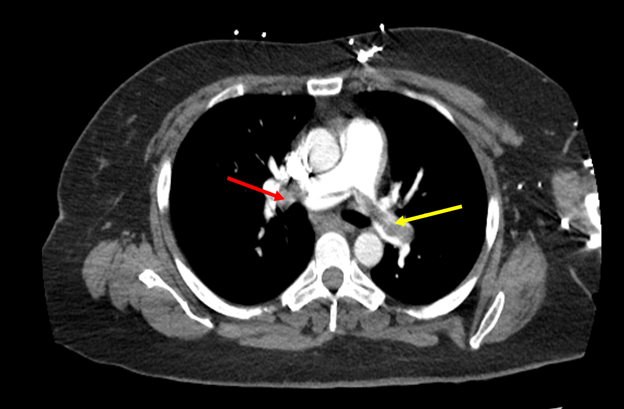
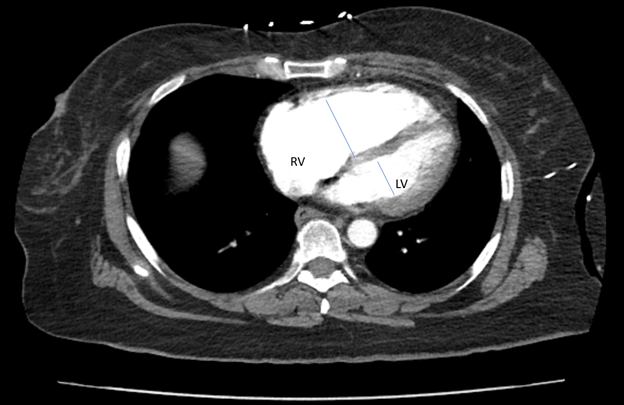
A 48-year-old woman presented to the emergency department with new onset shortness of breath—her past medical history of hyperlipidemia, hypertension, panic attacks, and generalized anxiety disorder. The dyspnea began suddenly and increased in severity within the last four hours. The dyspnea worsened with exertion and was associated with diaphoresis and palpitations. About a year ago, the patient had a similar episode that lasted a few minutes and was self-resolved. The patient denied any recent travel, hormonal treatment, history of thrombus, recent surgery or illness, trauma, smoking history, or active cancer. The risk factor she had for PE was her BMI of 30, which classified her as obese. On arrival, the patient's blood pressure was 135/90 mm Hg; oxygen saturation was 98% on room air, respirations were 14 breaths/min, and heart rate was 90 bpm. The physical exam was notable for generalized anxiety. The rest of the physical exam was unremarkable. The Well's score for PE was 0 points; the Geneva score was 3 points, the pulmonary embolism rule-out criteria (PERC) was 0, and the pulmonary embolism severity index (PESI) was 48 points. These tools to assess pre-test probability for PE were low. ECG showed normal sinus rhythm at 85 bpm with one pre-ventricular contraction. Significant laboratory workup showed troponin of 0.10, D-dimer of 8.10, and a B-type natriuretic peptide (BNP) of 1,160. Computed tomography angiography (CTA) showed extensive pulmonary emboli in the bilateral main and segmental pulmonary arteries, with findings consistent with right heart strain (Figure 1). The RV/LV ratio increased, measuring approximately 1.1 (Figure 2). In light of a submassive PE with cardiac strain, the patient received an unfractionated loading dose of heparin, followed by a drip. Then, the patient was transferred to a tertiary medical center for further management our center did not provide.
Figure 1. Computed tomography angiography. Cross-sectional view of CTA highlighting PE. The red arrow showed a thrombus on the right pulmonary artery. The yellow arrow showed a thrombus in the left pulmonary artery.
Figure 2. Computed tomography angiography. Cross-sectional view of CTA highlighting the RV/LV ratio measuring approximately 1.1.
Discussion
Patients with submassive PE have a high mortality risk. In this case, the patient was dyspneic and had an underlying diagnosis of generalized anxiety disorder. Her presentation was misleading at first. Moreover, all the Wells, PERC, PESI, and Geneva scores had a low pre-test probability indicating a PE was a low risk or even ruled out. The risk stratification did not aid the clinical decision. The tools mentioned above were unreliable in this setting.
Furthermore, the patient presented no physical findings such as leg swelling, pain, venous stasis, discoloration, tachycardia, or tachypnea. Fortunately, the clinical suspicion for PE suggested ordering a simple, fast, inexpensive screening test with a high-sensitivity D-dimer [5]. The D-dimer was 8.10 in this case. The next step was ordering a CTA based on the clinical correlation of PE and elevated D-dimer. The CTA showed a submassive PE with cardiac strain (Figure 1) and an increased RV/LV ratio measuring approximately 1.1 (Figure 2). The patient's BNP and troponin levels correlated with right ventricular strain. Patients with RV strain or instability benefit from systemic thrombolytics or mechanical thrombectomy [6]. The patient was managed with a heparin drip and then transferred to a tertiary center for the saddle pulmonary embolus thrombectomy.
This case highlights the importance of considering pulmonary embolism as a potential diagnosis in patients presenting with sudden onset shortness of breath and no risk factors. Although the patient physically did not look obese, her BMI categorized her as obese. Obese patients have an increased risk of venous thrombosis secondary to immobilization or sedentary lifestyles [7]. Most obese patients with BMI >30 have additional problems, such as osteoarthritis that impedes mobility. Prompt diagnosis and treatment can be life-saving and help prevent complications associated with PE.
Conclusion
The patient, in this case, had no notable risk factors or physical findings for PE. The case demonstrated that clinical suspicion is sufficient to work up a patient for PE. It also highlights that prompt diagnosis and management of submassive PE with cardiac strain in non-critically ill patients have a low mortality and morbidity rate.
Author’s Contributions
All authors participated in managing this patient, conceived the idea of the case report, and participated in the drafting of the study.
Acknowledgment
Dr. Miguel Diaz, MD, Program Director & Faculty, Department of Family Medicine, Larkin Community Hospital Palm Springs Campus, for his support in this publication and for inspiring us to be better physicians and contributors to the medical field.
Competing Interests
All authors in this case report declare that they have no competing interests.
References
2. Taylor, B.,Kabrhel, C. (2022). Overview of acute pulmonary embolism in adults. Uptodate. Retrieved April 9, 2023 from
3. https://www.uptodate.com/contents/overview-of-acute-pulmonary-embolism-in-adults?search=massive%20pulmonary%20embolism&source=search_result&selectedTitle=2~49&usage_type=default&display_rank=2
4. Bryce YC, Perez-Johnston R, Bryce EB, Homayoon B, Santos-Martin EG. Pathophysiology of right ventricular failure in acute pulmonary embolism and chronic thromboembolic pulmonary hypertension: a pictorial essay for the interventional radiologist. Insights into imaging. 2019 Dec;10(1):18.
5. Tarbox AK, Swaroop M. Pulmonary embolism. International Journal of Critical Illness and Injury Science. 2013;3(1):69-72.
6. Gao H, Liu H, Li Y. Value of D-dimer levels for the diagnosis of pulmonary embolism: An analysis of 32 cases with computed tomography pulmonary angiography. Experimental and Therapeutic Medicine. 2018 Aug 1;16(2):1554-60.
7. Biggs MD, Bell J, Park C. Pharmacological Management of Saddle Pulmonary Embolism in a High-Risk Patient With COVID-19. Cureus. 2022 Jun 22;14(6):e26211.
8. Cascio V, Hon M, Haramati LB, Gour A, Spiegler P, Bhalla S, et al. Imaging of suspected pulmonary embolism and deep venous thrombosis in obese patients. The British Journal of Radiology. 2018 Sep;91(1089):20170956.
9. Therriault C, Natkiel D, Stobart-Gallagher M. Saddle Pulmonary Embolus. Journal of Education and Teaching in Emergency Medicine. 2019;4(2):21-5.
10. Shekhda KM, Ameer N. Pulmonary Embolism and Right Heart Strain: Can It Be Diagnosed Based on Electrocardiographic Findings?. Chonnam Medical Journal. 2020 Sep;56(3):206-7.